Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3334
Revised: August 25, 2024
Accepted: August 28, 2024
Published online: October 27, 2024
Processing time: 117 Days and 1.4 Hours
Perivascular epithelioid cell tumor (PEComa) is a rare mesenchymal neoplasm that predominantly affects the kidney and uterus. The occurrence of this tumor in the liver, particularly with simultaneous involvement of the liver and kidney, is exceedingly uncommon. Pathological diagnosis is the gold standard. PEComas usually show positive immunohistochemical staining for melanocytic (HMB-45, Melan-A) and myoid (SMA, muscle-specific actin) markers.
We presented a noteworthy case of malignant PEComa affecting both the liver and kidney in a 53-year-old man with tuberous sclerosis complex (TSC). FAT2 and TP73 mutations in the kidney were identified and positive expression of diagnostic markers including HMB-45, Melan A, and TFE3 were detected. In addition, we demonstrated that hepatic artery perfusion chemotherapy was ineffective for hepatic PEComa, while surgery remained the most effective approach. Everolimus showed an excellent efficacy in the postoperative treatment of the tumor.
Surgical treatment is preferred for malignant PEComa affecting liver and kidney, especially with TSC; everolimus is effective postoperatively.
Core Tip: Perivascular epithelioid cell tumor (PEComa) is rare. Herein, we present the case of a 53-year-old man with the concurrent presence of malignant PEComa in both the liver and kidney, accompanied by tuberous sclerosis complex. During treatment, the hepatic artery perfusion chemotherapy was ineffective for hepatic PEComa, while surgery remained the most effective approach. Everolimus showed an excellent efficacy during the postoperative treatment of the patient. This report provides valuable insights for the diagnosis and treatment of PEComa, paving the way for future clinical practice.
- Citation: Yang HT, Wang FR, He N, She YH, Du YY, Shi WG, Yang J, Chen G, Zhang SZ, Cui F, Long B, Yu ZY, Zhu JM, Zhang GY. Massive simultaneous hepatic and renal perivascular epithelioid cell tumor benefitted from surgery and everolimus treatment: A case report. World J Gastrointest Surg 2024; 16(10): 3334-3342
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3334.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3334
Perivascular epithelioid cell tumor (PEComa) is a rare mesenchymal neoplasm, belonging to a member of the family (PEComas) including lymphangioleiomyomatosis, classic angiomyolipoma (AML) and clear epithelioid cell tumors. It is most frequently detected in the retroperitoneum, peritoneal and pelvic cavities, and occasionally found in somatic soft tissues and the skin[1,2]. The female-to-male ratio is approximately 6: 1 and it is more common in young and middle-aged patients[3]. This tumor, an epithelioid AML, mainly consists of epithelioid cells, and lacks typical fat tissues[4]. It is typically accompanied with focal infiltration of blood vessel walls and the cells are often arranged radially around the vascular lumen[5]. PEComas usually show immunoreactivity for melanocytic (HMB-45, Melan-A) and myoid (SMA, muscle-specific actin) markers. Melan-A is expressed in more than 50% cases and approximately 25% of patients show desmin expression[4]. Compared with other markers, positive HMB-45 is often considered the gold standard for the diagnosis of AML histopathologically[6]. Most patients are asymptomatic or complain of non-specific gastrointestinal manifestations. The tumor is usually of a large size and holds the malignant potential. The more aggressive are less common[7]. Here, we present a case with the concurrent presence of malignant PEComa in both the liver and kidney, accompanied by tuberous sclerosis complex (TSC).
A 53-year-old man with a one-year history of intermittent abdominal distention and anorexia was hospitalized, who also experienced fatigue and shortness of breath for 2 months.
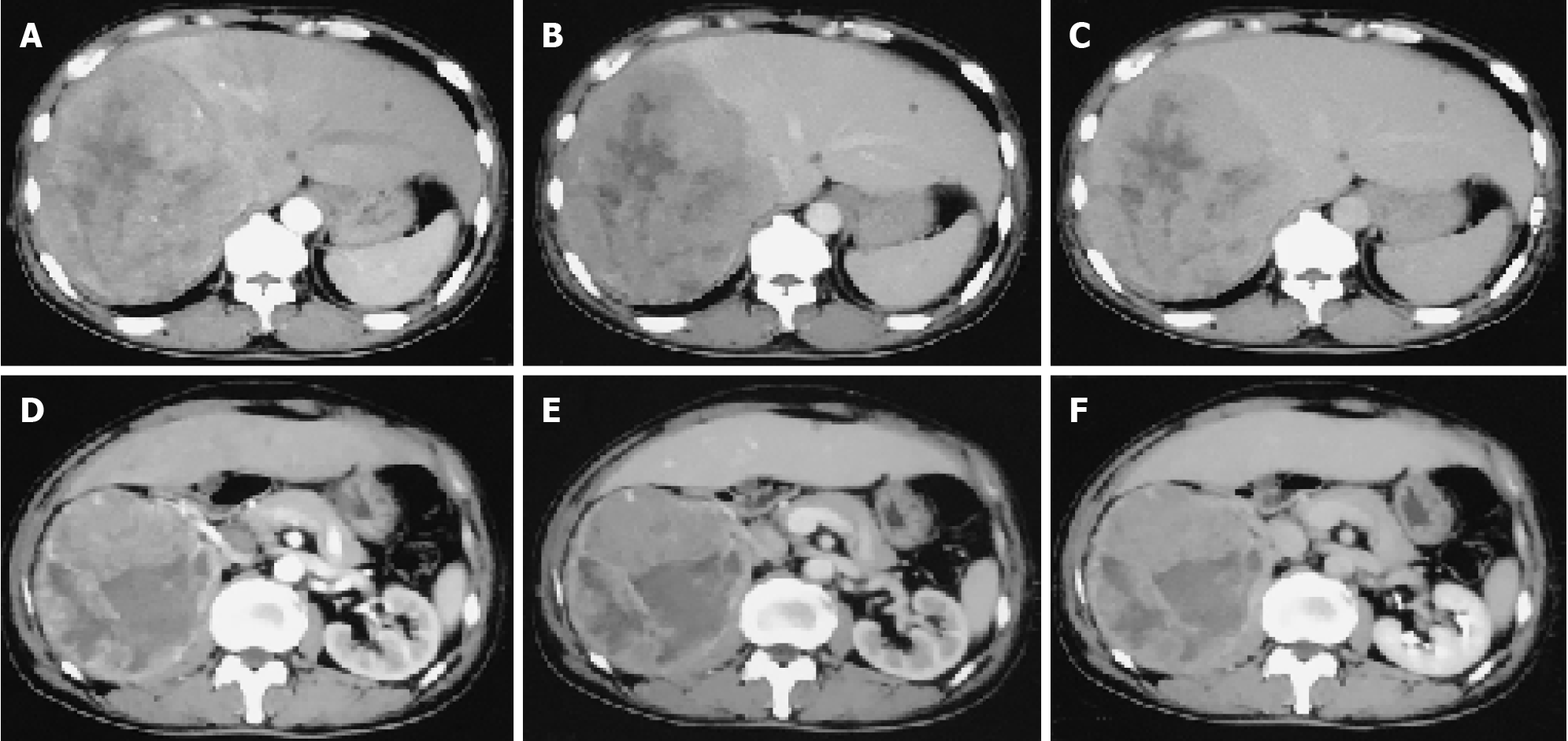
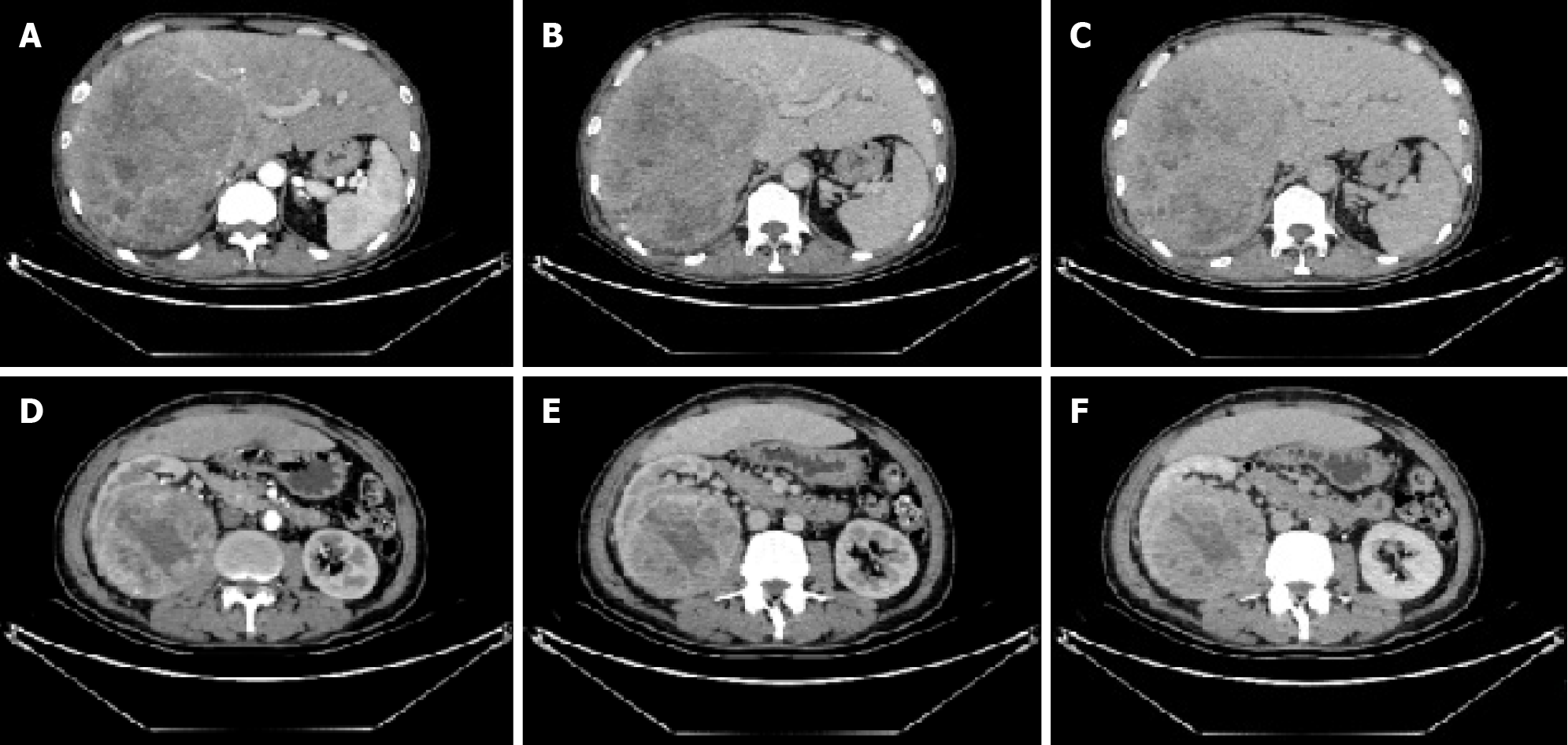
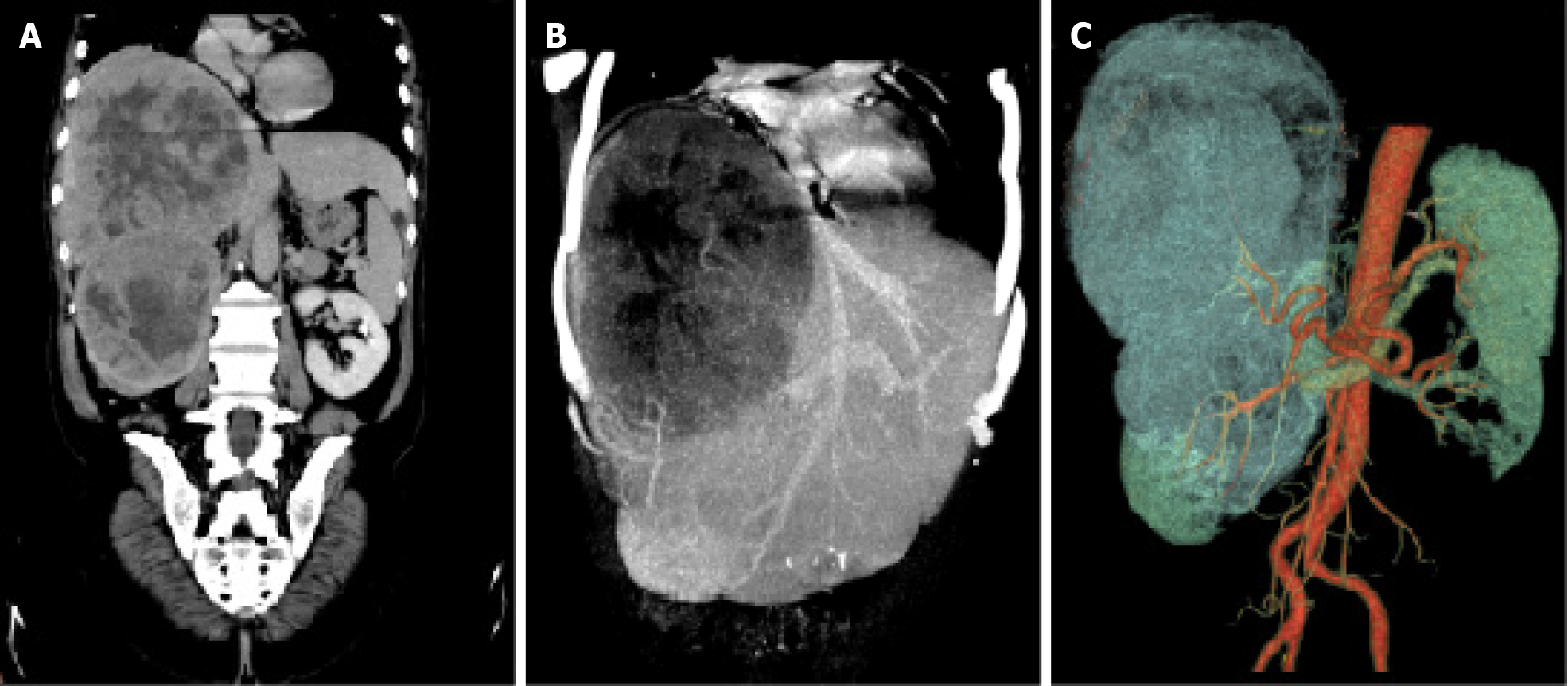
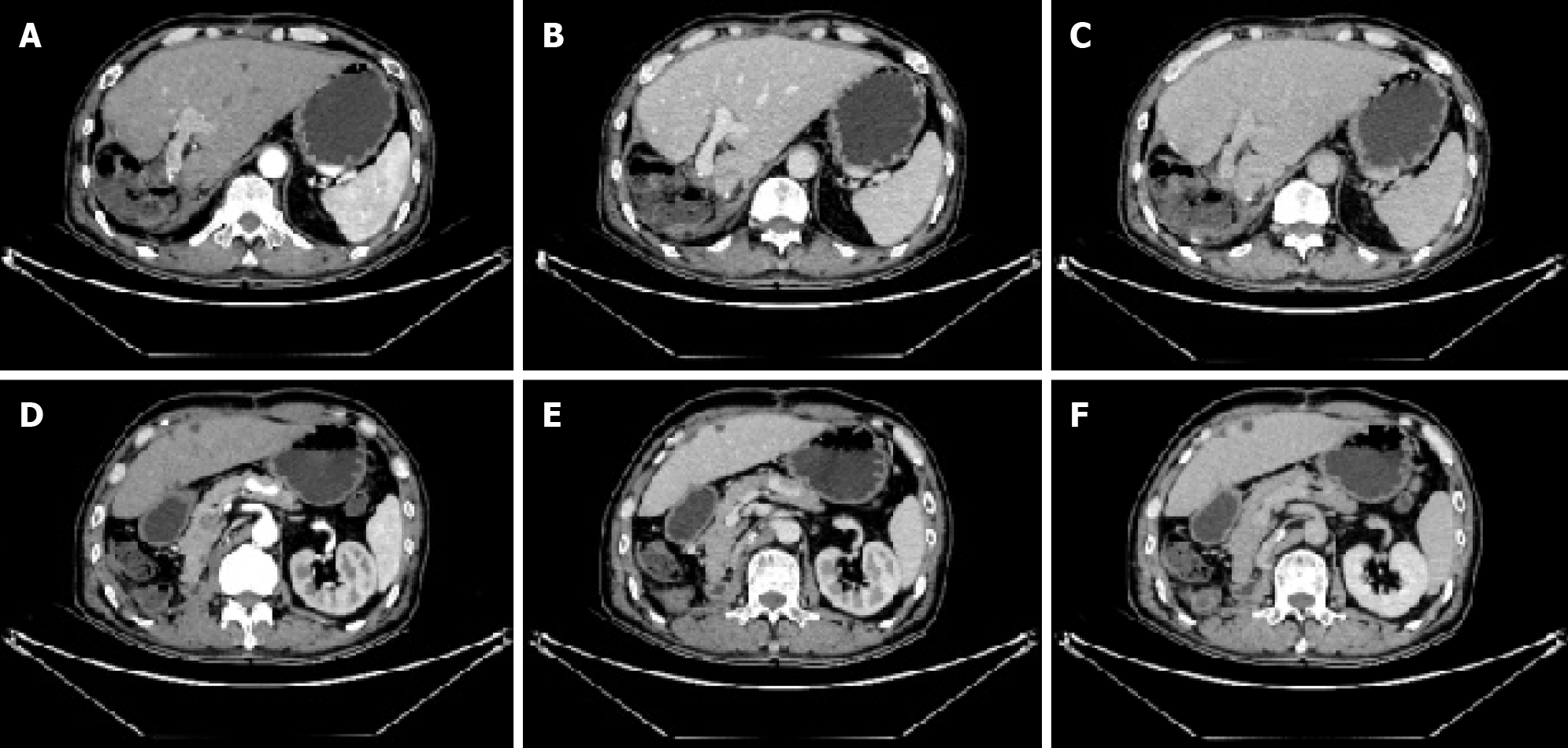
To control tumor progression and alleviate discomfort, we attempted to perform the hepatic artery perfusion chemotherapy with oxaliplatin, leucovorin and fluorouracil after obtaining informed consent from the patient and his family. Surprisingly, the size of the liver tumor increased from 14.0 cm × 16.0 cm before treatment (Figure 1) to 14.5 cm × 18.5 cm after treatment (Figure 2). And tumor vessels were observed (Figure 3). During hospitalization, the patient’s chest tightness and shortness of breath worsened and blood pressure was elevated intermittently, reaching up to 145/96 mmHg. Blood pressure was controlled with oral administration of phenoxybenzamine (10 mg, twice a day) and nifedipine (30 mg, once a day).
The patient had no history of diabetes, hypertension or other cardiovascular diseases.
The patient denied any family history of malignant tumors and TSC.
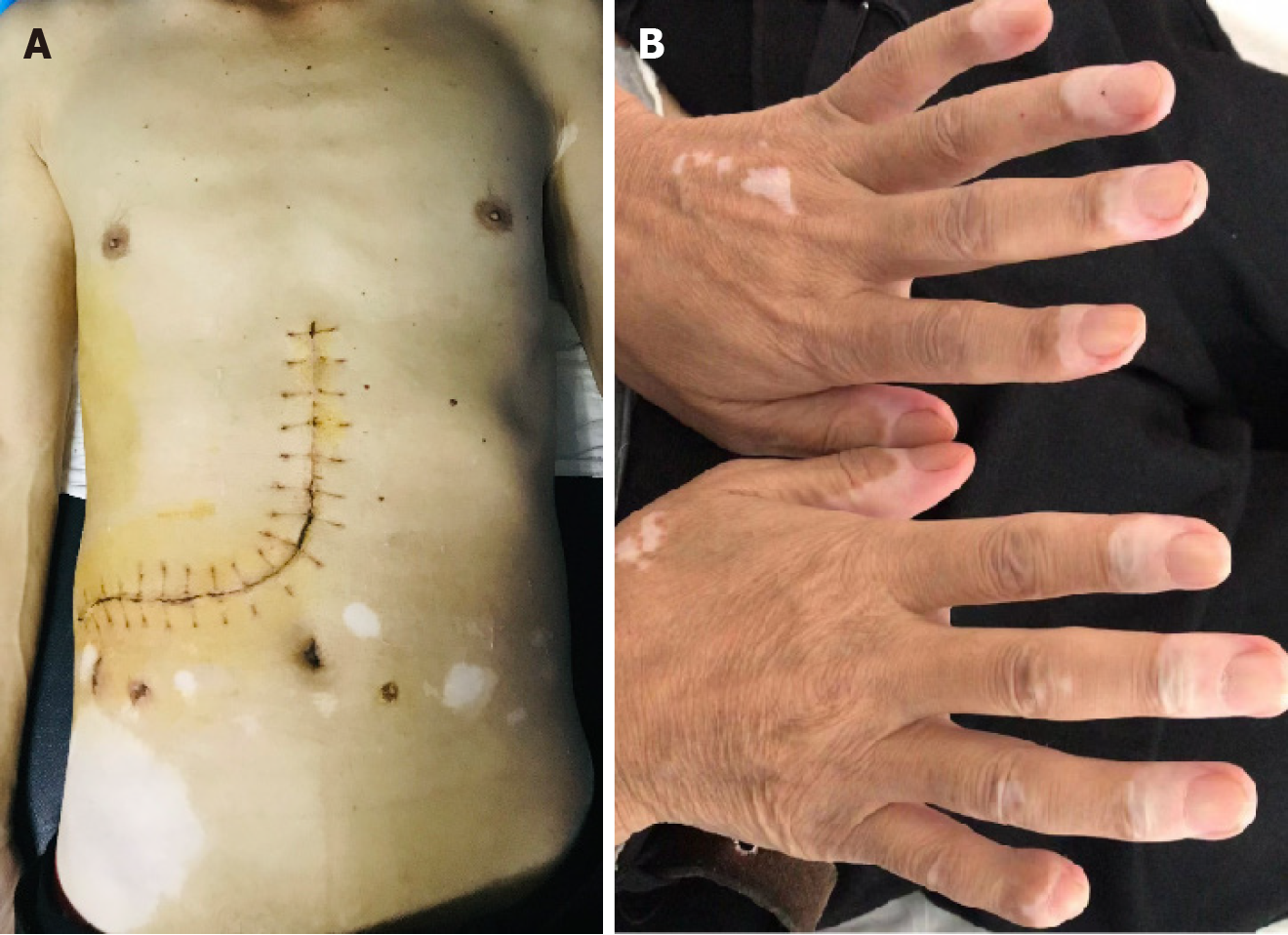
Physical examination revealed space-occupying lesions with non-tender, flexible texture, poor mobility and blurred boundaries in the right upper abdomen. Many depigmented macules were found in multiple regions of the body, especially in the abdomen (Figure 4).
Laboratory results showed an albumin concentration of 30.5 g/L, lactate dehydrogenase level of 665 U/L (120-250), interleukin-6 level of 207.00 pg/mL (0.00-7.00), PO2 of 66 mmHg (80-100), SO2 of 93% (95%-98%), methoxynorepinephrine level of 72.9 pg/mL, methoxyepinephrine level below 18.0 pg/mL and dopamine level of 35.9 pg/mL. Tumor markers revealed elevated levels of CA199 48.9 U/mL (0.00-27.00 U/mL), CA72-4 9.42 U/mL (0.00-6.90 U/mL) and neuronal-specific enolase 119.0 ng/mL (0.00-15.20 ng/mL).
He had undergone contrast-enhanced computed tomography (CT) scans of the abdomen, which confirmed the presence of neoplasms located in the right lobe of the liver and the upper pole of the right kidney (Figure 1).
Based on various clinical data, including patient complaints, physical, laboratory and imaging examinations, and pathology and immunohistochemistry findings, the patient was diagnosed with malignant PEComa.
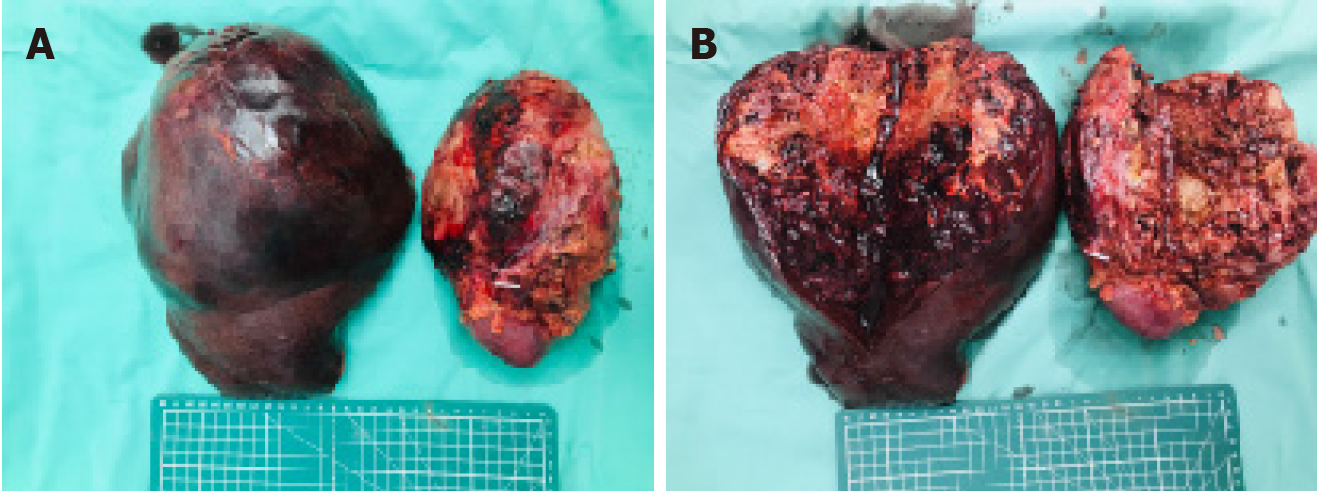
The patient underwent open surgical resection, including right hemihepatectomy, right nephrectomy and right adrenalectomy. Gross examination of the specimens revealed a liver tumor measuring approximately 24 cm × 17 cm × 14.5 cm and a kidney tumor measuring approximately 12 cm × 10 cm × 11.7 cm, both with gray-yellow or gray-red surfaces and focal hemorrhage (Figure 5). Finally, the patient recovered well and was discharged on the 15th postoperative day.
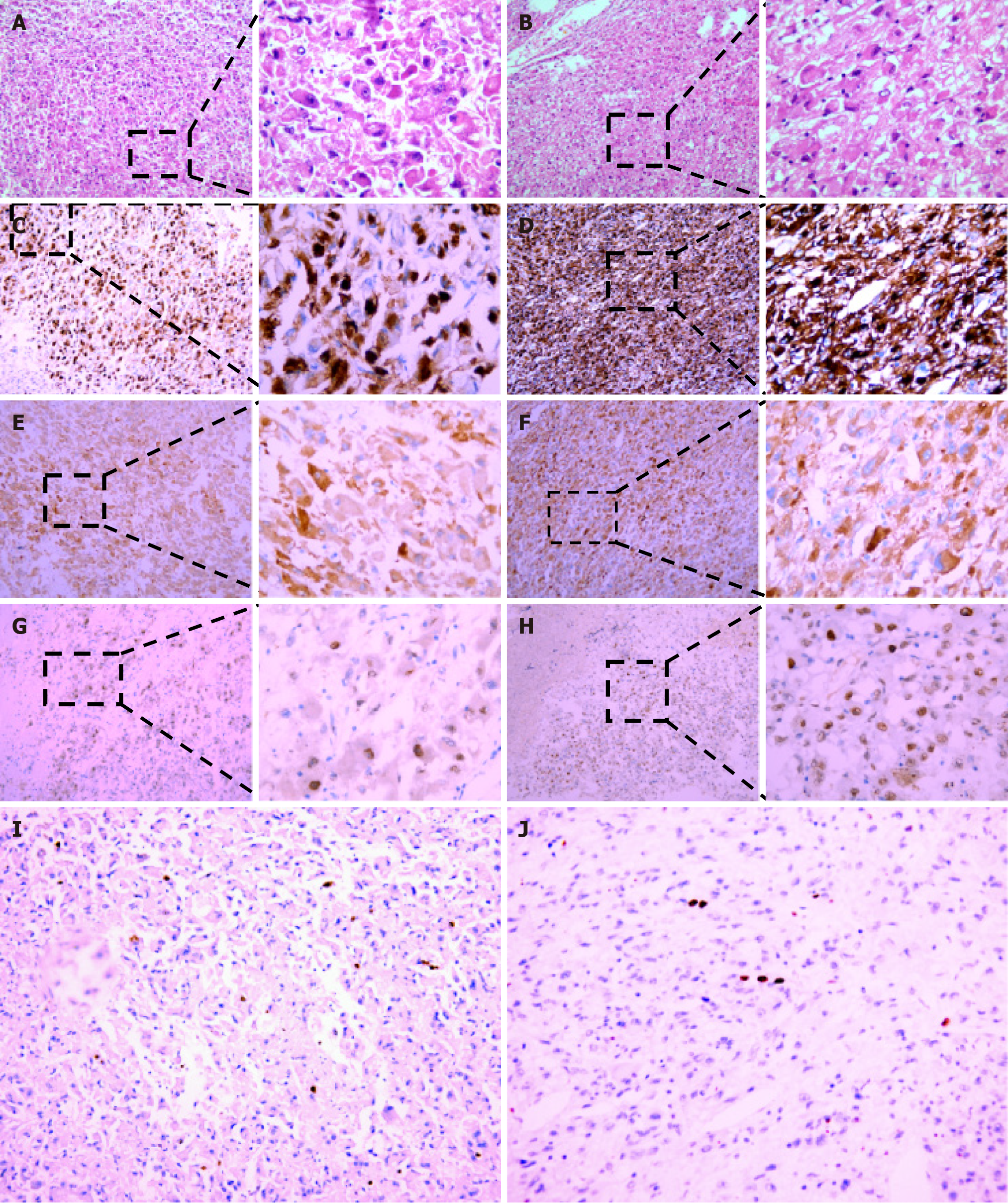
Histopathological analysis of surgical specimens showed that the tumors in the liver and kidney were about of the same type. The tumor cells were diffusely distributed, forming a sheet-like structure and characterized by epithelioid cell differentiation. The nuclei were enlarged, vacuolar, and showed overt atypia. In addition, the cytoplasm were abundant. The cells were strongly positive for HMB45 and Melan A, weakly positive for TFE3, but negative for Vimentin, SMA, Desmin, Calponin, S-100, EMA, PAX2, PAX8, CD10, CD34, CD117 and CK7. The Ki67 proliferation index was approximately 2% (Figure 6). The second-generation sequencing identified FAT2 and TP73 mutations in the kidney (Table 1).
| Gene | Transcript | Mutated base | Changed amino acid | Functional region | Mutation frequency (%) |
| FAT2 | NM_001447.2 | c.5986C>T | p.H1996Y | EX9 | 1.8 |
| TP73 | NM_005427.3 | c.94C>T | p.P32S | EX3 | 1.1 |
Approximately 1 year after discharge, a follow-up abdominal CT revealed no aberrant lesions in the surgical area (Figure 7). During the 1.5-year follow-up period, the patient took oral everolimus without experiencing obvious discomfort.
Malignant PEComa is extremely rare, accounting for only 4.6% cases of AML[7]. Compared to classic AML, PEComa is typically larger in size and contains less-fat, often accompanied by necrosis and hemorrhage[8]. When 2 or more of these characteristics are present, such as epithelioid cells ≥ 70%, tumor size > 5 cm, mitoses per 10 high-powered fields ≥ 2, aberrant mitosis and necrosis, and tumor-infiltrating growth and metastasis, the tumor is considered to behave in a highly malignant manner[9,10]. In a study of 36 patients, Tirumani et al[11] found that malignant PEComa tends to be a well-defined heterogeneous neoplasm. However, the diagnosis of PEComa can be challenging due to its rarity and lack of typical imaging characteristics. Kidneys and uterus are the most commonly affected organs, while involvement of the liver, especially simultaneous involvement of both the liver and kidney, is exceedingly rare[9,12]. Renal PEComa can be difficult to differentiate from renal cell carcinoma, lymphoma and transitional cell carcinoma. Uterine PEComa should be discriminated from fibroids and leiomyosarcomas. Hepatic PEComa is easily misdiagnosed as hepatocellular carcinoma, hepatic hemangioma, hepatic sarcoma, gastrointestinal stromal tumor, or other types of tumors. The diagnostic accuracy of hepatic PEComa using CT combined with MRI is only 20%[9]. Fine-needle aspiration and immunohistochemical staining for HMB45 and Melan-A are necessary for a definitive diagnosis. Patients with PEComa often presented to the clinic, due to weight loss, fatigue, abdominal discomfort or shortness of breath. Approximately one-third of patients may develop metastatic lesions in the liver, lung, and peritoneum/mesentery[4]. It is consistent with the findings of Nese et al[13]. In their study, the recurrence and mortality rates of PEComa patients were 17% and 33%, respectively, during a median follow-up of 24.5 months[13]. The malignancy of PEComa may be associated with vascular invasion and certain genetic changes. However, the detailed mechanism remains to be further explored.
TSC is a rare autosomal dominant disorder characterized by inactivating mutations in the TSC1 and TSC2 genes encoding the proteins hamartin and tuberin. Patients with TSC are predisposed to multiple tumor or cyst diseases, occurred frequently in central nervous system, skin, and kidney[14]. The incidence of TSC was reported to be around 1 in 6000-10000 individuals, and TSC-related lesions increase with age[6,15]. Renal involvement is present in approximately 60%-80% of TSC cases, with renal AML being the most common[14,16]. But the probability of having TSC in patients with renal AML is less than 20%[17]. Hepatic AML is uncommon, accounting for 6%-10% of TSC cases[18]. However, the incidence of hepatic AML has been reported to be as high as 13%-21% in female TSC patients with bilateral renal AML[19]. For the malignant PEComa affecting both the liver and kidney, reports are even rarer. It is well established that most PEComas are characteristic of TSC genetic alterations[20]. TSC2 mutations were detected in 80% of TFE3-negative PEComas[21]. In addition, mTOR inhibitors, such as everolimus or temsirolimus, are beneficial for some patients with advanced or recurrent PEComas[4,7,17,22].
In this study, we present a rare case of malignant PEComa affecting both the liver and kidney in a patient with TSC. FAT2 and TP73 mutations in the kidney were identified. Due to lower mutation frequencies of these genes, no relevant important targets for PEComa were detected. The tumor showed positive expression for markers including HMB-45, Melan A, and TFE3. TSC1 and TSC2 mutations were not detected in this case. The hepatic artery perfusion chemotherapy is not effective and the surgery is the most effective. During a 1.5-year postoperative follow-up period, the patient recovered well after long-term oral administration of everolimus.
The malignant PEComa affecting both the liver and kidney, especially accompanied by TSC, is exceedingly rare. Herein, we provided a valuable case study suggesting that the interventional therapy is undesirable and the surgical treatment is a better choice. In addition, we demonstrated that everolimus is highly effective in the postoperative treatment of this tumor, indicating the tight association of PEComa with TSC. However, the pathogenic mechanism of PEComa remains poorly defined and further studies are required.
We appreciate the cooperation of the patient and his family during the treatment.
| 1. | Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 318] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 2. | Lai CL, Hsu KF, Yu JC, Chen CJ, Hsieh CB, Chan DC, Li HS, Hsu HM. Malignant perivascular epithelioid cell tumor of the mesentery: a case report and literature review. Onkologie. 2012;35:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Son HJ, Kang DW, Kim JH, Han HY, Lee MK. Hepatic perivascular epithelioid cell tumor (PEComa): a case report with a review of literatures. Clin Mol Hepatol. 2017;23:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Torres Luna N, Mosquera JE, Comba IY, Kinaan M, Otoya J. A Primary Adrenal Epithelioid Angiomyolipoma (PEComa) in a Patient with Tuberous Sclerosis Complex: Report of a Case and Review of the Literature. Case Rep Med. 2020;2020:5131736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Adachi Y, Horie Y, Kitamura Y, Nakamura H, Taniguchi Y, Miwa K, Fujioka S, Nishimura M, Hayashi K. CD1a expression in PEComas. Pathol Int. 2008;58:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Lam HC, Siroky BJ, Henske EP. Renal disease in tuberous sclerosis complex: pathogenesis and therapy. Nat Rev Nephrol. 2018;14:704-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Saoud R, Kristof TW, Judge C, Chumbalkar V, Antic T, Eggener S, Modi P. Clinical and pathological features of renal epithelioid angiomyolipoma (PEComa): A single institution series. Urol Oncol. 2022;40:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Zhou H, Guo M, Gong Y. Challenge of FNA diagnosis of angiomyolipoma: A study of 33 cases. Cancer Cytopathol. 2017;125:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Jia J, Luo J, Pan CG, Ge G, Feng M, Zou B, Liu L, Zheng S, Yu J. Single-center Experience in the Diagnosis and Treatment of Hepatic Perivascular Epithelioid Cell Neoplasm. J Clin Transl Hepatol. 2022;10:72-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Lau SK. Malignant PEComa of the adrenal gland. Pathol Res Pract. 2012;208:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Tirumani SH, Shinagare AB, Hargreaves J, Jagannathan JP, Hornick JL, Wagner AJ, Ramaiya NH. Imaging features of primary and metastatic malignant perivascular epithelioid cell tumors. AJR Am J Roentgenol. 2014;202:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Nie P, Wu J, Wang H, Zhou R, Sun L, Chen J, Yang G. Primary hepatic perivascular epithelioid cell tumors: imaging findings with histopathological correlation. Cancer Imaging. 2019;19:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Nese N, Martignoni G, Fletcher CD, Gupta R, Pan CC, Kim H, Ro JY, Hwang IS, Sato K, Bonetti F, Pea M, Amin MB, Hes O, Svec A, Kida M, Vankalakunti M, Berel D, Rogatko A, Gown AM, Amin MB. Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: A clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol. 2011;35:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Bonsib SM, Boils C, Gokden N, Grignon D, Gu X, Higgins JP, Leroy X, McKenney JK, Nasr SH, Phillips C, Sangoi AR, Wilson J, Zhang PL. Tuberous sclerosis complex: Hamartin and tuberin expression in renal cysts and its discordant expression in renal neoplasms. Pathol Res Pract. 2016;212:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Lenci I, Angelico M, Tisone G, Orlacchio A, Palmieri G, Pinci M, Bombardieri R, Curatolo P. Massive hepatic angiomyolipoma in a young woman with tuberous sclerosis complex: significant clinical improvement during tamoxifen treatment. J Hepatol. 2008;48:1026-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Bissler JJ, Christopher Kingswood J. Renal manifestation of tuberous sclerosis complex. Am J Med Genet C Semin Med Genet. 2018;178:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Touloumis Z, Giannakou N, Sioros C, Trigka A, Cheilakea M, Dimitriou N, Griniatsos J. Retroperitoneal perivascular epithelioid cell tumours: A case report and review of literature. World J Clin Cases. 2019;7:3524-3534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Petrolla AA, Xin W. Hepatic angiomyolipoma. Arch Pathol Lab Med. 2008;132:1679-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Fricke BL, Donnelly LF, Casper KA, Bissler JJ. Frequency and imaging appearance of hepatic angiomyolipomas in pediatric and adult patients with tuberous sclerosis. AJR Am J Roentgenol. 2004;182:1027-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 21. | Agaram NP, Sung YS, Zhang L, Chen CL, Chen HW, Singer S, Dickson MA, Berger MF, Antonescu CR. Dichotomy of Genetic Abnormalities in PEComas With Therapeutic Implications. Am J Surg Pathol. 2015;39:813-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 22. | Wolff N, Kabbani W, Bradley T, Raj G, Watumull L, Brugarolas J. Sirolimus and temsirolimus for epithelioid angiomyolipoma. J Clin Oncol. 2010;28:e65-e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |