Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3321
Revised: August 24, 2024
Accepted: September 9, 2024
Published online: October 27, 2024
Processing time: 150 Days and 18.6 Hours
Peroral endoscopic myotomy (POEM) has been widely performed as a standard treatment for achalasia; however, its efficacy and safety for treating distal esopha
A 72-year-old male was referred to our hospital and complained of progressive dysphagia for two years. Endoscopy revealed a 2 cm long segment esophageal stenosis with intact mucosa and normal cardia. Computed tomography showed a right upper lung mass, and pathology of the right pleural effusion confirmed the diagnosis of right upper lung adenocarcinoma with multiple rib and mediastinal lymph node metastases and right malignant pleural effusion. Individualized POEM was performed first to alleviate dysphagia, and the final diagnosis was changed to esophageal muscle metastasis arising from lung adenocarcinoma. After treatment, the patient could eat soft solid food and received multiple rounds of pembrolizumab-combination chemotherapy. The patient’s progression-free survival was approximately 16 months. Long stable disease was obtained during the 24-month follow-up.
The incidence of distal esophageal segmental spasms induced by muscular metastasis arising from lung adenocarcinoma is extremely low. Individualized POEM can effectively improve a patient’s nutritional status before subsequent chemotherapy can be combined with immune checkpoint inhibitors.
Core Tip: The incidence of esophageal metastasis-induced segmental spasm is extremely low. Here, we report for the first time an individualized peroral endoscopic myotomy procedure for treating distal esophageal segmental spasm caused by muscular metastasis arising from lung adenocarcinoma, and this new technique can effectively improve a patient’s nutritional status before subsequent chemotherapy combined with immune checkpoint inhibitors.
- Citation: Shi H, Chen SY, Xie ZF, Lin LL, Jiang Y. Lung cancer metastasis-induced distal esophageal segmental spasm confirmed by individualized peroral endoscopic myotomy: A case report. World J Gastrointest Surg 2024; 16(10): 3321-3327
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3321.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3321
Peroral endoscopic myotomy (POEM) has been widely performed as a standard treatment for achalasia; however, its efficacy and safety for treating distal esophageal segmental spasms due to cancer metastasis remain unknown[1]. POEM, also known as one of the key branches of submucosal endoscopy, allows for a wide range of diagnostic or therapeutic interventions within the muscular layer of the gastrointestinal lumen. Here, we present a successful treatment for a patient with lung adenocarcinoma with esophageal muscle metastasis via individualized POEM followed by the addition of pembrolizumab[2,3] to standard chemotherapy consisting of pemetrexed under the guidance of a multidisciplinary team (MDT), which led to long-term progression-free survival.
A 72-year-old male was referred to our hospital because he had symptoms of progressive dysphagia, retrosternal pain and intermittent cough, with significant loss of body weight for two years that had worsened for one month.
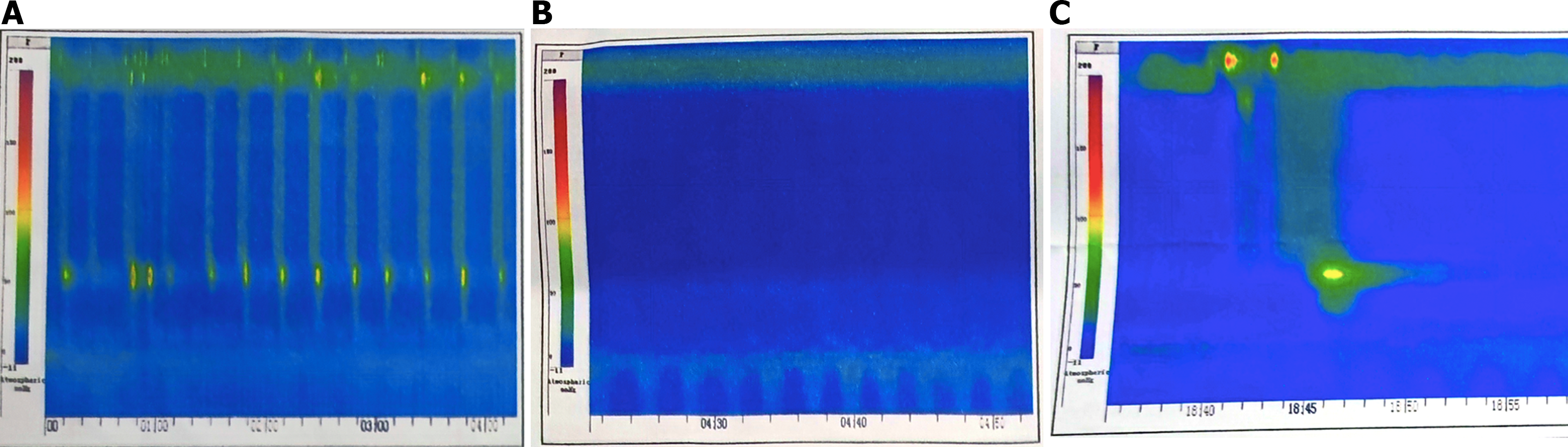
On July 4, 2022, the patient was admitted to our hospital for chest computed tomography (CT), which revealed a right upper lung mass and right pleural effusion. A right pleural puncture was performed, which confirmed lung adenocarcinoma by pathology, with immunohistochemical staining showing positive expression of cytokeratin (CK) 7, carcinoembryonic antigen, and thyroid transcription factor-1 (TTF-1). PIK3CA mutations were detected via next-generation sequencing. An endoscopy report at a local hospital led to a suspected diagnosis of jackhammer esophagus, based on an esophageal high-resolution manometry report, low resting pressure of the upper esophagus sphincter, normal pressure of the lower esophagus sphincter, and locally increased pressure in the middle and lower esophagus without normal peristalsis (Chicago classification II and III) (Figure 1).
The patient had been suffering from hypertension for several years.
No family history was identified.
The Karnofsky Performance Status[4] score remained above 90 throughout the treatment. Mild tenderness in the right chest was identified by palpation.
Routine blood and biochemical examinations revealed no obvious abnormalities. The tumor biomarkers were generally normal or slightly elevated: Carcinoembryonic antigen = 5.42 ng/mL and neuron-specific enolase = 19.22 ng/mL. The serum albumin level changed from below 40 g/L pre-POEM to above 40 g/L post-POEM.
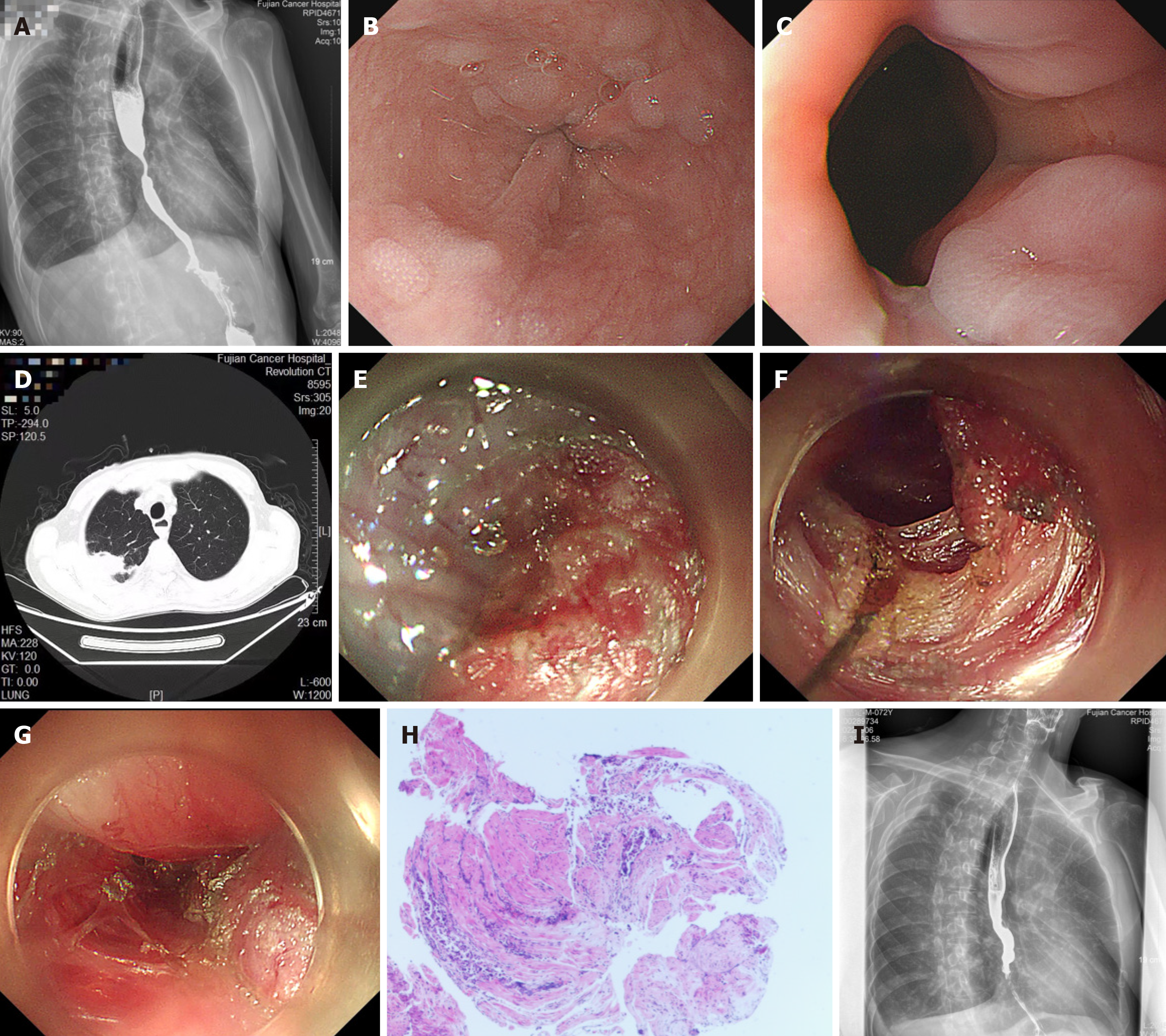
After admission to our hospital, the patient received an esophagogram revealing grade I dilation of the esophageal lumen, segmental stenosis of the distal esophagus, and delayed emptying of barium contrast agent from the esophagus (Figure 2A). Endoscopy revealed a 2 cm long segment of concentric esophageal stenosis 35–37 cm away from the incisors, with apparently normal overlying mucosa (Figure 2B). The cardia had normal contraction and relaxation (Figure 2C). CT revealed a right upper lung mass (Figure 2D), abnormality of the right 9th and 10th ribs, enlargement of mediastinal lymph nodes, right pleural effusion, and thickening of the wall in the lower esophagus far from the lung space-occupying lesion.
The pretreatment diagnosis: (1) Right upper lung adenocarcinoma with multiple rib and mediastinal lymph node metastases and right malignant pleural effusion. The tumor was clinically staged as cT1bN0M1c, IVB; and (2) Distal esophageal segmental stenosis of unknown cause. The post-POEM diagnosis was confirmed as esophageal muscle metastasis arising from lung adenocarcinoma.
After the first MDT discussion, an individualized POEM procedure (Video) was performed first to alleviate the patient’s dysphagia before chemotherapy. On July 14, 2022, under general anesthesia, a submucosal injection was performed first at the level of the middle esophagus, 5 cm proximal to the level of stenosis, rather than 13 cm proximal to the gastroesophageal junction (GEJ), as usual. After the submucosal space was entered, a longitudinal tunnel was created until the level of the stenosis was reached. Because it was difficult to dissect the submucosal space at the stenotic segment due to the inability to identify the right posterior wall of the muscular layer (Figure 2E), we had to perform inner circular myotomy of the esophagus to expand the tunnel space, beginning 3 cm distal to the mucosal entry. The circular muscle was thickened. Then, muscle biopsies were taken. Subsequently, myotomy at the narrow segment was performed, extending 2 cm distal to the stenosis segment (Figure 2F). The length of the entire myotomy was approximately 6 cm (33–39 cm away from the incisors) (Figure 2G). After myotomy, the endoscope could be passed smoothly through the distal segment stenosis with minimal resistance. No perioperative adverse events occurred. Esophageal muscle biopsy pathology revealed poorly differentiated adenocarcinoma metastasis from lung adenocarcinoma, with immunohistochemical staining showing positive expression of CK, CK7, CK8/18, NapsinA, and TTF-1 (Figure 2H).
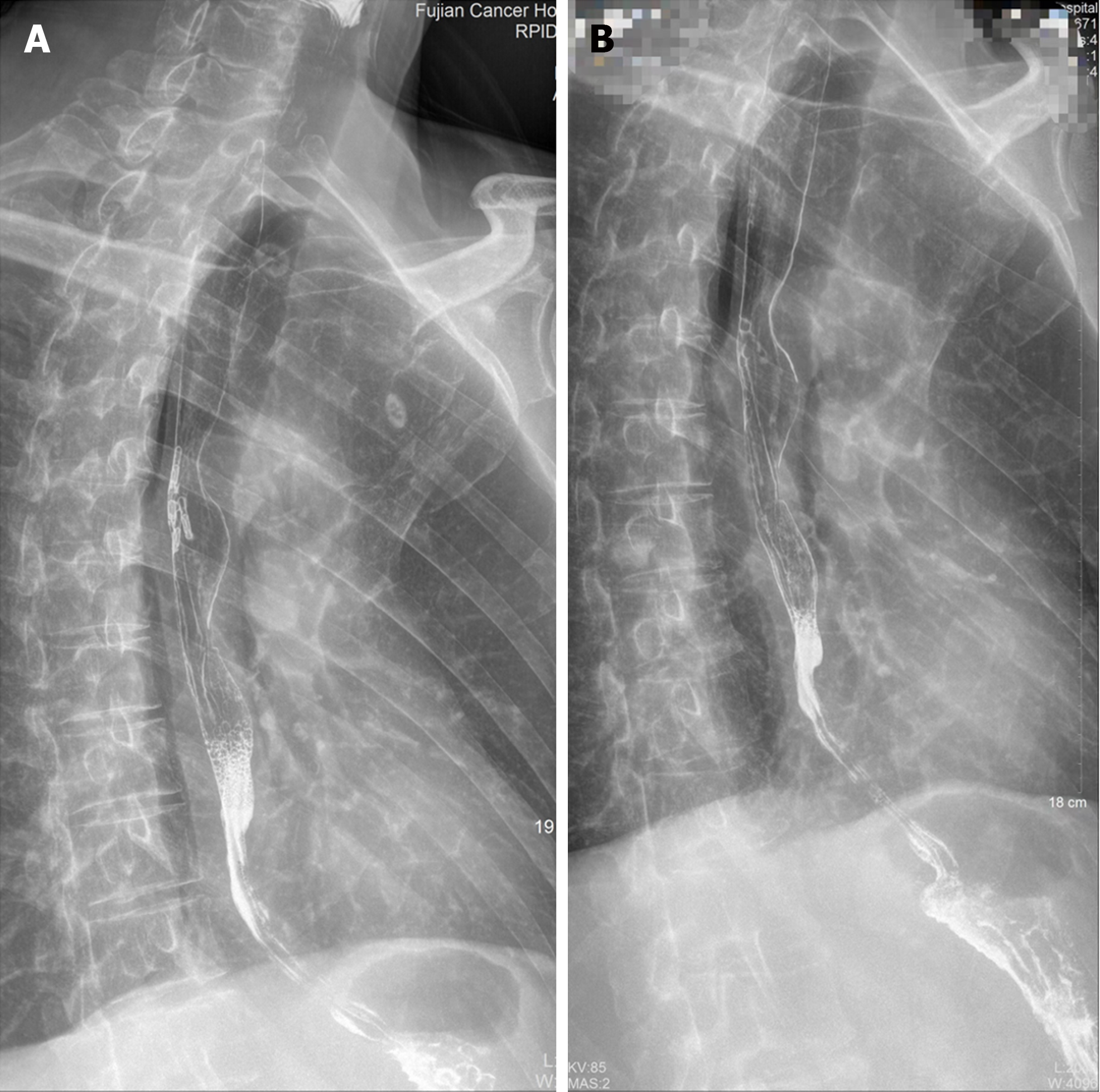
After treatment, the patient’s Eckardt score[5] changed from 9 before POEM to 3 after POEM. Repeated esophagography indicated that the segmental stenosis had vanished and that barium flowed smoothly into the stomach on postoperative days 18 and 54 (Figure 2I), 203 and 745, respectively. Additionally, the esophageal mucosal folds were not interrupted or destroyed even on postoperative day 745 (Figure 3). The patient could eat soft solid food and received multiple rounds of pembrolizumab-combination chemotherapy. Pembrolizumab plus pemetrexed[6] was selected as the chemotherapy regimen for 4 cycles, followed by metronomic oral vinorelbine[7] for up to 14 cycles, resulting in a progression-free survival (PFS) of sixteen months. After the progression of pleural metastasis, docetaxel[8] was given as an intravenous infusion for 4 cycles.
The patient’s progression-free survival was approximately 16 months. Long stable disease was obtained during the 24-month follow-up. The patient has been monitored regularly in the outpatient department, and no dysphagia recurrence has been noted thus far.
To the best of our knowledge, this is the first and only case of lung adenocarcinoma with esophageal muscle metastases that presented with progressive dysphagia as the initial sign. Although poorly differentiated adenocarcinoma of the right upper lung was confirmed by pathology of the right pleural effusion, an accurate diagnosis of segmental concentric stenosis of the distal esophagus with intact mucosa and cardia could not be made via CT or endoscopy. To alleviate the patient’s dysphagia, an individualized POEM[9] procedure was performed first formulated by the MDT team. During the process of establishing the esophageal submucosal tunnel, a muscle biopsy was taken from the right posterior wall of the muscular layer at the stenotic segment, revealing esophageal muscle metastasis from lung adenocarcinoma via pathology combined with immunohistochemical staining. The final diagnosis was adjusted accordingly. Metastatic esophageal tumors are relatively rare[10], and most metastatic esophageal tumors take the form of submucosal solid tumors in appearance under endoscopic ultrasound.
The POEM[11-14] procedure, as first described in 2008, is a submucosal tunneling endoscopy technique that allows access to the muscle layers throughout the gastrointestinal tract. By cutting pathological muscle fibers, POEM can be treated not only for motility disorders of unknown cause but also for structural pathologies, such as esophageal diverticula. Regardless of the location, there are four steps to the procedure: Mucosal incision, submucosal tunneling, myotomy, and entry closure. It is flexible in terms of the tunnel entry position, the length and depth of the myotomy. In our case, dissection of the inner circular muscle began at the narrow segment of the esophagus, extending 2 cm distal to the stenosis segment. The GEJ remained unchanged due to normal contraction and relaxation of the cardia. Overall, the location of the mucosal incision and the length and depth of the myotomy differed from those of traditional POEM prescribed by Inoue et al[11]. No procedure-related complications occurred. Both the short-term and long-term outcomes of individualized POEM were excellent.
As reported, needle tract seeding following endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is a rare but serious complication that may lead to poor prognosis[15]. To reduce the risk of tumor dissemination, EUS-FNA should be performed only when the results obtained via this procedure are useful for therapeutic decision-making. From a technical perspective, the procedure of our individualized POEM is more like an endoscopic unroofing technique for gastric gastrointestinal subepithelial tumors[16] rather than EUS-FNA. Although tumor translocation caused by endoscopic unroofing gastric muscle biopsy has not yet been reported, the risk of tumor dissemination to the mucosal incision must be estimated. Fortunately, all post-POEM esophagography examinations revealed continuous mucosal folds.
Obviously, for the palliative treatment of benign or malignant esophageal strictures, flexible self-expanding metal (SEMS)[17,18] can effectively provide rapid relief of dysphagia. However, despite the specific characteristics of recently developed stents, recurrent dysphagia due to food impaction, tumoral and granulation tissue overgrowth, or stent migration remains a major challenge. In our case, the smooth mucosa at the stenotic segment was more likely to lead to stent migration in a short period because of insufficient friction between the SEMS and the esophageal wall.
As reported previously[2,19], in patients with previously untreated metastatic non-squamous non-small cell lung cancer (NSCLC) without epidermal growth factor receptor or alkaline mutations, the addition of pembrolizumab to standard chemotherapy consisting of pemetrexed-platinum resulted in significantly longer overall survival and progression-free survival than did chemotherapy alone. Pembrolizumab plus pemetrexed-platinum improved ovarian stimulation cycles and PFS, regardless of programmed cell death ligand-1 expression. Moreover, the metronomic formulation and safety of vinorelbine[7,20] reduces the length of hospitalization. In our case, pembrolizumab plus pemetrexed was selected as the first-line regimen, followed by pembrolizumab plus oral vinorelbine. Arrieta et al[21] described that the combination of pembrolizumab plus docetaxel was well tolerated and improved the objective response rate and PFS in patients with previously treated advanced NSCLC. Our patient received intravenous docetaxel to control tumor progression.
Here, we are the only one to report an individualized POEM procedure for treating distal esophageal segmental spasm induced by muscular metastasis arising from lung adenocarcinoma, and this new technique can effectively improve a patient’s nutritional status before subsequent chemotherapy combined with immune checkpoint inhibitors.
| 1. | Feng J, Ali RW, Hao JY, Kong GX, Yang LH, Huang XJ. Peroral endoscopic myotomy for esophageal motility disorders. Esophagus. 2020;17:11-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC; KEYNOTE-189 Investigators. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3449] [Cited by in RCA: 4786] [Article Influence: 683.7] [Reference Citation Analysis (0)] |
| 3. | Rao S, Min L, Zhao J, Su J, Ye L. Efficacy of consolidation of immune checkpoint inhibitor after chemoradiation for unresectable, locally advanced PDL1 negative nonsmall cell lung cancer: A systematic review and metaanalysis. Oncol Lett. 2024;27:242. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Ramírez-Ferrer E, Aguilera-Pena MP, Duffau H. Functional and oncological outcomes after right hemisphere glioma resection in awake versus asleep patients: a systematic review and meta-analysis. Neurosurg Rev. 2024;47:160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Ren Y, Tang X, Chen Y, Chen F, Zou Y, Deng Z, Wu J, Li Y, Huang S, Jiang B, Gong W. Pre-treatment Eckardt score is a simple factor for predicting one-year peroral endoscopic myotomy failure in patients with achalasia. Surg Endosc. 2017;31:3234-3241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, Hochmair MJ, Powell SF, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Kurata T, Gray JE, Schwarzenberger P, Jensen E, Pietanza MC, Rodríguez-Abreu D. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol. 2023;41:1992-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 311] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 7. | Vergnenegre A, Monnet I, Ricordel C, Bizieux A, Curcio H, Bernardi M, Corre R, Guisier F, Hominal S, Le Garff G, Bylicki O, Locher C, Geier M, Chouaïd C, Robinet G; GFPC team. Safety and efficacy of second-line metronomic oral vinorelbine-atezolizumab combination in stage IV non-small-cell lung cancer: An open-label phase II trial (VinMetAtezo). Lung Cancer. 2023;178:191-197. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, Pluzanski A, Arrieta O, Frontera OA, Chiari R, Butts C, Wójcik-Tomaszewska J, Coudert B, Garassino MC, Ready N, Felip E, García MA, Waterhouse D, Domine M, Barlesi F, Antonia S, Wohlleber M, Gerber DE, Czyzewicz G, Spigel DR, Crino L, Eberhardt WEE, Li A, Marimuthu S, Brahmer J. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol. 2021;39:723-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 403] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 9. | Chen SY, Xie ZF, Jiang Y, Lin J, Shi H. Modified endoscopic submucosal tunnel dissection for large esophageal submucosal gland duct adenoma: A case report. World J Gastrointest Surg. 2023;15:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 10. | Kimura S, Onishi I, Kobayashi M. A Rare Case of Esophageal Metastasis of Invasive Mucinous Adenocarcinoma of the Lung. ACG Case Rep J. 2022;9:e00857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1232] [Article Influence: 82.1] [Reference Citation Analysis (1)] |
| 12. | Shimamura Y, Fujiyoshi Y, Fujiyoshi MRA, Inoue H. Evolving field of third-space endoscopy: Derivatives of peroral endoscopic myotomy. Dig Endosc. 2023;35:162-172. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Mu D, Li YY, Zhang MM, Zhang Y, Li Z, Li YQ. POEM for special patient cohorts: A review. J Dig Dis. 2017;18:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Maselli R, Spadaccini M, Pellegatta G, Repici A. Peroral Endoscopic Myotomy Technique, from Mouth to Anus. Gastrointest Endosc Clin N Am. 2023;33:127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Minaga K, Takenaka M, Katanuma A, Kitano M, Yamashita Y, Kamata K, Yamao K, Watanabe T, Maguchi H, Kudo M. Needle Tract Seeding: An Overlooked Rare Complication of Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Oncology. 2017;93 Suppl 1:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Yamamoto M, Nishida T, Uema R, Kanesaka T, Ogawa H, Kitamura S, Iijima H, Nagai K, Tsutsui S, Komori M, Yamamoto K, Tsujii Y, Hayashi Y, Takehara T. Utility and advantage of the unroofing technique for gastrointestinal subepithelial tumors: A multicenter retrospective cohort study. DEN Open. 2024;4:e332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Hirdes MM, Vleggaar FP, Siersema PD. Stent placement for esophageal strictures: an update. Expert Rev Med Devices. 2011;8:733-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Sáez de la Fuente I, Chacón Alves S, Molina Collado Z. Pleural migration of esophageal stent. Med Intensiva (Engl Ed). 2024;48:64-65. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Horinouchi H, Nogami N, Saka H, Nishio M, Tokito T, Takahashi T, Kasahara K, Hattori Y, Ichihara E, Adachi N, Noguchi K, Souza F, Kurata T. Pembrolizumab plus pemetrexed-platinum for metastatic nonsquamous non-small-cell lung cancer: KEYNOTE-189 Japan Study. Cancer Sci. 2021;112:3255-3265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Rossi D. Metronomic oral vinorelbine and lung cancer therapy during the COVID 19 pandemic: A single-center experience. Lung Cancer. 2020;145:83-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Arrieta O, Barrón F, Ramírez-Tirado LA, Zatarain-Barrón ZL, Cardona AF, Díaz-García D, Yamamoto Ramos M, Mota-Vega B, Carmona A, Peralta Álvarez MP, Bautista Y, Aldaco F, Gerson R, Rolfo C, Rosell R. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |