Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3312
Revised: August 30, 2024
Accepted: September 9, 2024
Published online: October 27, 2024
Processing time: 162 Days and 2.5 Hours
Most patients with hepatocellular carcinoma (HCC) have lost the opportunity for direct surgery at the time of diagnosis. Transarterial chemoembolization (TACE) combined with immune checkpoint inhibitors or tyrosine kinase inhibitors (TKI) can partially transform some unresectable HCC and improve the prognosis ef
In this study, we describe two successful cases of "conversion therapy for un
These cases demonstrate that the addition of palliative surgery to conversion therapy in a selected population with a high tumor burden could benefit patients with initially unresectable HCC.
Core Tip: We herein present two successful cases of "conversion Therapy for unresectable hepatocellular carcinoma (HCC)" achieved mainly by palliative surgery combined with transarterial chemoembolization (TACE) plus immunotherapy and tyrosine kinase inhibitors. For some selected advanced HCC with a high risk of tumor overload and metastasis, if liver function permits, early palliative surgery combined with local subsequent TACE, along with targeted and immunotherapy, may be a tolerable and effective strategy, which may provide the opportunity for the ultimate cure for the initially unresectable HCC and effectively improve the long-term survival based on the promising prospects of combined targeted and immunotherapy.
- Citation: Zhu YB, Qin JY, Zhang TT, Zhang WJ, Ling Q. Reassessment of palliative surgery in conversion therapy of previously unresectable hepatocellular carcinoma: Two case reports and review of literature. World J Gastrointest Surg 2024; 16(10): 3312-3320
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3312.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3312
Hepatocellular carcinoma (HCC) is currently the sixth most common malignancy and the third leading cause of patient mortality worldwide[1]. Hepatectomy and liver transplantation are the most effective radical treatments for HCC. However, approximately 70% of HCC patients cannot undergo direct surgery due to the advanced stage at the time of initial diagnosis[2]. For unresectable patients, simple epidermal growth factor receptor-tyrosine kinase inhibitor (TKI) targeted therapy and/or immunotherapy can also control tumor progression to a certain extent, prolong the survival time, and significantly improve the current status and prognosis of HCC treatment[3-5]. Furthermore, the emerging "conversion therapy" has achieved a better prognosis for patients with HCC, that is, achieving tumor regression in un
At present, the current conversion therapy is a comprehensive program that mainly relies on transarterial chemoembolization (TACE), mostly combined with targeted and/or immunotherapy[8,9]. However, the cure rate of HCC still remains low even some studies have confirmed that complete tumor necrosis can be achieved in some patients[10,11]. Moreover, new therapeutic drugs such as immune checkpoint inhibitors (ICIs) and TKIs are emerging and indications are expanding continuously[11-13]. The clinical trials of conversion therapy have not been completed currently which lacks effective studies on large sample, and have not established international expert consensus standards. Yet it's worth noting that the positive role of palliative surgery in conversion therapy has always been overlooked and more in-depth re
In this study, two patients with advanced HCC achieving conversion therapy with firstly palliative surgical resection and radiofrequency ablation (+/-) were reported. Considering its excessive tumor burden and limited location in the liver with a high risk of metastasis, a palliative surgical resection plan was firstly adopted. Early postoperative combined TACE with targeted and/or immunotherapy were followed effectively to achieve successful imaging complete remission (CR) and improve the prognosis of HCC.
Case 1: On February 10, 2021, a 48-year-old male patient was admitted to the hospital due to computed tomography (CT) scan showed a mass in the right lobe of the liver, and he has a history of hepatitis B virus infection for more than 30 years without liver cirrhosis.
Case 2: On December 10, 2021, a 47-year-old male patient was admitted to the hospital because his routine examination showed a huge mass in the left liver who had a history of hepatitis B virus infection for more than 20 years and ac
Review of systems was negative.
Case 1: The patient has a history of hepatitis B virus infection for more than 30 years without liver cirrhosis.
Case 2: The patient had a history of hepatitis B virus infection for more than 20 years and accompanied with liver cirrhosis.
All other personal and family medical history was noncontributory.
Case 1: Physical examination revealed a large palpable mass in the right upper quadrant, with no other obvious concerning findings. Abdomen was soft, non-tender, non-distended, and without other palpable masses; bowel sounds were present.
Case 2: Physical examination revealed a large mass in the left upper quadrant, with no other obvious concerning findings. Abdomen was soft, non-tender, non-distended, and without other palpable masses; bowel sounds were present.
Case 1: The hepatobiliary enzymes were normal with a Child-Pugh score of A, and tumor markers were elevated with his serum alpha-fetoprotein (AFP) 2.2 ng/mL, des-γ-carboxyprothrombin (DCP) 811 mAU/mL, and albumin 4.4 g/L.
Case 2: The hepatobiliary enzymes were normal with a Child-Pugh score of A, and tumor markers were elevated with his serum AFP > 80000.0 ng/mL, DCP 24132 mAU/mL, and albumin 4.8 g/L on admission.
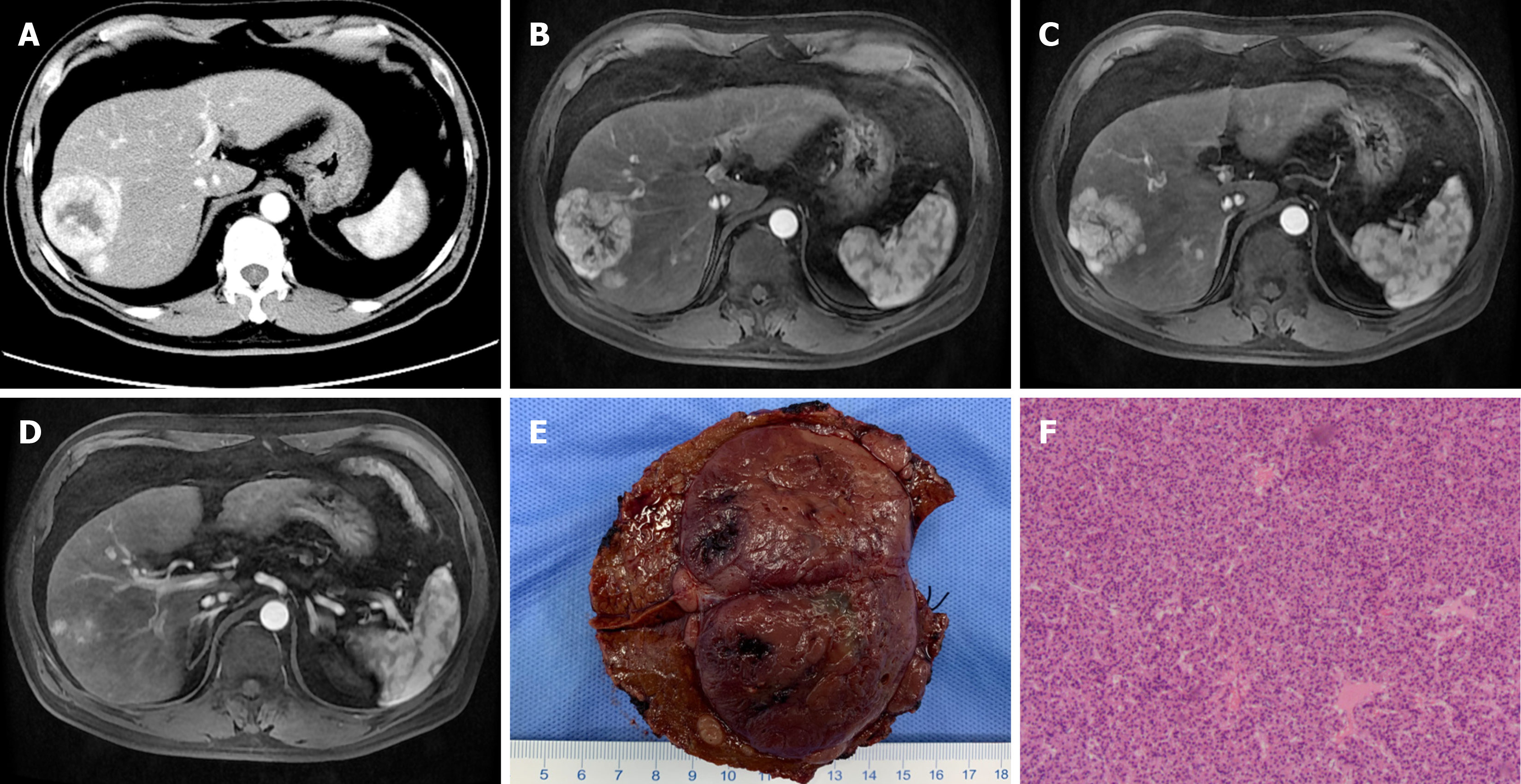
Case 1: Dynamic contrast-enhanced CT scan and magnetic resonance imaging (MRI) of the liver revealed a right hepatic mass in segments VII/VIII (S7/8, 6.5 cm × 5.5 cm × 6.2 cm) with multiple intrahepatic metastasis, without large vessel invasion or extrahepatic metastasis (Figure 1A-D).
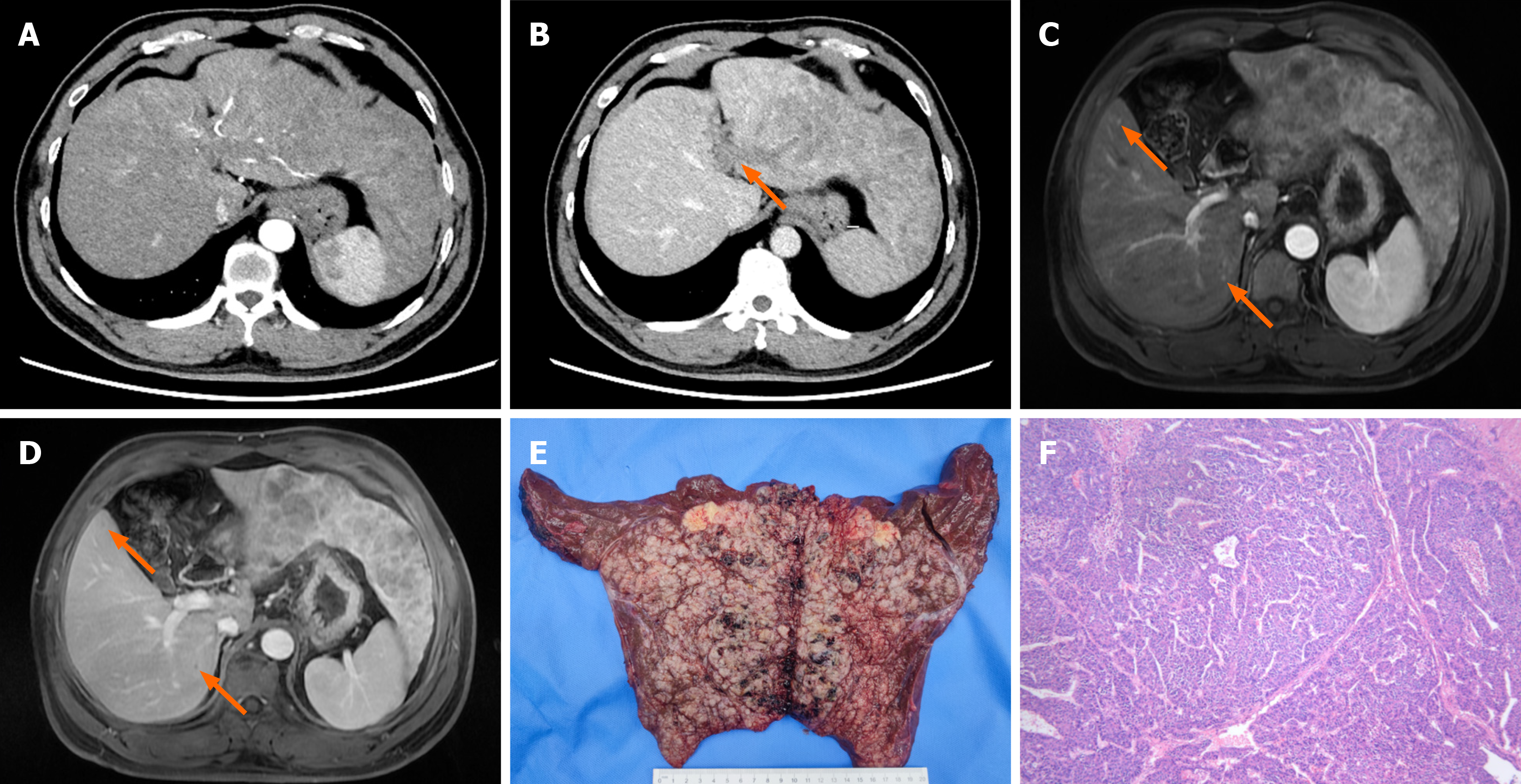
Case 2: Dynamic contrast-enhanced CT and MRI scans of the liver revealed massive HCC (18 cm × 15 cm × 4.5 cm) in the left liver with multiple intrahepatic foci, tumor thrombus formation in the left branch of the portal vein, involvement of the left hepatic vein, liver cirrhosis, splenomegaly, and no extrahepatic metastasis (Figure 2A-D).
HCC with multiple intrahepatic metastasis, hepatitis B virus infection.
HCC with multiple intrahepatic foci, tumor thrombus formation in the left branch of the portal vein, liver cirrhosis, splenomegaly.
After excluding contraindications, radiofrequency ablation of intrahepatic lesions was performed on February 18, 2021. A Cool-Tip needle was punctured into 4 nodules in the liver (two in liver S4, one S4/8, and one S6) and continuous ablated for 7 minutes (power 150 W), stopped radiofrequency ablation, and ablated the needle tract when met hyperechoic covering mass. On February 25, 2021, resection of the main lesion of HCC was performed under general anesthesia. The pathological dissection showed that the major lesion was single with a size of 5.5 cm × 4.5 cm × 6 cm, surrounded by 5 satellite lesions (with a diameter of 0.3 cm-1 cm). Postoperative pathological diagnosis is moderately differentiated HCC with negative surgical margins (Figure 1E and F).
The patient's liver function recovered after postoperative reexamination and preventive TACE treatment was subsequently performed twice on March 8, 2021 and April 13, 2021, respectively. After angiography, microcatheter superselection was used to perform TACE on the tumor supplying artery and its branches, and chemotherapy (150 mg oxaliplatin, 4 mg raltitrexed, 30 mg epirubicin), 5 mL iodized oil suspoemulsion, and appropriate amount of polyvinyl alcohol particles (diameter 100-300 um) for embolization were injected through the catheter until the tumor staining disappeared after re-contrasted imaging. During the treatment, the patient had no obvious adverse reactions except for mild fever. Two weeks after surgery (March 8, 2021), the patient received PD-1 immunization (sintilimab injection) every 3 weeks, combined with TKIs via oral lenvatinib.
Left hepatectomy palliative surgery for liver cancer including resection of the middle hepatic vein was performed on December 13, 2021 after excluding contraindications. Pathological dissection showed that the main lesion was solitary, with a size of 20 cm × 15 cm × 3.5 cm, accompanied by multiple subfocal lesions with microvascular tumor thrombus more than 5 foci. The postoperative pathological diagnosis was moderately differentiated HCC, with negative surgical margins (Figure 2E and F).
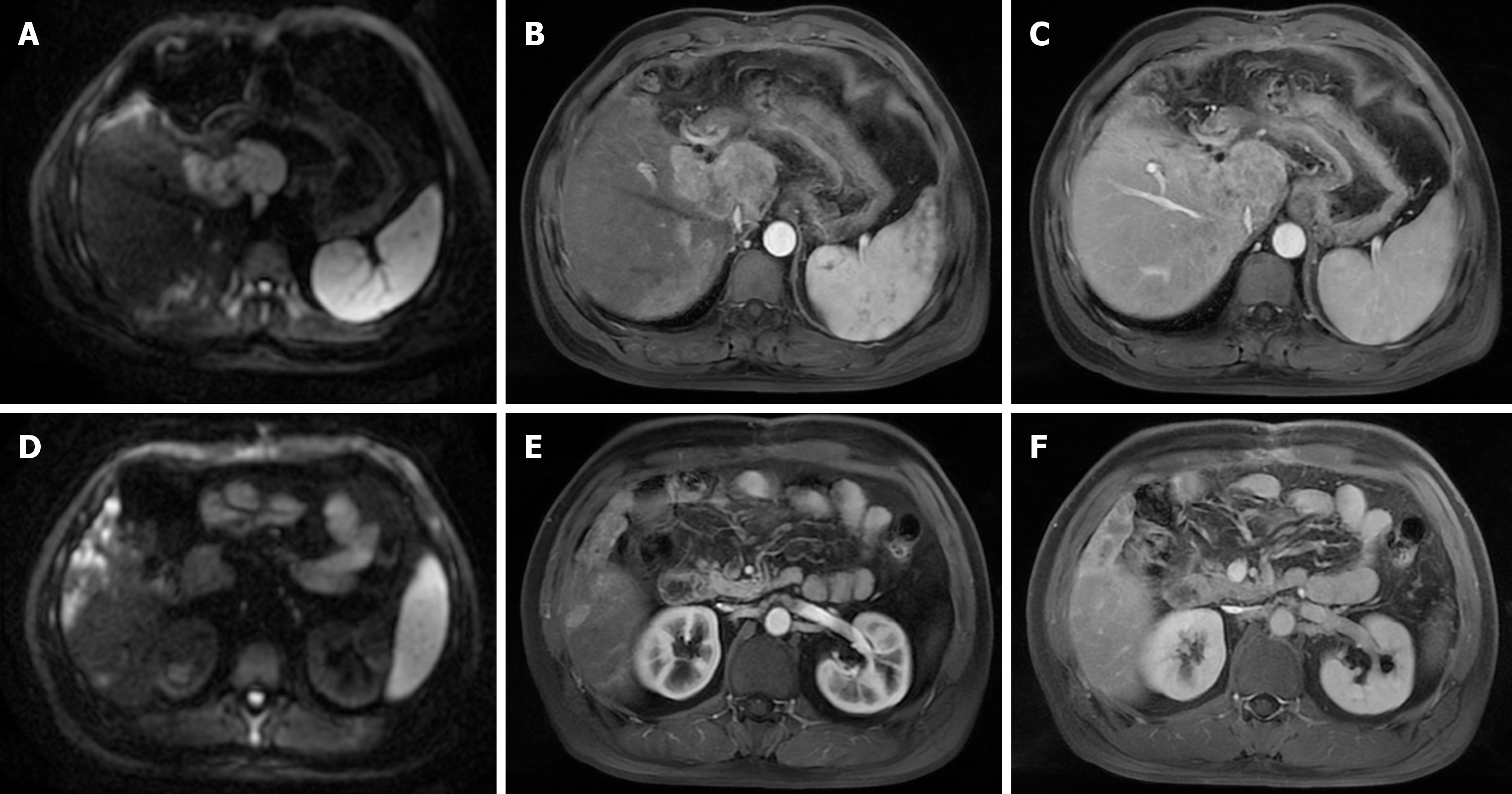
The patient recovered and began to take a long-term oral lenvatinib therapy 2 weeks later after the operation. After a month, the patient changed to regorafenib due to skin rash and new focal HCC recurrence mainly in caudate lobe and multiple intrahepatic foci in right liver (Figure 3). On January 24, March 10, May 20 in 2022, TACE was performed respectively with 150 mg oxaliplatin chemotherapy perfusion through microcatheter, followed by chemoembolization with 10 mg of idarubicin, 3 mL of liquefied lipiodol suspension emulsion, and an appropriate amount of 300-500 μm micron Meriton blank microspheres and gelatin sponge for embolism until the tumor staining disappeared in the post
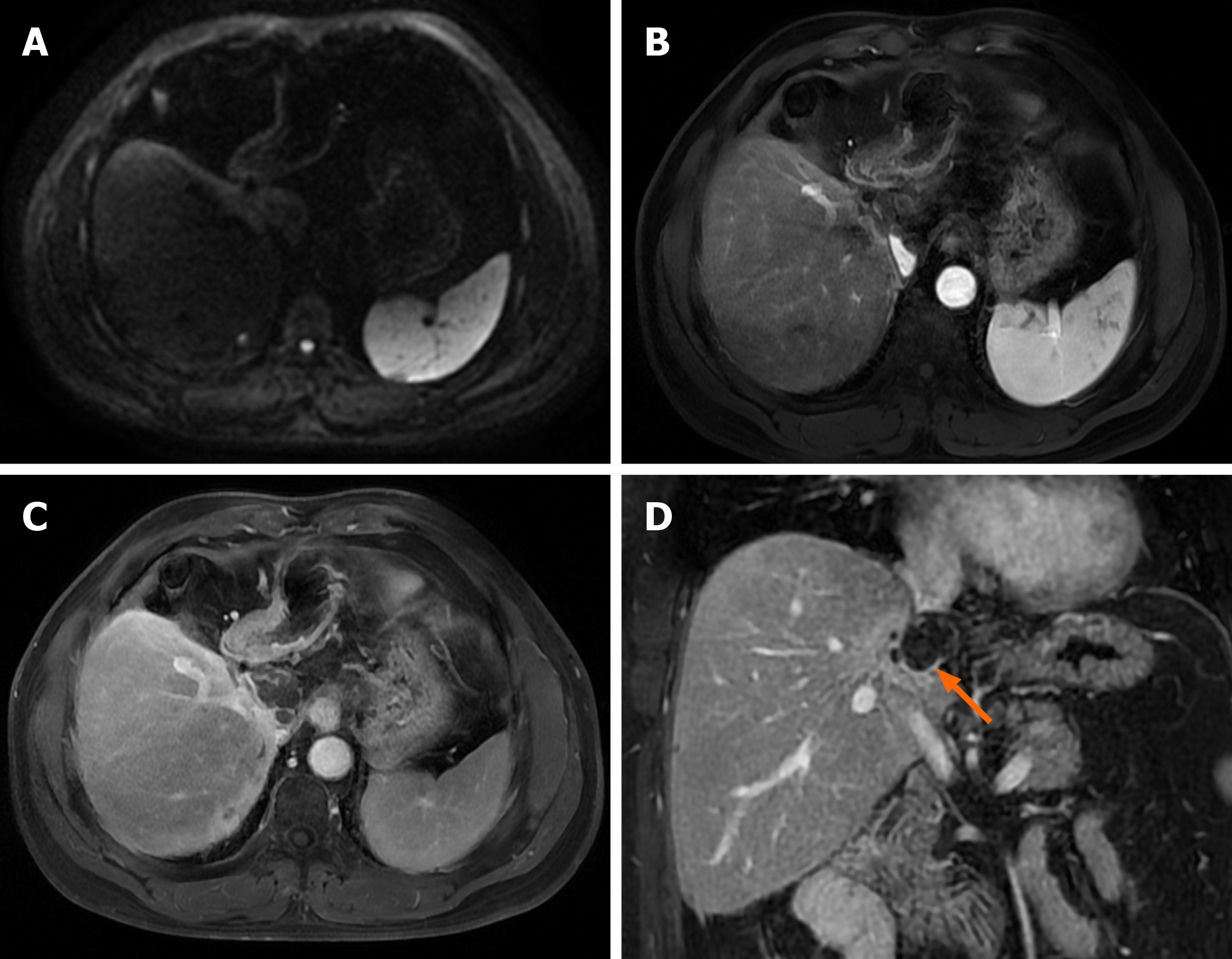
The patient was re-examined with serial AFP and DCP every month and Liver contrast-enhanced CT scan or MRI every 3 months after surgery. The contrast-enhanced MRI of the liver did not indicate tumor recurrence until 11 months since the hepatectomy surgery. Liver contrast-enhanced MRI was performed when blood sampling showed an increase in DCP to 300 mAU/mL after 12 months of the operation (March 20, 2022) and discovered a new intrahepatic focus in S2 Lobe (0.9 cm × 0.8 cm), suggesting HCC recurrence. On March 25, 2022, radiofrequency ablation of the S2 focus of the liver was performed. During follow-up until December 25, 2022, no tumor recurrence was indicated based on serum AFP and DCP levels combined with enhanced MRI of the liver.
After the operation, serum AFP and DCP were rechecked monthly and Liver contrast-enhanced CT scan or MRI per
Based on the current consensus guidelines[14-17] for the diagnosis and treatment of liver cancer, the first-line treatment for unresectable HCC is usually TACE, radiofrequency and targeted- or immune therapy, such as sorafenib, lenvatinib, atezolizumab -bevacizumab or durvalumab-tremelimumab, etc., while the second-line drugs, including regorafenib, cabozantinib, and tislelizumab injection are used if the tumor progresses during first line treatment. At present, the commonly used TKIs or ICIs themselves are only about 20% effective, and the combination of ICIs and targeted drugs can partially improve the treatment efficiency of HCC[13,17,18]. In previous studies, direct surgery is usually not particularly recommended or controversial for HCC with more than 4 intrahepatic lesions[19,20], and TACE combined with other comprehensive treatments is usually first-line recommended[8,21]. However, based on the advantages of combined targeted and immunotherapy in the new era, we chose these 2 patients with excessive tumor burden and high risk of metastasis, performed palliative resection of the main HCC lesion plus TACE, radiofrequency ablation combined with targeted and immunotherapy, thus successfully achieved the goal of eradicating HCC in imaging. Through reasonable case screening, for those patients with excessive tumor burden and high risk of metastasis, this treatment strategy, which mainly relies on palliative surgical resection and supplemented by other comprehensive treatments, may provide a new option for some selected patients with unresectable HCC.
As the first-line treatment of the advanced HCC currently, TACE could significantly prolong the patients’ overall survival time with its advantage in controlling tumor progression and preventing tumor recurrence[7,19]. Pembrolizumab, which is the first PD-1 inhibitor included in the HCC treatment guidelines, had a significant therapeutic effect on some HCC but at a high cost[22]. In recent years, a variety of low-cost PD-1 inhibitor have been developed in China with similar efficacy[23-25]. Two patients in this study selected the relatively cheaper PD-1 inhibitor sintilimab injection combined with lenvatinib for targeted therapy. Relevant studies have confirmed that combined TACE with immunotherapy drugs play a synergistic anti-tumor effect compared with TACE alone. In the procedure of Conversion Therapy for unresectable HCC, TACE could cause tumor ischemia and necrosis by embolizing tumor blood vessels, and utilize the toxicity of chemotherapy drugs to kill tumor cells, while Sintilimab injection induced tumor cell apoptosis by sensitizing endogenous anti-tumor T cell responses[19,23,26]. In this study, these two patients underwent early preventive TACE assistance after palliative surgery of HCC in a month, combined with targeted and immune therapy, and both patients achieved successful tumor CR in the subsequent follow-up imaging features.
Nowadays, there is still no clear standard for how to best evaluate the efficacy of conversion therapy, and imaging response evaluation criteria in solid tumors (RECIST) and modified RECIST (mRECIST) criteria are commonly used for evaluation[27,28]. Compared with the RECIST, mRECIST criteria focuses more on the blood supply of tumors, which is beneficial for displaying tumor active ingredients and more accurate in reflecting the therapeutic effect[29]. Therefore, in this study, following with TACE and the other comprehensive therapies on postoperative patients for HCC, we selected the mRECIST criteria to evaluate the therapeutic efficacy. During the follow-up process until tumor recurrence, both patients were observed that the serum AFP and DCP decreased to baseline levels, and finally achieved imaging CR for HCC for 11 and 10 months separately. This study proved that mRECIST criteria could be effective in assessing the effect of Conversion Therapy for HCC, however, if combined with tumor biomarkers and pathological biopsy results, the accuracy of evaluation on therapeutic effect could be further improved, which needs to be confirmed by further large-scale research.
Concerning the palliative surgery for HCC, based on the multiple intrahepatic lesions and high risk of tumor dissemination during the removal of the main liver cancer lesion, open surgery may be relatively safer[30]. Although some studies have shown that laparoscopic hepatectomy for HCC is superior to open hepatectomy in terms of surgical effect and hospital stay, which also reduces the difficulty for reoperation and the incidence of reoperation complications after tumor recurrence, and does not affect the long-term survival of patients[30-33]. However, these findings are more suitable for simple or selected patients with cirrhosis undergoing major hepatectomy in laparoscopic surgery, rather than the complicated surgical resection of those huge liver cancers with strict requirements for incisal margins[30].
In this study, considering the difficulty in surgical negative incisal margins for the palliative surgery of HCC, one case with a tumor size greater than 18 cm, and the other with multiple sub-foci around the main lesion, making it difficult to ensure the resection margins negative, thus forcible laparoscopy surgery may increase the risk of metastasis and the incidence of surgical complications. Therefore, on the basis of the patient's surgical safety, we ultimately chose open hepatectomy surgery. However, it is not inaccessible or impossible to perform palliative surgery with laparoscopy if reasonable patient screening, sufficient surgical experience, skilled surgical techniques and precise tumor localization are available. We performed left hemi-hepatectomy for the second patient which did not involve the resection of the caudate lobe in order to preserve the remnant liver volume as much as possible. However, we suffered with early tumor recurrence that mainly located in the caudate lobe, which could not rule out the possibility of tumor cell dissemination in the portal vein tumor thrombus during the surgery. Subsequently, after multiple times of TACE, the tumor was basically controlled, which indicated that for patients with HCC invading the left branch of the portal vein, left hemi-hepatectomy combined with resection of the caudate lobe could effectively improve the prognosis and reduce the risk of recurrence if the residual liver volume and function permitted.
Nevertheless, liver transplantation is currently the treatment of choice for unresectable HCC which confined to the liver without distant metastasis or extrahepatic vascular invasion[34,35]. The prognosis of liver transplantation is better than resection because it could cure patients along with liver cirrhosis completely. However, considering the high risk of late recurrence and shortage of donor livers, if we could identified those unresectable HCC patients sensitive to con
These two cases indicate that for some selected advanced HCC with a high risk of tumor overload and metastasis, if the liver function permits, early palliative surgery combined with local subsequent TACE, along with targeted and immunotherapy, may be a tolerable and effective strategy, which can provide the opportunity for ultimate cure for the initially unresectable HCC and effectively improve the long-term survival. However, the screening criteria for selected patients and the positive role of palliative surgery in the conversion therapy of unresectable HCC still need to be further con
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64673] [Article Influence: 16168.3] [Reference Citation Analysis (176)] |
| 2. | Thiesen AL. Hepatocellular Carcinoma in Adults. In: Liver Cancer [Internet]. Brisbane (AU): Exon Publications; 2021-Apr-6. [PubMed] |
| 3. | Shindoh J, Kawamura Y, Kobayashi M, Akuta N, Okubo S, Matsumura M, Suzuki Y, Hashimoto M. Prognostic Advantages of Individual Additional Interventions After Lenvatinib Therapy in Patients with Advanced Hepatocellular Carcinoma. J Gastrointest Surg. 2022;26:1637-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Ielasi L, Tovoli F, Tonnini M, Stefanini B, Tortora R, Magini G, Sacco R, Pressiani T, Trevisani F, Garajová I, Piscaglia F, Granito A. Prognostic Impact of Metastatic Site in Patients Receiving First-Line Sorafenib Therapy for Advanced Hepatocellular Carcinoma. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Laschtowitz A, Roderburg C, Tacke F, Mohr R. Preoperative Immunotherapy in Hepatocellular Carcinoma: Current State of the Art. J Hepatocell Carcinoma. 2023;10:181-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 6. | Hatzidakis A, Müller L, Krokidis M, Kloeckner R. Local and Regional Therapies for Hepatocellular Carcinoma and Future Combinations. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Arita J, Ichida A, Nagata R, Mihara Y, Kawaguchi Y, Ishizawa T, Akamatsu N, Kaneko J, Hasegawa K. Conversion surgery after preoperative therapy for advanced hepatocellular carcinoma in the era of molecular targeted therapy and immune checkpoint inhibitors. J Hepatobiliary Pancreat Sci. 2022;29:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Hatanaka T, Yata Y, Naganuma A, Kakizaki S. Treatment Strategy for Intermediate-Stage Hepatocellular Carcinoma: Transarterial Chemoembolization, Systemic Therapy, and Conversion Therapy. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Gan L, Lang M, Tian X, Ren S, Li G, Liu Y, Han R, Zhu K, Li H, Wu Q, Cui Y, Zhang W, Fang F, Li Q, Song T. A Retrospective Analysis of Conversion Therapy with Lenvatinib, Sintilimab, and Arterially-Directed Therapy in Patients with Initially Unresectable Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2023;10:673-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 10. | Shimose S, Iwamoto H, Shirono T, Tanaka M, Niizeki T, Kajiwara M, Itano S, Yano Y, Matsugaki S, Moriyama E, Noda Y, Nakano M, Kuromatsu R, Koga H, Kawaguchi T. The impact of curative conversion therapy aimed at a cancer-free state in patients with hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Cancer Med. 2023;12:12325-12335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 11. | Bai J, Huang M, Song B, Luo W, Ding R. The Current Status and Future Prospects for Conversion Therapy in the Treatment of Hepatocellular Carcinoma. Technol Cancer Res Treat. 2023;22:15330338231159718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Wang L, Wang H, Cui Y, Liu M, Jin K, Xu D, Wang K, Xing B. Sintilimab plus Lenvatinib conversion therapy for intermediate/locally advanced hepatocellular carcinoma: A phase 2 study. Front Oncol. 2023;13:1115109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 13. | Suresh D, Srinivas AN, Prashant A, Harikumar KB, Kumar DP. Therapeutic options in hepatocellular carcinoma: a comprehensive review. Clin Exp Med. 2023;23:1901-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, Zhao M, Bi X, Li G, Bai X, Ji Y, Xu L, Zhu XD, Bai D, Chen Y, Chen Y, Dai C, Guo R, Guo W, Hao C, Huang T, Huang Z, Li D, Li G, Li T, Li X, Li G, Liang X, Liu J, Liu F, Lu S, Lu Z, Lv W, Mao Y, Shao G, Shi Y, Song T, Tan G, Tang Y, Tao K, Wan C, Wang G, Wang L, Wang S, Wen T, Xing B, Xiang B, Yan S, Yang D, Yin G, Yin T, Yin Z, Yu Z, Zhang B, Zhang J, Zhang S, Zhang T, Zhang Y, Zhang Y, Zhang A, Zhao H, Zhou L, Zhang W, Zhu Z, Qin S, Shen F, Cai X, Teng G, Cai J, Chen M, Li Q, Liu L, Wang W, Liang T, Dong J, Chen X, Wang X, Zheng S, Fan J; Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11:227-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 15. | Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, Toyoda H, Imai Y, Hiraoka A, Ikeda M, Izumi N, Moriguchi M, Ogasawara S, Minami Y, Ueshima K, Murakami T, Miyayama S, Nakashima O, Yano H, Sakamoto M, Hatano E, Shimada M, Kokudo N, Mochida S, Takehara T. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021;10:181-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 455] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 16. | Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, Miyayama S, Cheng AL. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer. 2020;9:245-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 17. | Leowattana W, Leowattana T, Leowattana P. Systemic treatment for unresectable hepatocellular carcinoma. World J Gastroenterol. 2023;29:1551-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 18. | Kudo M. A Novel Treatment Strategy for Patients with Intermediate-Stage HCC Who Are Not Suitable for TACE: Upfront Systemic Therapy Followed by Curative Conversion. Liver Cancer. 2021;10:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 19. | Falette Puisieux M, Pellat A, Assaf A, Ginestet C, Brezault C, Dhooge M, Soyer P, Coriat R. Therapeutic Management of Advanced Hepatocellular Carcinoma: An Updated Review. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Xu B, Zhu XD, Shen YH, Zhu JJ, Liu J, Li ML, Tang PW, Zhou J, Fan J, Sun HC, Huang C. Criteria for identifying potentially resectable patients with initially oncologically unresectable hepatocellular carcinoma before treatment with lenvatinib plus an anti-PD-1 antibody. Front Immunol. 2022;13:1016736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Meshari AA. Role of Trans-Arterial Chemoembolization (TACE) in patients with hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2022;26:6764-6771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Li Q, Han J, Yang Y, Chen Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front Immunol. 2022;13:1070961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 23. | Liu K, Zhu Y, Zhu H. Immunotherapy or targeted therapy as the first-line strategies for unresectable hepatocellular carcinoma: A network meta-analysis and cost-effectiveness analysis. Front Immunol. 2022;13:1103055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Wu J, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Wang J, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 672] [Article Influence: 168.0] [Reference Citation Analysis (1)] |
| 25. | Lei Q, Yan X, Zou H, Jiang Y, Lai Y, Ung COL, Hu H. Efficacy and safety of monotherapy and combination therapy of immune checkpoint inhibitors as first-line treatment for unresectable hepatocellular carcinoma: a systematic review, meta-analysis and network meta-analysis. Discov Oncol. 2022;13:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Li S, Jiang S, Rahman MSU, Mei J, Wang X, Jiang J, Chen Y, Xu S, Liu Y. Pre-Induced ICD Membrane-Coated Carrier-Free Nanoparticles for the Personalized Lung Cancer Immunotherapy. Small Methods. 2023;7:e2201569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 27. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 28. | Biondetti P, Lanza C, Carriero S, Arrigoni F, Bevilacqua M, Ruffino A, Triggiani S, Sorce A, Coppola A, Ierardi AM, Venturini M, Guzzardi G, Carrafiello G. Efficacy and Safety of Transarterial Chemoembolization with DC Beads LUMI in the Treatment of HCC: Experience from a Tertiary Centre. Technol Cancer Res Treat. 2023;22:15330338231184840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Takada J, Hidaka H, Nakazawa T, Kondo M, Numata K, Tanaka K, Matsunaga K, Okuse C, Kobayashi S, Morimoto M, Ohkawa S, Koizumi W. Modified response evaluation criteria in solid tumors is superior to response evaluation criteria in solid tumors for assessment of responses to sorafenib in patients with advanced hepatocellular carcinoma. BMC Res Notes. 2015;8:609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Alvikas J, Lo W, Tohme S, Geller DA. Outcomes and Patient Selection in Laparoscopic vs. Open Liver Resection for HCC and Colorectal Cancer Liver Metastasis. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Yang Z, Hu Z, Fu Y, Hu D, Zhou Z, Chen M, Pan Y, Zhang Y. Laparoscopic Hepatectomy versus Open Hepatectomy After Conversion Therapy Using Transarterial Chemoembolization or Hepatic Arterial Infusion Chemotherapy for Patients with Initially Unresectable Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2023;10:1157-1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Yoon YI, Kim KH, Cho HD, Kwon JH, Jung DH, Park GC, Song GW, Ha TY, Lee SG. Long-term perioperative outcomes of pure laparoscopic liver resection versus open liver resection for hepatocellular carcinoma: a retrospective study. Surg Endosc. 2020;34:796-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Di Sandro S, Danieli M, Ferla F, Lauterio A, De Carlis R, Benuzzi L, Buscemi V, Pezzoli I, De Carlis L. The current role of laparoscopic resection for HCC: a systematic review of past ten years. Transl Gastroenterol Hepatol. 2018;3:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Firl DJ, Kimura S, McVey J, Hashimoto K, Yeh H, Miller CM, Markmann JF, Sasaki K, Aucejo FN. Reframing the approach to patients with hepatocellular carcinoma: Longitudinal assessment with hazard associated with liver transplantation for HCC (HALTHCC) improves ablate and wait strategy. Hepatology. 2018;68:1448-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Yang PC, Ho CM, Hu RH, Ho MC, Wu YM, Lee PH. Prophylactic liver transplantation for high-risk recurrent hepatocellular carcinoma. World J Hepatol. 2016;8:1309-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Doycheva I, Thuluvath PJ. Systemic Therapy for Advanced Hepatocellular Carcinoma: An Update of a Rapidly Evolving Field. J Clin Exp Hepatol. 2019;9:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (1)] |