Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3261
Revised: August 22, 2024
Accepted: September 3, 2024
Published online: October 27, 2024
Processing time: 57 Days and 20.5 Hours
Older patients are prone to postoperative cognitive decline after laparoscopic rectal cancer surgery, which may be associated with increased intraoperative intracranial pressure (ICP). This study investigated the correlation between intra
To evaluate changes in ICP and associated postoperative neurocognition in older adults after laparoscopic radical resection for rectal cancer.
We included 140 patients who visited the Mianyang Central Hospital for malig
In patients with an ONSD greater than 5.00 mm (group A1), the MMSE scores at 1 day and 4 days after surgery were significantly lower than those of patients with an ONSD less than or equal to 4.00 mm (group A2) (P < 0.05). The CAM scores of group A1 were significantly higher than those of group A2 (P < 0.05). The MMSE scores of group A1 on days 1 and 4 after surgery were significantly lower than those 1 day before and 7 days after surgery (P < 0.05), while the CAM scores 1 day and 4 days after surgery were significantly higher than those 1 day before and 7 days after surgery.
Decline in cognitive function among older adults after the procedure may be related to intracranial hypertension during surgery.
Core Tip: This study investigated the correlation between intraoperative intracranial pressure (ICP) changes measured by optic nerve sheath diameter via ultrasound and postoperative neurocognitive function in older patients undergoing laparoscopic radical resection for rectal cancer. These findings indicate that increased ICP during surgery is associated with significant postoperative declines in cognitive function, emphasizing the need to monitor and manage ICP to mitigate postoperative neurocognitive disorders.
- Citation: Song B, Li LP, Wang XL, Guo Y, Li J. Relationship between intracranial pressure and neurocognitive function among older adults after radical resection of rectal cancer. World J Gastrointest Surg 2024; 16(10): 3261-3268
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3261.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3261
Cognitive dysfunction refers to the impaired ability of the human brain to acquire or apply knowledge when receiving external information and transforming it into internal mental activity through processing[1,2]. The function of various organs declines with age, leading to a decline in postoperative recovery ability and resulting in postoperative neurocognitive disorders (PNDs). Research has shown that older patients are more likely to develop PND after laparoscopic surgery under general anesthesia[3,4]. Laparoscopic radical resection of rectal cancer requires CO2 pneumoperitoneum and the Trendelenburg position, which causes significant physiological changes in cerebral hemodynamics and increases intracranial pressure (ICP)[5,6]. The optic nerve sheath diameter (ONSD) determined by noninvasive ocular ultrasound examination has been proven to be a measure to determine whether ICP is increased, and a diameter greater than 5.00 mm is the threshold that has been recognized worldwide[7,8]. Therefore, this study aimed to investigate the correlation between intraoperative ICP changes and postoperative cognitive function in such patients through intraoperative dynamic ultrasound measurement of ONSD.
Patients who underwent laparoscopic radical resection for rectal cancer at Mianyang Central Hospital between January 2020 and December 2021 were selected (the Ethics Committee of Mianyang Central Hospital approved the study, No. S-2021-007). The inclusion criteria were as follows: (1) Age 65-75 years and good mental state; (2) American Society of Anesthesiologists classification of I-II; (3) No serious diseases of the heart, liver, spleen, lungs, etc.; and (4) Understanding and voluntarily signing the informed consent form. The exclusion criteria were as follows: (1) Patients with hypertension or diabetes; (2) Patients taking preoperative or dependent psychotropic drugs or alcoholism; (3) Patients with mental illness or abnormal consciousness who could not cooperate with the research; and (4) Refusal to sign the informed consent form. ONSD was measured in 140 patients undergoing laparoscopic radical resection for rectal cancer 30 and 60 minutes after the Trendelenburg positioning. Patients with a diameter greater than 5.0 mm belonged to the intracranial hypertension group A1 (42 cases), those with a diameter less than or equal to 4.0 mm belonged to the normal ICP group A2 (63 cases), and those with a diameter greater than 4.0 mm and less than or equal to 5.0 mm were excluded (29 cases). Generally, it is believed that an ONSD diameter of 5.0 mm is the threshold for determining whether ICP is elevated; a diameter greater than 5.0 mm is considered elevated ICP, and a diameter less than 4.0 mm is considered normal[9]. However, an ONSD between 4.0 mm and 5.0 mm still, cannot clearly determine whether the ICP is normal. Therefore, the interval population is excluded.
Routine electrocardiogram monitoring was performed after the patient entered the operating room; simultaneously, a mask was employed to ensure the patient inhaled oxygen and the SpO2 was maintained at ≥ 97%[9] to avoid hypoxia causing brain cell edema, which will affect ICP. The venous channel was opened, the crystalloid solution was infused at a rate of 15 mL/kg∙h, and the blood pressure was dynamically monitored by radial artery puncture. In addition, an insulation blanket and bispectral index monitoring were added during routine surgery to reduce the influence of temperature and anesthesia on postoperative cognitive function. Anesthesia was routinely induced using sufentanil 0.3 μg/kg, propofol 2 mg/kg, cisatracurium 0.2 mg/kg, and midazolam 0.03 mg/kg. Two to three minutes after induction, intubation was performed using a visual laryngoscope when circulation was stable. The tidal volume of mechanical ventilation was set to 6-8 mL/kg, propofol and remifentanil (0.2 μg/kg∙min) were used to maintain intraoperative anesthesia, and the amount of propofol was adjusted and recorded to keep the depth of intraoperative anesthesia at approximately 50. The average arterial pressure did not fluctuate more than 20% of the baseline, and the body temperature was maintained at approximately 37 °C.
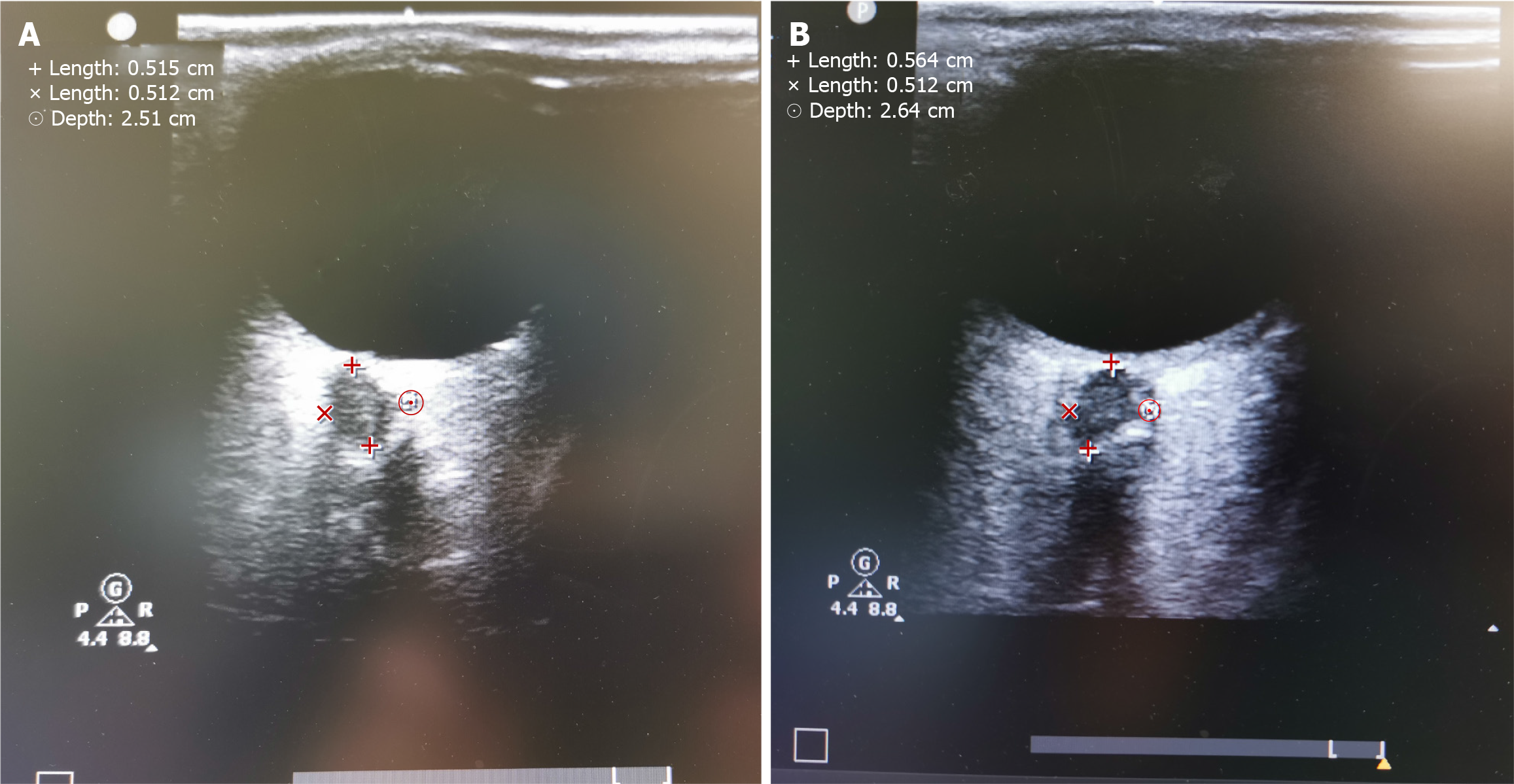
ONSD was measured by the same physician[10,11] (Figure 1). The couplant was applied to the patients’ upper eyelid after the preoperative supine position and the intraoperative Trendelenburg position[12]. A 7.5 MHz high-frequency probe (Philips) was placed on the couplant, did not press too much, and was adjusted to an appropriate angle to display the best contrast between the echogenic fat tissue behind the ball and the vertical hypoechoic zone. The ultrasonic beam was focused with the lowest sound power for measuring ONSD at the posterior zone of the optic disc, which was 3 mm behind the optic disc. The cross-sectional and sagittal planes of bilateral eyes were measured, and the average of four measurements at each time point was used.
Intraoperative and postoperative conditions: The time of surgery, intraoperative blood loss, and urine output of the two groups were observed and recorded. Intraoperative vital signs: The time points before anesthesia, 30 minutes after Trendelenburg positioning, 60 minutes after Trendelenburg positioning, and after extubation were marked as h1, h2, h3, and h4, respectively, and the mean arterial pressure (MAP), heart rate, and end-tidal carbon dioxide partial pressure of the two groups were recorded at four time points.
Mini-Mental State Examination (MMSE) and confusion assessment method (CAM) scores: The two groups were scored using the MMSE and CAM 1 day before surgery and 1, 4, and 7 days after surgery. MMSE score: A full score of 30 points, a score ≥ 28 means normal cognitive function; a score of 24 to 27 means mild cognitive impairment; a score of 19 to 23 means a moderate impairment of cognitive function; and a score ≤ 18 means severe cognitive impairment. CAM score: A full score of 30 points; 19 points or less indicates that the patient has no delirium; 19-22 points indicate that the patient may have delirium, and 22 points or more indicates that the patient has delirium.
We conducted a power analysis using G*Power to determine our sample size, aiming for a statistical power of 0.80 and a significance level of 0.05 to detect a medium effect size in cognitive function changes. Based on our pilot data and assuming a dropout rate of 10%, we calculated that 42 participants in the intracranial hypertension group and 63 participants in the normal pressure group would be necessary. The sample size ensured that our study was adequately powered to reveal significant differences in postoperative cognitive outcomes related to intraoperative ICP changes.
Statistical analyses were performed using SPSS version 22.0. First, describe the data in general, mean ± SD was used to represent measurement data, then the analysis of variance of repeated measurement design was used. The analysis of the repeated measurement design mainly includes Mauchly’s spherical test, interaction judgment, main-effect analysis, or single-effect analysis. Age, body weight, operation time, blood loss, urine volume, etc., were measured by unpaired t test; α = 0.05 for the inspection level.
A total of 140 patients with malignant rectal tumors were selected for this study. After 35 cases were based on ultrasound measurement of the optic nerve sheath, there were no statistically significant differences in age, weight, time of surgery, intraoperative blood loss, or urine output among the remaining 105 cases (Table 1).
| Item | Group A1 | Group A2 | F | P value |
| Age | 67.83 ± 2.09 | 68.49 ± 1.57 | 3.64 | 0.059 |
| Weight (kg) | 57.35 ± 2.19 | 57.29 ± 1.65 | 0.026 | 0.873 |
| Time of surgery (minutes) | 222.50 ± 11.82 | 224.17 ± 12.54 | 0.471 | 0.494 |
| Intraoperative blood loss (mL) | 150.33 ± 12.14 | 153.16 ± 15.75 | 0.968 | 0.328 |
| Urine volume (mL) | 376.86 ± 14.03 | 381.78 ± 22.84 | 1.555 | 0.215 |
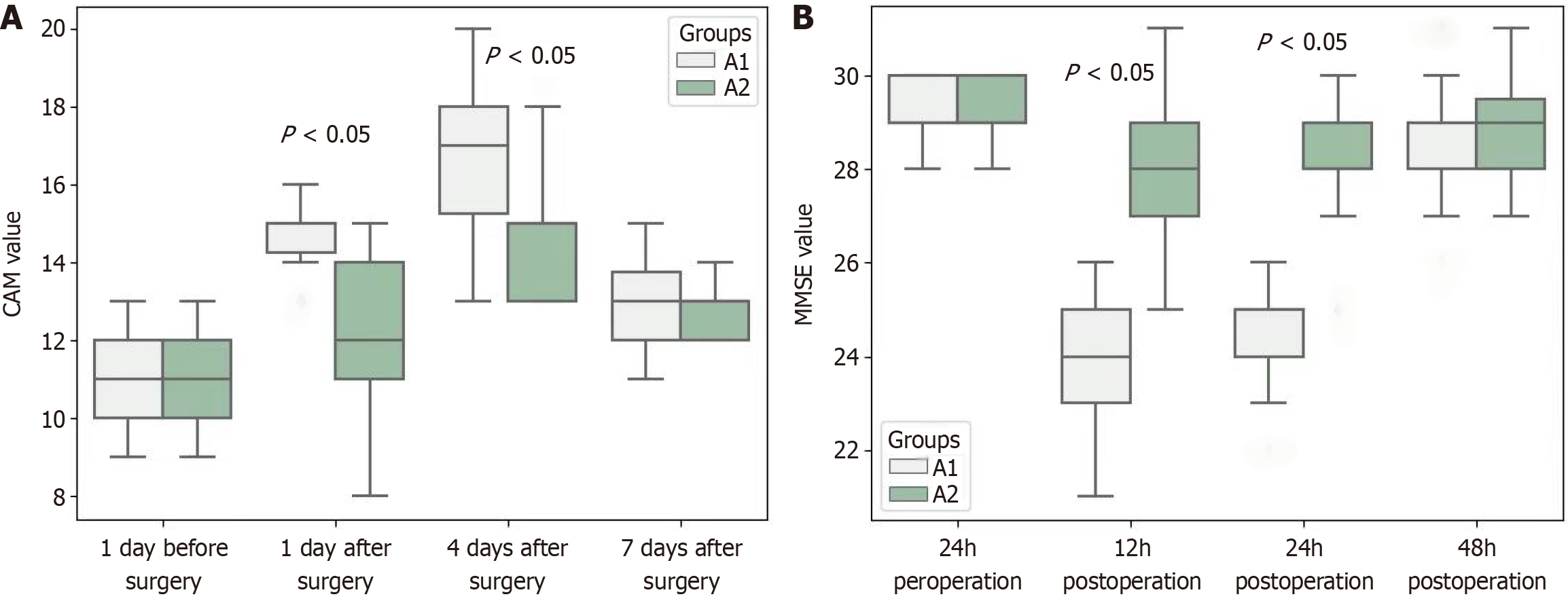
The preoperative MMSE scores of the two groups were not significantly different (P > 0.05). At 1 and 4 days after surgery, the MMSE scores of group A1 (patients with increased ICP) were significantly lower than those of group A2 (patients with no increase in ICP) (P < 0.05). Intragroup comparison: The scores of group A1 at 1 and 4 days after surgery were lower than those at 7 days after surgery and before surgery (P < 0.05) (Figure 2A and Table 2).
| Timepoints/ANOVA factors | Group A1 | Group A2 | F value | P value | |
| CAM | 1 day before surgery | 10.98 ± 1.00 | 11.08 ± 0.99 | 0.272 | 0.603 |
| 1 day after surgery | 14.93 ± 0.81 | 12.37 ± 1.64 | 88.2 | < 0.001 | |
| 4 days after surgery | 16.62 ± 1.78 | 14.62 ± 1.38 | 41.736 | < 0.001 | |
| 7 days after surgery | 12.93 ± 0.92 | 12.76 ± 0.61 | 1.239 | 0.268 | |
| Time effect | 213.73a | 0.012a | |||
| Group effect | 66.18a | 0.031a | |||
| Interaction effect | 21.41a | 0.022a | |||
| MMSE | 1 day before surgery | 29.29 ± 0.51 | 29.37 ± 0.60 | 0.493 | 0.484 |
| 1 day after surgery | 23.79 ± 1.32 | 27.97 ± 1.26 | 268.665 | < 0.001 | |
| 4 days after surgery | 24.64 ± 1.10 | 28.08 ± 0.79 | 347.358 | < 0.001 | |
| 7 days after surgery | 28.74 ± 1.06 | 28.89 ± 0.88 | 0.626 | 0.431 | |
| Time effect | 261.59 | 0.003 | |||
| Group effect | 312.85 | 0.025 | |||
| Interaction effect | 113.94 | 0.025 |
The preoperative CAM scores were not significantly different between the two groups (P > 0.05). At 1 and 4 days after surgery, the CAM scores of group A1 (patients with increased ICP) were significantly higher than those of group A2 (patients with no increase in ICP) (P < 0.05). Intragroup comparison: The scores of group A1 at 1 and 4 days after surgery were higher than those at 7 days after surgery and before surgery (P < 0.05) (Figure 2B and Table 2).
The MAP of group A1 was significantly higher than that of group A2 at h2 and h3 (P < 0.05), whereas the difference at h1 and h4 was not statistically significant (P > 0.05). The heart rate and end-tidal carbon dioxide partial pressure at each time point were not significantly different between the two groups (P > 0.05) (Table 3).
| Timepoints/ANOVA factors | Group A1 | Group A2 | F value | P value | |
| MAP | Before anesthesia | 75.60 ± 2.33 | 76.40 ± 1.93 | 3.68 | 0.058 |
| 30 minutes after the Trendelenburg position | 90.19 ± 1.55 | 77.11 ± 1.28 | 2212.42 | < 0.001 | |
| 60 minutes after the Trendelenburg position | 91.62 ± 1.34 | 78.41 ± 1.55 | 2028.42 | < 0.001 | |
| After extubation | 78.74 ± 1.11 | 78.59 ± 0.93 | 0.571 | 0.452 | |
| Time effect | 703.67a | 0.024a | |||
| Group effect | 993.59a | 0.014a | |||
| Interaction effect | 535.37a | 0.033a | |||
| HR | Before anesthesia | 81.95 ± 2.24 | 82.40 ± 1.62 | 1.39 | 0.241 |
| 30 minutes after the Trendelenburg position | 75.26 ± 1.62 | 75.81 ± 1.96 | 2.251 | 0.137 | |
| 60 minutes after the Trendelenburg position | 73.79 ± 2.07 | 74.70 ± 2.54 | 3.752 | 0.055 | |
| After extubation | 85.14 ± 1.76 | 85.79 ± 2.35 | 2.342 | 0.129 | |
| Time effect | 592.05 | 0.015 | |||
| Group effect | 7.59 | 0.011 | |||
| Interaction effect | 0.07 | 0.981 | |||
| PETCO2 | Before anesthesia | 31.07 ± 1.09 | 30.70 ± 1.35 | 2.229 | 0.139 |
| 30 minutes after the Trendelenburg position | 41.05 ± 1.06 | 40.76 ± 1.19 | 1.589 | 0.211 | |
| 60 minutes after the Trendelenburg position | 42.38 ± 0.49 | 41.97 ± 1.78 | 2.148 | 0.146 | |
| After extubation | 34.43 ± 1.35 | 34.24 ± 1.47 | 0.453 | 0.502 | |
| Time effect | 1553.13 | 0.004 | |||
| Group effect | 4.61 | 0.041 | |||
| Interaction effect | 0.16 | 0.921 |
PND refers to complications of the central nervous system in the older after surgery that manifest as confusion, anxiety, personality changes, and memory impairment. PND is a common postoperative complication that occurs primarily in older patients following anesthesia. The pathogenesis of PND is unknown, and the patients’ quality of life is affected in severe cases. According to Feinkohl et al[13], PND is related to the surgery itself, and anesthetics are also considered related to PND because they may be neurotoxic to the aging brain[14]. This study mainly used ONSD determined by noninvasive ocular ultrasound to reflect ICP[15] and to explore postoperative cognitive function after intraoperative ICP increases. During the laparoscopic radical resection of rectal cancer under general anesthesia, surgeons often place the patient in the Trendelenburg position to obtain a good surgical field. However, as the time of surgery increases, this position changes the systemic hemodynamics, which is believed to increase ICP[16]. Studies have shown that after adopting the Trendelenburg position, the patient’s internal jugular vein valve is not completely closed under pressure, resulting in increased intracranial blood flow and ICP[17]. After pneumoperitoneum is established, the long-term absorption of carbon dioxide gas will cause PaCO2 to increase and pH to decrease, leading to cerebral vasodilation, which further increases cerebral blood flow and ultimately causes ICP to increase. In this experiment, MAP at time points h2 and h3 in the group increased significantly, and increased ICP could induce PND[18]. According to previous studies, intracranial hypertension can damage brain function. Intracranial hypertension leads to damage to the blood-brain barrier, secretion of permeability factors, and an increase in osmotic pressure, which in turn leads to angioedema and ultimately brain cell damage or death[19,20]. In addition, intracranial hypertension, ischemia, and hypoxia cause brain cell ion channel disorders, which in turn cause cell swelling and cytotoxic edema[21]. Therefore, it is suggested that intracranial hypertension may be related to the decline in postoperative cognitive function. This study showed that during laparoscopic radical resection of rectal cancer under general anesthesia, if the patient has increased ICP during surgery, a decline in cognitive function will occur one and four days after surgery, which is consistent with the research mentioned above. In the non-increased ICP group, the patients’ postoperative MMSE and CAM scores were significantly better than those in the increased ICP group, suggesting that higher ICP levels are not conducive to protecting brain neurons and reducing cognitive impairment[16].
However, in this study, only MMSE and CAM scores were used to assess postoperative cognitive function, which lacked accuracy. For example, the pathophysiological mechanism of postoperative delirium is unknown, and only the incidence and epidemiological data are known[18]. Additional discoveries may be made if additional evaluation methods are used. Moreover, there are few studies on the correlation between postoperative cognitive function and ICP as reflected in ONSD. Therefore, this study lacked a specific experimental research basis.
Our study, while identifying a link between intraoperative ICP changes and postoperative cognition in older patients undergoing rectal cancer surgery, is limited by its single-center design. Future multicenter trials with diverse patient groups will enhance the validity of our results and explore variations in patient responses across healthcare settings. We are committed to expanding our research through multicenter studies to better understand and generalize our findings.
Cognitive function decline after laparoscopic radical resection for rectal cancer in older patients may be related to intraoperative intracranial hypertension. During surgery, factors that increase intraoperative ICP should be avoided as much as possible to reduce the probability of PND.
| 1. | Bickel H, Gradinger R, Kochs E, Wagner K, Förstl H. [Incidence and risk factors of delirium after hip surgery]. Psychiatr Prax. 2004;31:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Mahida JB, Asti L, Deans KJ, Minneci PC, Groner JI. Laparoscopic pyloromyotomy decreases postoperative length of stay in children with hypertrophic pyloric stenosis. J Pediatr Surg. 2016;51:1436-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Closhen D, Treiber AH, Berres M, Sebastiani A, Werner C, Engelhard K, Schramm P. Robotic assisted prostatic surgery in the Trendelenburg position does not impair cerebral oxygenation measured using two different monitors: A clinical observational study. Eur J Anaesthesiol. 2014;31:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Udwadia TE. Diagnostic laparoscopy. Surg Endosc. 2004;18:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Neira VM, Kovesi T, Guerra L, Campos M, Barrowman N, Splinter WM. The impact of pneumoperitoneum and Trendelenburg positioning on respiratory system mechanics during laparoscopic pelvic surgery in children: a prospective observational study. Can J Anaesth. 2015;62:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Ozcan MF, Akbulut Z, Gurdal C, Tan S, Yildiz Y, Bayraktar S, Ozcan AN, Ener K, Altinova S, Arslan ME, Balbay MD. Does steep Trendelenburg positioning effect the ocular hemodynamics and intraocular pressure in patients undergoing robotic cystectomy and robotic prostatectomy? Int Urol Nephrol. 2017;49:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Schramm P, Treiber AH, Berres M, Pestel G, Engelhard K, Werner C, Closhen D. Time course of cerebrovascular autoregulation during extreme Trendelenburg position for robotic-assisted prostatic surgery. Anaesthesia. 2014;69:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Kim SE, Hong EP, Kim HC, Lee SU, Jeon JP. Ultrasonographic optic nerve sheath diameter to detect increased intracranial pressure in adults: a meta-analysis. Acta Radiol. 2019;60:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Witherspoon B, Ashby NE. The Use of Mannitol and Hypertonic Saline Therapies in Patients with Elevated Intracranial Pressure: A Review of the Evidence. Nurs Clin North Am. 2017;52:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Moretti R, Pizzi B. Ultrasonography of the optic nerve in neurocritically ill patients. Acta Anaesthesiol Scand. 2011;55:644-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Seo H, Kim YK, Shin WJ, Hwang GS. Ultrasonographic optic nerve sheath diameter is correlated with arterial carbon dioxide concentration during reperfusion in liver transplant recipients. Transplant Proc. 2013;45:2272-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Mavrocordatos P, Bissonnette B, Ravussin P. Effects of neck position and head elevation on intracranial pressure in anaesthetized neurosurgical patients: preliminary results. J Neurosurg Anesthesiol. 2000;12:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Feinkohl I, Winterer G, Spies CD, Pischon T. Cognitive Reserve and the Risk of Postoperative Cognitive Dysfunction. Dtsch Arztebl Int. 2017;114:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Geng YJ, Wu QH, Zhang RQ. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: A randomized controlled trial. J Clin Anesth. 2017;38:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Bekker AY, Weeks EJ. Cognitive function after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol. 2003;17:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Dang Q, Simon J, Catino J, Puente I, Habib F, Zucker L, Bukur M. More fateful than fruitful? Intracranial pressure monitoring in elderly patients with traumatic brain injury is associated with worse outcomes. J Surg Res. 2015;198:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Chen K, Wang L, Wang Q, Liu X, Lu Y, Li Y, Wong GTC. Effects of pneumoperitoneum and steep Trendelenburg position on cerebral hemodynamics during robotic-assisted laparoscopic radical prostatectomy: A randomized controlled study. Medicine (Baltimore). 2019;98:e15794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med. 2017;377:1456-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 654] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 19. | Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2:492-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 461] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 20. | Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22:E1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Rungta RL, Choi HB, Tyson JR, Malik A, Dissing-Olesen L, Lin PJC, Cain SM, Cullis PR, Snutch TP, MacVicar BA. The cellular mechanisms of neuronal swelling underlying cytotoxic edema. Cell. 2015;161:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |