Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3239
Revised: August 17, 2024
Accepted: September 6, 2024
Published online: October 27, 2024
Processing time: 84 Days and 20.8 Hours
Gallbladder cancer (GBC) is the most common malignant tumor of the biliary system, and is often undetected until advanced stages, making curative surgery unfeasible for many patients. Curative surgery remains the only option for long-term survival. Accurate postsurgical prognosis is crucial for effective treatment planning. tumor-node-metastasis staging, which focuses on tumor infiltration, lymph node metastasis, and distant metastasis, limits the accuracy of prognosis. Nomograms offer a more comprehensive and personalized approach by visually analyzing a broader range of prognostic factors, enhancing the precision of treatment planning for patients with GBC.
To identify risk factors and develop a predictive model for GBC prognosis.
A retrospective study analyzed the clinical and pathological data of 93 patients who underwent radical surgery for GBC at Peking University People's Hospital from January 2015 to December 2020. Kaplan-Meier analysis was used to calculate the 1-, 2- and 3-year survival rates. The log-rank test was used to evaluate factors impacting prognosis, with survival curves plotted for significant variables. Single-factor analysis revealed statistically significant differences, and multivariate Cox regression identified independent prognostic factors. A nomogram was developed and validated with receiver operating characteristic curves and calibration curves.
Among 93 patients who underwent radical surgery for GBC, 30 patients survived, accounting for 32.26% of the sample, with a median survival time of 38 months. The 1-year, 2-year, and 3-year survival rates were 83.87%, 68.82%, and 53.57%, respectively. Univariate analysis revealed that carbohydrate antigen 19-9 expre
Lymph node metastasis, tumor differentiation, extrahepatic bile duct invasion, and perineural invasion are independent risk factors. A nomogram based on these factors can be used to personalize and improve treatment strategies.
Core Tip: Gallbladder cancer (GBC) is the most prevalent malignant tumor in the biliary system, with curative surgery being the only viable option for long-term survival. Accurate postoperative prognosis assessment is essential for effective treatment planning. Our study identifies lymph node metastasis, tumor differentiation, extrahepatic bile duct invasion, and neural invasion as independent risk factors for postoperative prognosis in GBC patients. we developed a nomogram model that demonstrates strong internal validation consistency.
- Citation: Li XF, Ma TT, Li T. Risk factors and survival prediction model establishment for prognosis in patients with radical resection of gallbladder cancer. World J Gastrointest Surg 2024; 16(10): 3239-3252
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3239.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3239
Gallbladder cancer (GBC), the most common type of malignant tumor in the biliary tract, often shows nonspecific symptoms and signs during its initial stages. Its onset is gradual and highly malignant, with a median survival time of less than one year and a five-year survival rate ranging from 5% to 13%[1,2]. The main treatment for GBC is radical resection, which offers the possibility of long-term survival[3,4]. However, patients with locally advanced disease, the survival rate after surgery alone is low, with a high recurrence rate. Therefore, accurate prognosis assessment after surgery is critical for developing effective treatment plans. Tailoring individualized treatment strategies on the basis of different prognostic risk factors is key to enhancing patient outcomes. While the tumor-node-metastasis (TNM) staging system is commonly used for guiding treatment, its focus on tumor extent, lymph node status, and distant metastasis has limitations in prognostic evaluation. Identifying independent risk factors and creating predictive models are crucial for evaluating survival and treatment outcomes. Currently, the existing predictive models for GBC prognosis are limited and often require advanced statistical understanding, hindering their clinical utility. This study aimed to gather pathological data from GBC patients who underwent radical surgery, identify independent risk factors influencing patient prognosis, and develop a predictive nomogram model. This model will serve as a valuable tool for prognosis assessment and personalized treatment planning for patients.
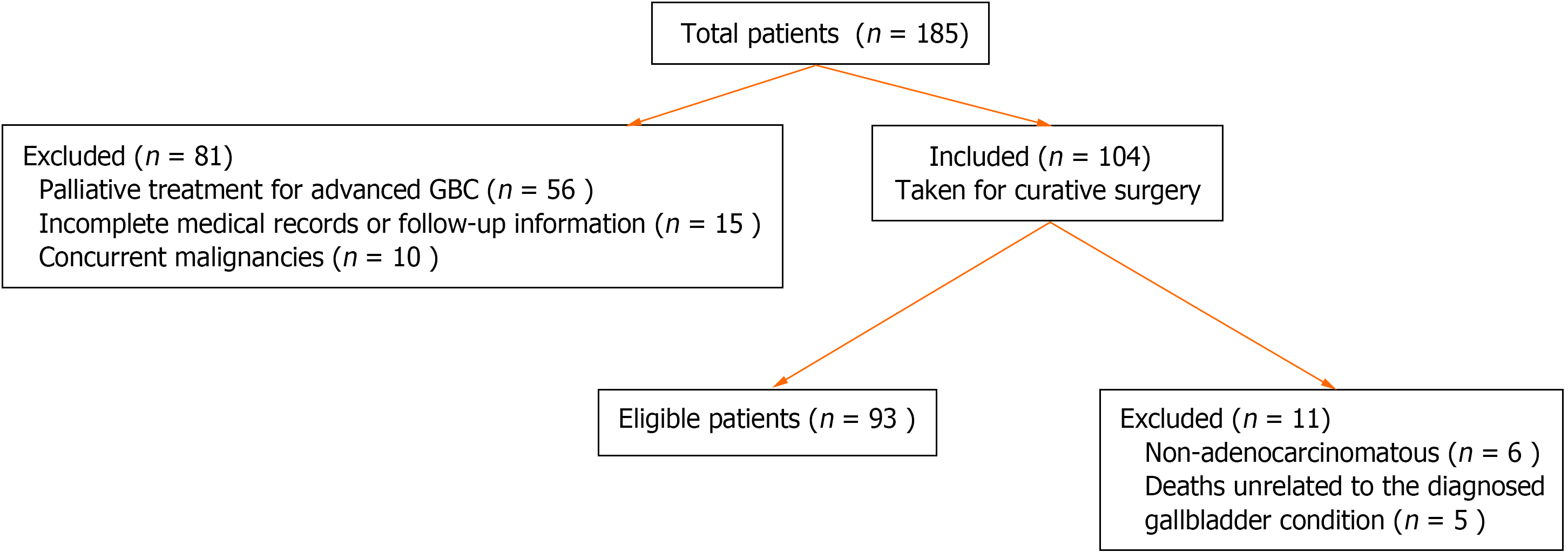
A retrospective analysis of 185 GBC patients at Peking University People's Hospital from 2015 to 2020 revealed that 104 patients underwent curative resections. Ninety-three patients were included in the statistical analyses (Figure 1).
Surgical treatment details were obtained from operative records, whereas information on adjuvant therapy was acquired through hospitalization records and subsequent follow-up. All patients were maintained on regular follow-up, every 3 months for the first 2 years, and every 6 months for the next 3 years. Follow-up was conducted via either outpatient visits or telephone calls. The follow-up period extended until December 31, 2023, with patient mortality serving as the endpoint of the event.
Age, which was initially viewed as a continuous variable, was discretized to facilitate optimized statistical analysis. Using SPSS software (version 25.0), a threshold age of 57 years was determined through receiver operating characteristic curves (ROC) curve analysis. This led to the classification of age into two groups: < 57 years and ≥ 57 years. Categorical data are presented as percentages (%) and were compared via the χ2 test. Survival rates were assessed via the Kaplan-Meier method with GraphPad Prism software, and corresponding survival curves were generated. Various prognostic factors including age, sex, carbohydrate antigen 19-9 (CA19-9) Levels, histological differentiation, invasion depth (pT stage), lymph node metastasis, liver involvement, bile duct invasion, vascular or neural infiltration, and the presence of cancer cells within 1 cm of the gallbladder resection margin (recognized as a positive surgical margin), were scrutinized via the log-rank test. Univariate Cox regression analysis was carried out in R software to identify statistically significant variables (P < 0.05). These significant variables were then subjected to stepwise backward regression employing the step Akaike information criterion (AIC) function from the MASS package in R, which is based on the AIC, to select the model with the lowest AIC value for inclusion in the multivariable Cox regression analysis. The independent prognostic factors identified (P < 0.05) were utilized to develop a survival prediction nomogram. The model's reliability was assessed through internal validation employing the bootstrap resampling method (bootstrap = 200). Model performance was evaluated by generating ROC curves and calibration curves. A significance level (alpha) of 0.05 was established, with P values lower than 0.05 considered statistically significant.
Patient clinical data, including age, sex, presence of gallbladder stones, preoperative full blood count, biochemical markers, liver and kidney function assessments, and CA19-9 levels, were recorded. Values exceeding the standard range were considered positive. Details on postoperative adjuvant treatment are outlined in Table 1.
| Factor | n = 93 |
| Age (mean ± SD) | 64.18 ± 12.05 |
| Age distribution | |
| < 40 | 2 (2.15) |
| 40-49 | 9 (9.68) |
| 50-59 | 23 (24.73) |
| 60-69 | 21 (22.58) |
| 70-80 | 32 (34.41) |
| > 80 | 6 (6.45) |
| Sex | |
| Male | 38 (40.86) |
| Female | 55 (59.14) |
| Gallstones | |
| Yes | 42 (45.16) |
| No | 51 (54.84) |
| Hematologic parameters | |
| Red blood cell (1012/L) | 4.15 ± 1.82 |
| White blood cell (109/L) | 7.60 ± 3.27 |
| Hemoglobin (g/L) | 122.62 ± 16.83 |
| Neutrophils (%) | 41.04 ± 12.91 |
| Lymphocytes (%) | 18.99 ± 10.91 |
| Liver function test | |
| ALT (U/L) | 43.71 ± 35.03 |
| AST (U/L) | 43.13 ± 40.61 |
| ALP (U/L) | 89.15 ± 74.44 |
| ALP (U/L) | 71.99 ± 82.92 |
| GGT (U/L) | 35.94 ± 6.19 |
| Albumin (g/L) | 43.71 ± 35.03 |
| Bilirubin Level | |
| TB (umol/L) | 33.84 ± 56.43 |
| DB (umol/L) | 6.01 ± 3.62 |
| CA19-9 marker | |
| Positive | 41 (44.09) |
| Negative | 52 (55.91) |
| Adjuvant therapy | |
| Yes | 44 (47.31) |
| No | 49 (52.69) |
The pathological characteristics of the tumors are described including the T stage, lymph node involvement, histological type of the tumor, degree of tumor differentiation, and the presence of liver, bile duct, neural, or vascular invasion. These features are detailed in Table 2. The TNM staging for these patients is based on the American Joint Committee on Cancer (AJCC) 8th edition GBC staging criteria.
| Patient characteristic | n = 93 |
| pT stage | |
| T1-T2 | 29 (31.18) |
| T3-T4 | 64 (68.82) |
| Lymph node metastasis | |
| N0 | 54 (58.06) |
| N1 | 34 (36.56) |
| N2 | 5 (5.38) |
| Pathological staging (AJCC 8th) | |
| I-II | 25 (26.88) |
| III-IV | 68 (73.12) |
| Degree of tissue differentiation | |
| Well differentiated | 25 (26.88) |
| Moderately differentiated | 23 (24.73) |
| Poorly differentiated | 45 (48.39) |
| Surgical margin | |
| Positive | 10 (10.75) |
| Negative | 83 (89.25) |
| Liver invasion | |
| Positive | 43 (46.24) |
| Negative | 50 (53.76) |
| Extrahepatic bile duct invasion | |
| Positive | 36 (38.71) |
| Negative | 57 (61.29) |
| PNI | |
| Positive | 29 (31.18) |
| Negative | 64 (68.82) |
| LVI | |
| Positive | 33 (35.48) |
| Negative | 60 (64.52) |
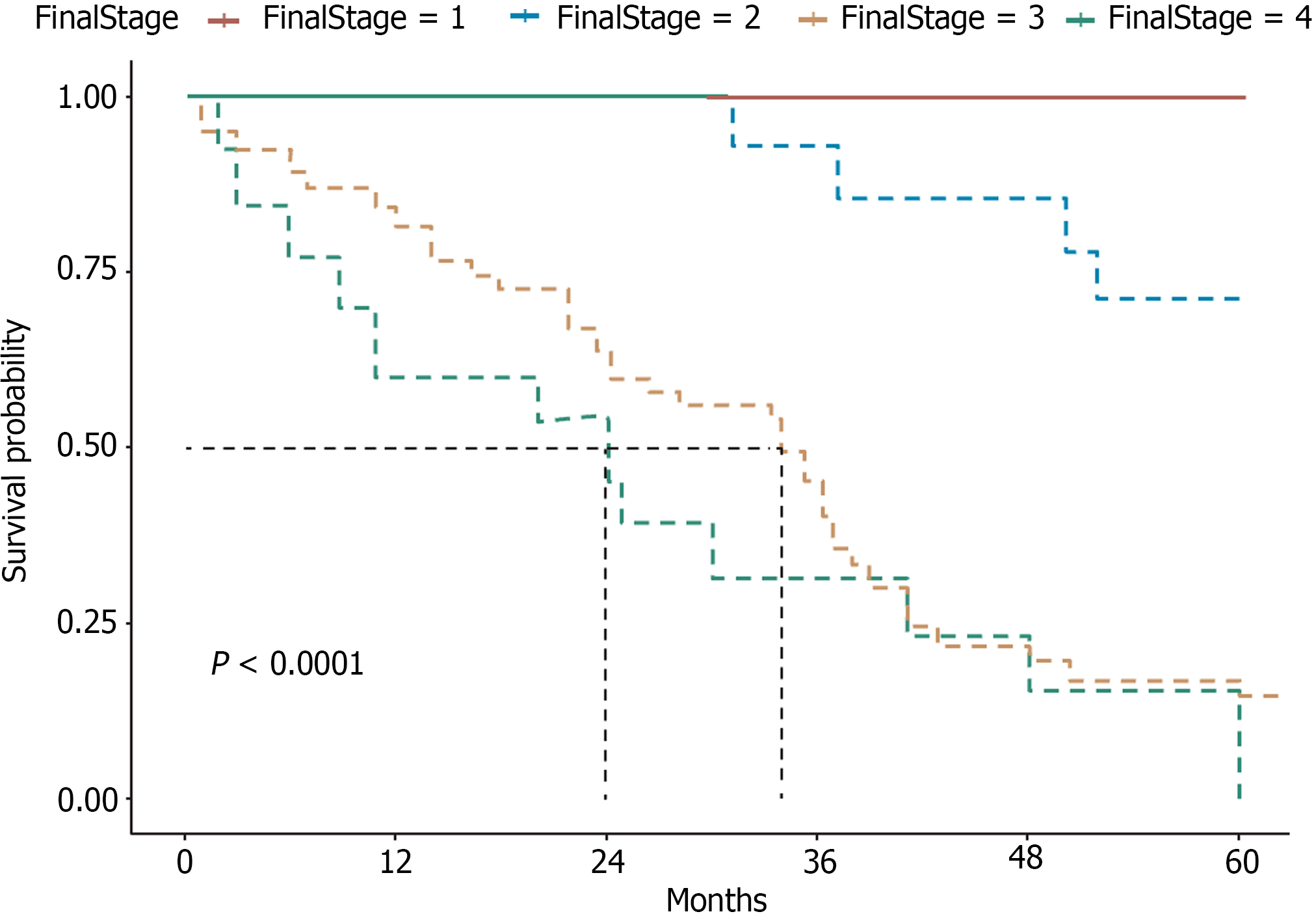
As of December 31, 2023, among the cohort of 93 patients, the median follow-up time was 60 months. At the end of this period, 30 patients (32.26%) remained alive, whereas 63 (67.74%) had died from the disease, with a median survival duration of 38 months. The analysis provides a clear depiction of the prognostic impact of the disease staging at diagnosis on patient outcomes (Figure 2). These data underscore the variable progression patterns and survival outcomes across different stages of the disease.
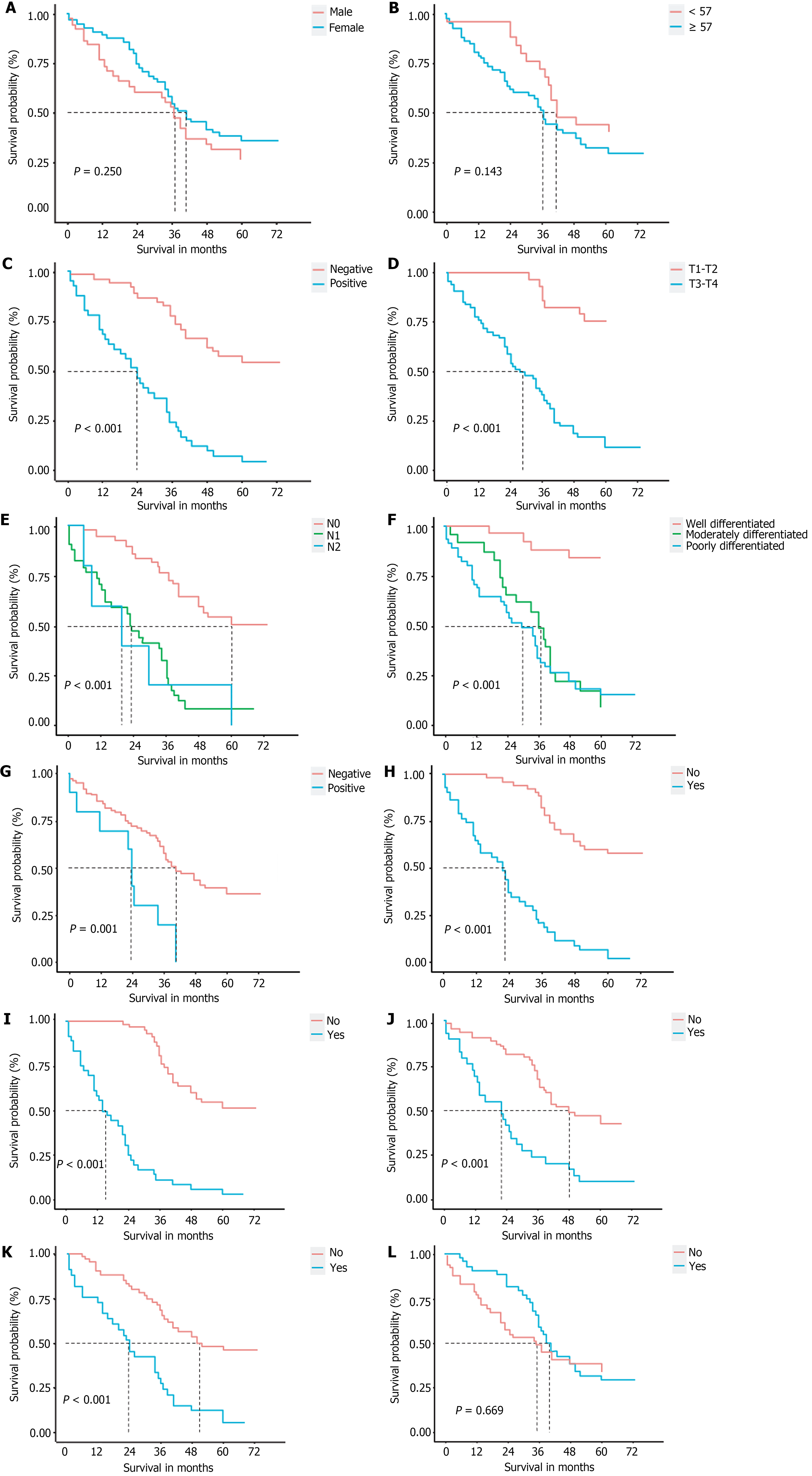
The analysis of the clinical and pathological variables in a cohort of 93 patients demonstrated that factors such as sex, age, and adjuvant therapy following surgery did not have a significant influence on postoperative survival rates. Conversely, notable indicators of postoperative survival included CA19-9 levels, T staging, lymph node metastasis, tissue differentiation, surgical margins, liver invasion, bile duct invasion, perineural invasion, and lymphovascular invasion (P < 0.05; Figure 3).
A study using univariate Cox regression analysis was conducted to assess twelve potential prognostic factors in patients with GBC who underwent curative surgery. The variables under investigation included sex, age, CA19-9 positivity, T stage, lymph node metastasis, tissue differentiation, surgical margin status, liver invasion, extrahepatic bile duct invasion, neural invasion, vascular invasion, and receipt of adjuvant therapy. The findings demonstrated statistically significant associations with overall survival (OS) for CA19-9 positivity, T stage, lymph node metastasis, tissue differentiation, surgical margin status, liver invasion, extrahepatic bile duct invasion, neural invasion, and vascular invasion (P ≤ 0.001) as indicated in Table 3. A stepwise backward regression analysis was performed to choose the best variable combination, including liver invasion, extrahepatic bile duct invasion, degree of tissue differentiation, lymph node metastasis, and neural invasion. These factors were used in a multivariable Cox regression analysis, which revealed that lymph node metastasis (P = 0.03), degree of tissue differentiation (P < 0.05), neural invasion (P = 0.036), and extrahepatic bile duct invasion (P = 0.014) were significant independent risk factors affecting GBC prognosis after surgery. Liver invasion was also noted as an important prognostic factor (P = 0.063; Table 4).
| Factor | Frequency/percentage | HR (95%CI) | P value |
| Sex | |||
| Male | 38 (40.9) | - | |
| Female | 55 (59.1) | 0.75 (0.45-1.23) | P = 0.248 |
| Age | |||
| < 57 | 25 (26.9) | - | |
| ≥ 57 | 68 (73.1) | 1.51 (0.84-2.70) | P = 0.165 |
| CA19-9 | |||
| Negative | 52 (55.9) | - | |
| Positive | 41 (44.1) | 5.05 (2.97-8.60) | P < 0.001 |
| T stage | |||
| T1-T2 | 29 (31.2) | - | |
| T3-T4 | 64 (68.8) | 7.62 (3.44-16.87) | P < 0.001 |
| Lymph node metastasis | |||
| N0 | 54 (58.1) | - | |
| N1 | 34 (36.6) | 4.18 (2.44-7.15) | P < 0.001 |
| N2 | 5 (5.4) | 4.69 (1.79-12.26) | P = 0.002 |
| Differentiation degree | |||
| Well-differentiated | 25 (26.9) | - | |
| Moderately differentiated | 23 (24.7) | 10.28 (3.50-30.21) | P < 0.001 |
| Poorly differentiated | 45 (48.4) | 11.38 (4.03-32.11) | P < 0.001 |
| Surgical margin | |||
| Negative | 83 (89.2) | - | |
| Positive | 10 (10.8) | 3.10 (1.54-6.22) | P = 0.002 |
| Liver invasion | |||
| Absent | 50 (53.8) | - | |
| Present | 43 (46.2) | 6.91 (3.99-11.97) | P < 0.001 |
| Variable | HR | 95%CI | P value |
| Lymph node metastasis | |||
| N1 | 1.12 | 0.39-3.22 | P = 0.835 |
| N2 | 1.93 | 1.07-3.48 | P = 0.030 |
| Degree of differentiation | |||
| Moderately differentiated | 4.08 | 1.32-12.64 | P = 0.015 |
| Poorly differentiated | 4.33 | 1.35-13.89 | P = 0.014 |
| Surgical margin | |||
| Positive | 0.39 | 0.20-0.74 | P = 0.004 |
| Liver invasion | |||
| Present | 1.97 | 0.96-4.03 | P = 0.063 |
| Extrahepatic bile duct invasion | |||
| Present | 2.39 | 1.20-4.77 | P = 0.014 |
| Perineural invasion | |||
| Present | 1.83 | 1.04-3.22 | P = 0.036 |
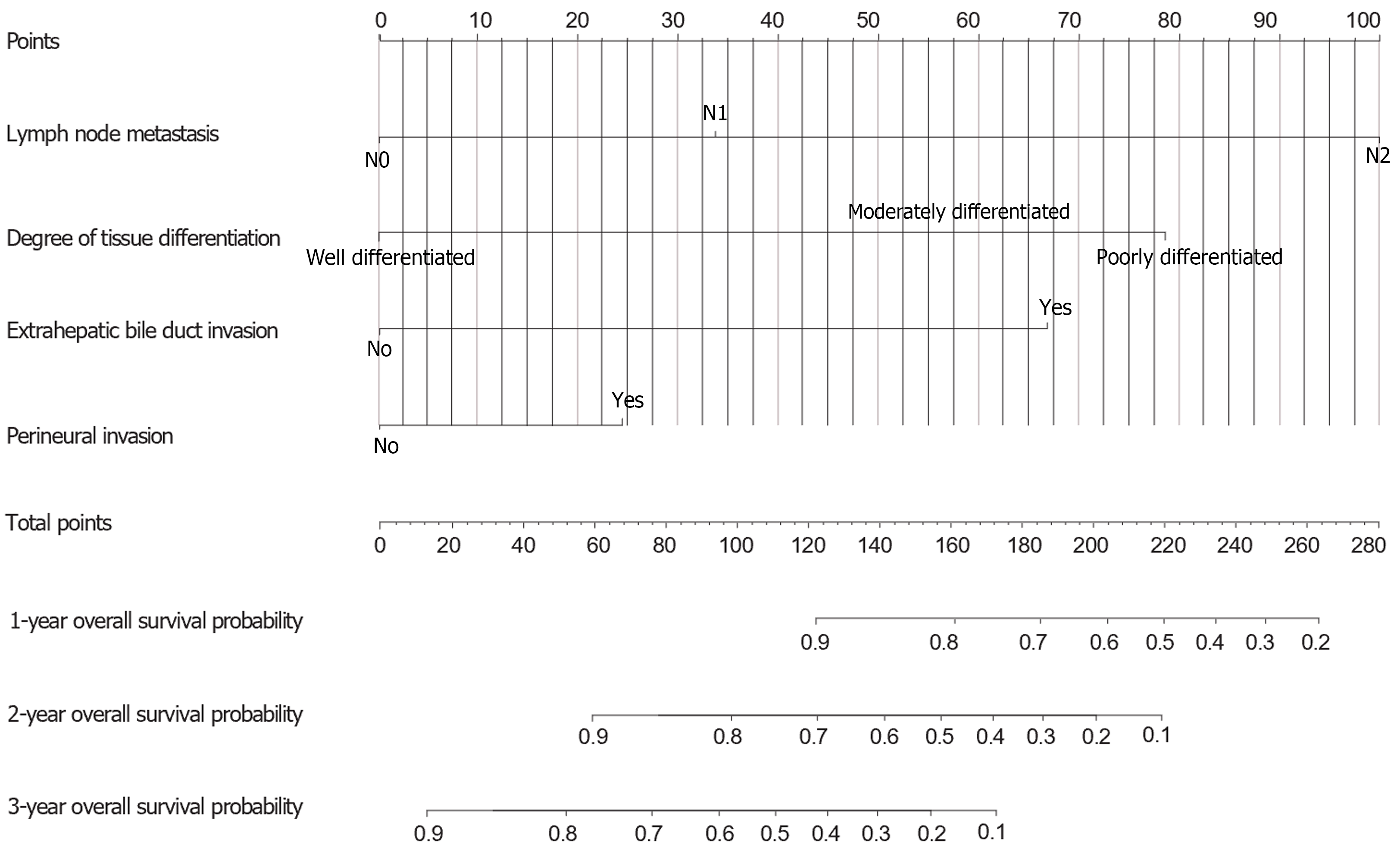
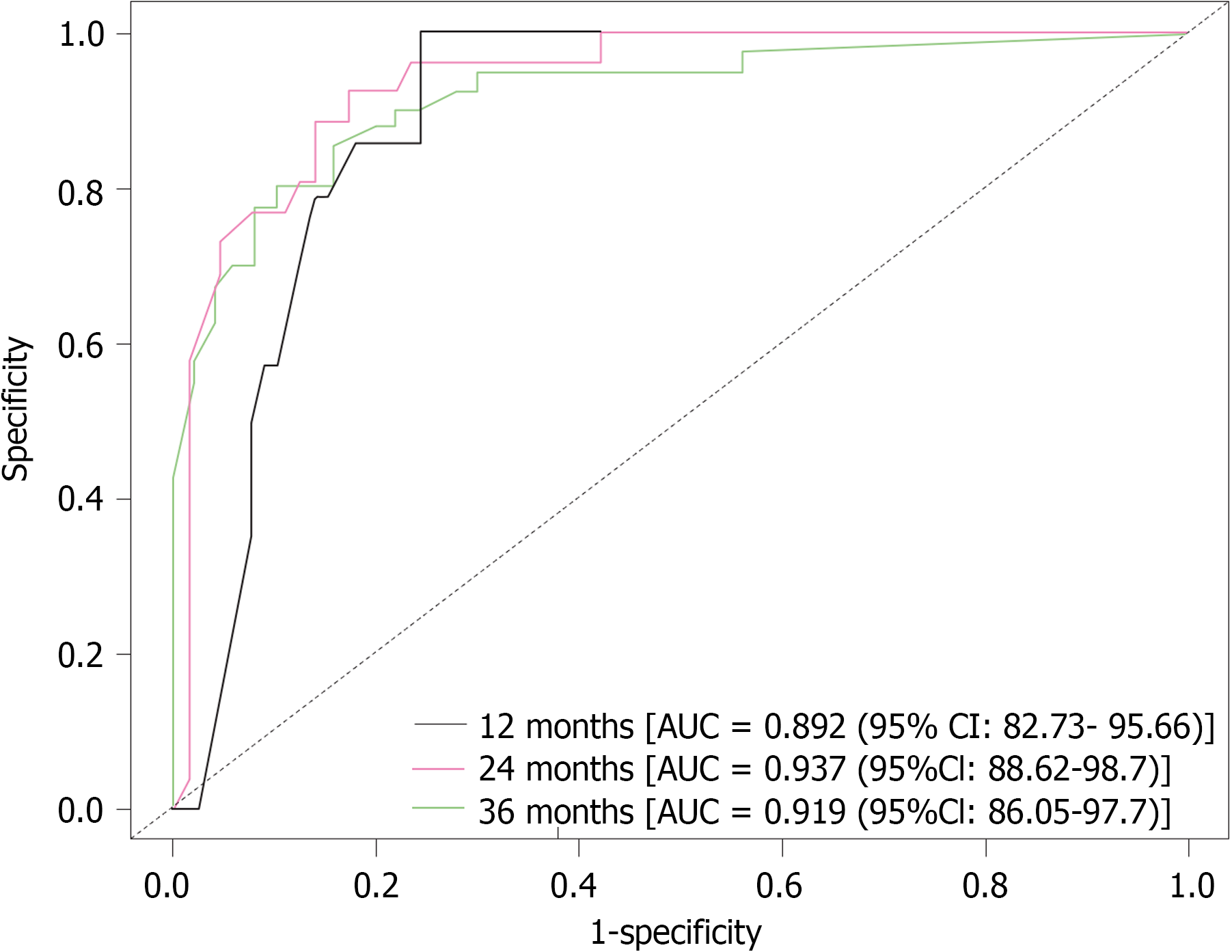
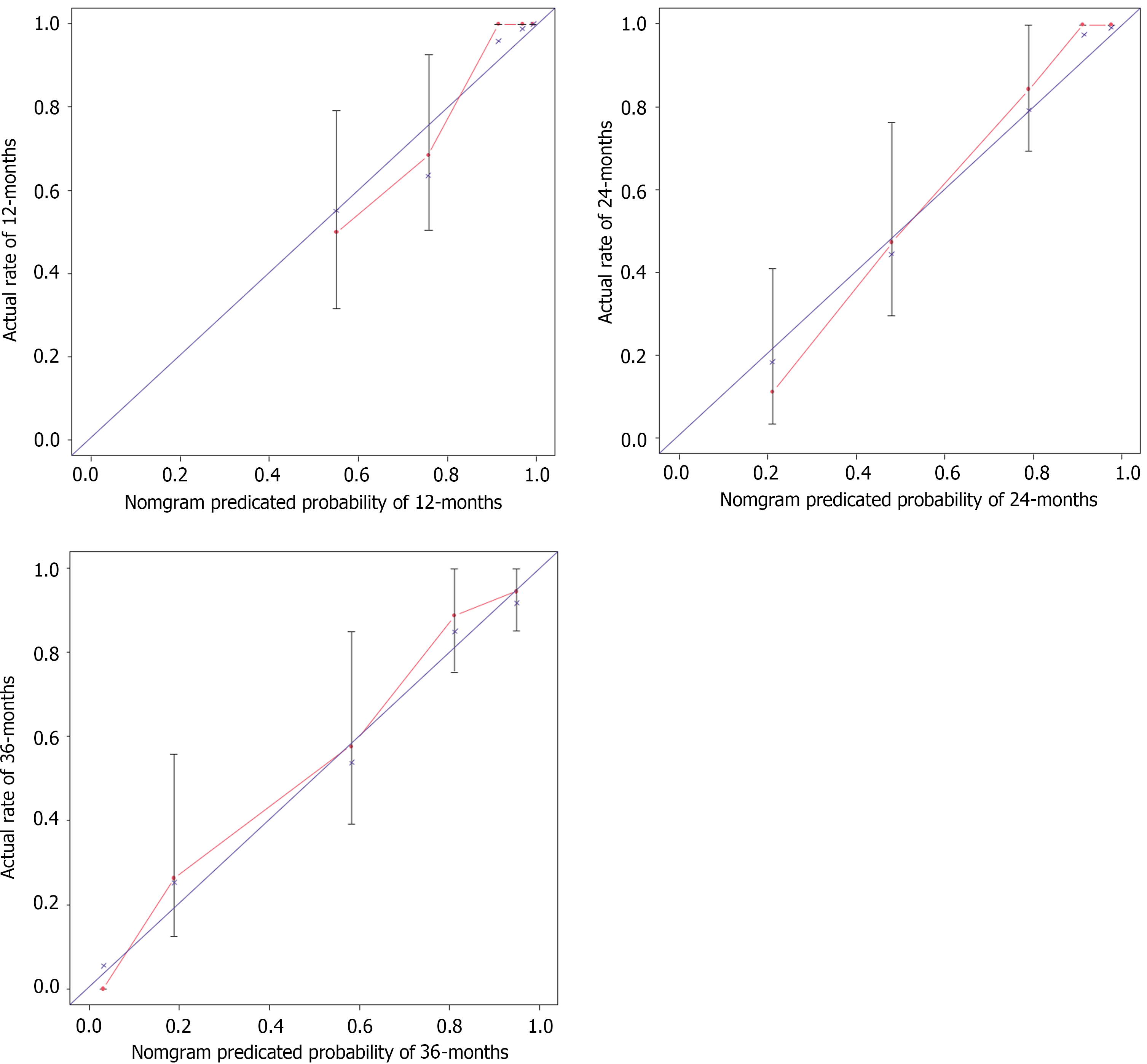
Using the AIC and multivariate Cox regression, this research revealed five key variables for a nomogram for predicting 1-year, 2-year, and 3-year OS (Figure 4). The ROC curves for 1-, 2-, and 3- year OS were 0.892, 0.937, and 0.919, respectively, highlighting the model's accuracy, stability, and discriminative ability (Figure 5). Moreover, the model was internally validated, showing a concordance index (C-index) of 0.838, indicating good agreement between the predicted and observed survival rates (Figure 6).
GBC holds the sixth position among gastrointestinal neoplasms, representing 1.2% of all cancer cases. Its frequency is progressively increasing, and the overall prognosis is generally unfavorable, typically resulting in a median survival of less than one year in advanced stages[1]. Despite these challenges, comprehensive investigations regarding the clinical and pathological prognostic determinants in patients with GBC are scarce[5,6]. This study constructed a clinical prediction nomogram utilizing four distinct risk factors: The presence of lymph node metastasis, tumor grade, invasion of the bile duct, and neural invasion. Through internal validation, the feasibility of the nomogram was confirmed, providing an alternative to the conventional TNM models for predicting survival. The prognosis of GBC patients is shaped by a multifaceted interplay of elements, leading to discrepancies in findings across previous studies due to variations in assessment criteria and research emphases[7].
Lymph node metastasis is a common occurrence in advanced GBC and significantly impacts prognosis[8-10]. The N classification in the 8th edition of the AJCC Cancer Staging Manual prioritizes the number of affected nodes over their location, underscoring its prognostic importance[10]. Metastasis to lymph nodes in patients with GBC poses an independent risk factor for postoperative survival due to various factors: Disease progression to advanced stages, necessitating aggressive treatments like extensive surgeries and intensified chemotherapy or radiotherapy[11], reflecting heightened tumor aggressiveness, lowering long-term survival rates, and potential presence of specific biomarkers like differentially expressed proteins[12]. Overall, lymph node metastasis in GBC is a crucial prognostic factor indicating disease spread, tumor aggressiveness, treatment complexity, and recurrence risk. Vigilant post-treatment monitoring and follow-up are necessary to promptly detect and manage lymph node involvement and other signs of recurrence.
Tumor differentiation plays a critical role in treatment and prognosis, as evidenced by various studies indicating its impact on postoperative outcomes[9,13,14].
Tumor differentiation affects the tumor microenvironment, influencing factors such as angiogenesis, immune evasion, and cell interactions, thus affecting cancer growth and treatment responses[15,16].
Patients with moderately and poorly differentiated GBC in the study demonstrated lower survival rates, underscoring the aggressive nature of less differentiated cancers. Conversely, those with well-differentiated tumors had higher survival rates, showing a clear link between differentiation and patient survival. The degree of differentiation not only correlates with tumor behavior, affecting growth, invasion, and metastasis, but also significantly impacts clinical management. For patients with moderately and poorly differentiated tumors, more aggressive treatments such as radiotherapy and chemotherapy are necessary due to their high metastatic potential. Despite postoperative adjuvant therapy, poorly differentiated tumors may not respond as effectively due to their aggressiveness. Therefore, tumor differentiation is vital for determining treatment strategies, monitoring tumor progression, and designing personalized therapeutic approaches for GBC.
Extrahepatic bile duct invasion, which is often associated with biliary obstruction and jaundice, is a significant independent prognostic factor in GBC patients. In our research, 36 patients (38.71%) presented with this condition and underwent resection of the extrahepatic bile duct. At the conclusion of the follow-up period, the mortality rate was alarmingly high at 97.22%, with only one patient surviving. A fraction of these individuals exhibit obstructive jaundice as a clinical manifestation. The degree of invasion along the extrahepatic bile duct, which is dictated by biliary anatomy, plays a critical role in determining resectability in advanced stages of the disease. Evaluating both the presence and extent of bile duct invasion is imperative for accurately assessing tumor resectability. Patients with preoperative obstructive jaundice experienced notably poorer prognoses, with a median survival of 5 months in contrast to 23.81 months for those without jaundice.
Curative resection represents a potential treatment option for advanced GBC featuring extrahepatic bile duct invasion and/or jaundice, provided that there is no distant metastasis and that R0 resection is feasible[17]. Nevertheless, the intricate nature of such cases presents challenges to achieving curative resection. Surgical strategies should be effective if the intervention does not surpass the liver or bile duct. For example, when the resection of neighboring organs, such as hepatopancreatoduodenectomy, is necessary, meticulous patient selection is paramount[18,19]. Further investigations are warranted to elucidate the contraindications for curative surgery in these patients, aiming to optimize treatment outcomes.
Perineural invasion (PNI) in GBC is considered a harmful pathological factor, that is correlated with increased tumor aggressiveness, increased recurrence rates, and notably poorer outcomes, leading to substantially reduced survival rates[20]. PNI commonly occurs in tumors near the gallbladder neck or cystic duct, and is often associated with higher T stages and various clinicopathological characteristics, such as the tumor size, lymph node metastasis, and overall tumor staging[21,22]. Within our patient group, individuals with PNI had significantly reduced OS, with a median OS of 22 months compared with 48 months for those without PNI. Moreover, neural invasion is linked to higher T stages, increased lymph node metastasis rates, worsening prognoses, and complicating disease management. The majority of stage III/IV patients presented with PNI (28/29), whereas only one stage I/II patient presented with PNI. Patients with neural invasion also demonstrated a considerably greater probability of lymph node metastasis than did those without neural invasion (65.52% vs 31.25%, P < 0.05). Despite its significant prognostic impact, the influence of neural invasion on survival can be alleviated. Among the 29 patients with neural involvement, those who underwent postoperative adjuvant therapy had a median survival time of 43.5 months, which was significantly longer than the 13.87 months observed in patients who did not receive such treatment. This underscores the importance of timely and appropriate therapeutic interventions, such as postoperative adjuvant therapy, in enhancing patient outcomes. These findings indicate that clinicians should adopt a proactive, multidisciplinary approach for the treatment of patients with PNI-positive GBC. Future research should concentrate on delving deeper into the mechanisms of neural invasion and its implications for therapeutic approaches, with the goal of increasing survival rates and enhancing the post surgery quality of life for these patients.
Sex: GBC occurrence varies between sexes and is more prevalent in women than in men, with ratios ranging from 2:1 to 6:1[23]. In our analysis, we noted a ratio of 1.45:1, comprising 55 females and 38 males. This difference is likely attributed to the association between estrogen-induced gallstone formation and an increased risk of GBC in females. Studies indicate that estrogen can stimulate gallstone formation by increasing cholesterol levels in bile[24]. This, combined with chronic cholecystitis, is a well-established risk factor for GBC, impacting approximately 70%-88% of patients[25]. In our dataset, approximately half of the patients had concurrent gallstones, potentially indicating specific environmental and genetic susceptibilities within the studied population. The gallstone prevalence was notably greater in female patients (64.29%) than in male patients (35.71%), highlighting sex-specific factors related to GBC etiology. This disparity underscores the necessity for targeted investigation into biological mechanisms and the establishment of sex-specific preventive strategies in GBC care.
Age distribution: In this study, GBC onset occurred mostly between the ages of 70 and 80. However, data from the Global Cancer Observatory show that the highest GBC rates are in individuals above 85 years of age[26], with the main age range for gallbladder and bile duct cancer in domestic cases being 80 to 84 years[26,27]. The difference in age distribution could be due to the small sample size and the limited focus of our center's group, which is not representative of the general population. Higher rates in older age groups might stem from a lack of awareness or reluctance to seek treatment for tumors, leading to delayed medical consultation, resulting in statistical bias from underrepresentation of this age group.
Surgical margins in nonmetastatic GBC: For nonmetastatic GBC, radical cholecystectomy with nonanatomical wedge resections of liver segments IVb/V and regional lymphadenectomy are standard. Ensuring negative surgical margins is crucial as they greatly impact OS. Studies indicate that positive margins (R1 resection) are linked to a shorter median OS and increased risks of mortality [hazard ratio (HR) = 4.08, 95%CI: 1.22-13.64, P < 0.001] and recurrence (HR = 4.13, 95%CI: 1.22-13.9, P < 0.001)[3,28]. When negative margins are not achieved, two common scenarios are positive liver margins and positive extrahepatic bile duct margins. Positive liver margins usually occur when the tumor invades the middle hepatic vein, and surgeons may choose to preserve the vein to avoid liver failure, especially in elderly patients, resulting in positive margins. Positive bile duct margins occur when the tumor extends into the pancreatic segment of the common bile duct and reaches the hepatic hilum. An R0 resection in these cases would require both a-pancreatoduodenectomy and liver resection, but many patients cannot undergo such extensive surgery, leading to positive bile duct margins.
Liver invasion: Liver invasion greatly impacts the prognosis of GBC patients and is crucial in assessing patient outcomes after surgery[29,30]. Univariate analysis validated its significant influence on survival. Advanced imaging methods, such as computed tomography with three-dimensional reconstruction are advised before surgery to evaluate the extent of liver invasion. This assessment helps in determining the required resection extent during surgery and ensuring sufficient remaining liver volume for postoperative liver function maintenance.
Adjuvant therapy: Although adjuvant therapy improves survival rates for advanced GBC patients, uptake is low, with less than one-third of eligible patients receiving it[21]. In our study, fewer than half of the patients received adjuvant therapy, mainly due to contraindications such as advanced age, liver and cardiopulmonary dysfunction, other comorbidities, and concerns about drug side effects and resistance, complicating treatment delivery. A retrospective analysis conducted at the Mayo Clinic revealed a notable increase in survival rates among individuals receiving adjuvant treatment[31]. In contrast, in our research, the limited number of participants did not exhibit evident advantages from adjuvant therapy, mainly because of the patients' initial frail health status and the aggressive characteristics of their advanced-stage ailment. Consequently, adjuvant therapy did not prove to be a standalone influential factor impacting postoperative results within our group.
Nomograms, which leverage statistical and medical principles, provide a succinct, two-dimensional visual tool that helps healthcare professionals and individuals grasp prognostic results effortlessly. When utilized in GBC, nomograms offer significant advantages over traditional prognostic models. They enhance accuracy, individualization, and accessibility, thereby simplifying prognosis tracking and postoperative care. Consequently, nomograms play a pivotal role in assessing GBC patients postsurgery[32-34].
Nomograms represent a significant advancement from the conventional TNM staging system by offering a detailed, visual overview of specific patient data and prognostic factors. This approach enables a comprehensive, multifaceted assessment of disease status and risks, overcoming the limitations of TNM staging, which focuses primarily on tumor characteristics, such as the depth of infiltration, lymph node involvement, and metastasis. TNM often fails to account for subtle differences among patients and the full spectrum of biological responses in GBC.
In contrast, nomograms provide personalized prognostic scores and survival predictions tailored to individual attributes, allowing healthcare providers to develop precise treatment plans. They advocate for more aggressive therapies for patients with poorer prognoses while minimizing overtreatment for those with better outcomes, reducing unnecessary side effects and financial burdens. Furthermore, nomograms offer the flexibility to dynamically adjust prognoses on the basis of treatment response or disease progression, ensuring up-to-date and relevant prognostic information. This adaptability supports the continuous refinement of treatment approaches, enhancing patient care through targeted, personalized interventions.
Nomograms offer a clear and simple alternative to the complex TNM staging system by visually illustrating how various factors, such as lymph node metastasis and invasion of the extrahepatic bile duct, influence prognosis. The position and length of each factor on the graph directly indicate its impact on patient outcomes, enabling both physicians and patients to identify key prognostic elements easily.
This visual simplicity distinguishes nomograms as user-friendly tools. They enable clinicians to conduct quantitative risk assessments, such as predicting the 3-year survival rate, without intricate calculations or extensive statistical knowledge. This facilitates straightforward clinical decision-making and enhances patient care management.
This model supports real-time monitoring, aiding in the early detection of disease recurrence or progression, and facilitating timely interventions. Physicians can tailor follow-up plans on the basis of individual patient risk profiles, scheduling less frequent visits for patients with favorable prognoses and more intensive monitoring for high-risk individuals. This individualized approach not only enhances monitoring efficiency but also optimizes the utilization of healthcare resources.
Previous investigations into GBC primarily utilized conventional models such as TNM staging, placing minimal emphasis on nomograms[35,36]. Our research pinpointed essential elements, such as lymph node metastasis and tumor differentiation and constructed a more precise nomogram to predict patient survival. Nonetheless, our research is limited by its retrospective, single-center design, limited sample size, and likely data biases. Subsequent studies should encompass multicenter trials and incorporate additional variables to bolster the model's precision.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55773] [Article Influence: 7967.6] [Reference Citation Analysis (132)] |
| 2. | Krell RW, Wei AC. Gallbladder cancer: surgical management. Chin Clin Oncol. 2019;8:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Patkar S, Ostwal V, Ramaswamy A, Engineer R, Chopra S, Shetty N, Dusane R, Shrikhande SV, Goel M. Emerging role of multimodality treatment in gall bladder cancer: Outcomes following 510 consecutive resections in a tertiary referral center. J Surg Oncol. 2018;117:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Creasy JM, Goldman DA, Gonen M, Dudeja V, O'Reilly EM, Abou-Alfa GK, Cercek A, Harding JJ, Balachandran VP, Drebin JA, Allen PJ, Kingham TP, D'Angelica MI, Jarnagin WR. Evolution of surgical management of gallbladder carcinoma and impact on outcome: results from two decades at a single-institution. HPB (Oxford). 2019;21:1541-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Wu D, Jin W, Zhang Y, An Y, Chen X, Chen W. Insights From the Analysis of Clinicopathological and Prognostic Factors in Patients With Gallbladder Cancer. Front Oncol. 2022;12:889334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Zatonski WA, Lowenfels AB, Boyle P, Maisonneuve P, Bueno de Mesquita HB, Ghadirian P, Jain M, Przewozniak K, Baghurst P, Moerman CJ, Simard A, Howe GR, McMichael AJ, Hsieh CC, Walker AM. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Feroz Z, Gautam P, Tiwari S, Shukla GC, Kumar M. Survival analysis and prognostic factors of the carcinoma of gallbladder. World J Surg Oncol. 2022;20:403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Higuchi R, Yazawa T, Uemura S, Matsunaga Y, Ota T, Araida T, Furukawa T, Yamamoto M. Examination of Prognostic Factors Affecting Long-Term Survival of Patients with Stage 3/4 Gallbladder Cancer without Distant Metastasis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Chen C, Rui Z, Yuhan W, Yongjie Z, Yinghe Q, Ning Y, Tianqiang S, Jianying L, Jiangtao L, Xianhai M, Shengping L, Shubin S, Zhiqiang C, Zhaohui T, Zhimin G. Optimal Lymph Node Staging System in Evaluating Prognosis of Gallbladder Carcinoma: A Multi-institutional Study. Ann Surg Oncol. 2021;28:8142-8151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Jiang W, Zhao B, Li Y, Qi D, Wang D. Modification of the 8th American Joint Committee on Cancer staging system for gallbladder carcinoma to improve prognostic precision. BMC Cancer. 2020;20:1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Kamada Y, Hori T, Yamamoto H, Harada H, Yamamoto M, Yamada M, Yazawa T, Tani M, Sato A, Tani R, Aoyama R, Sasaki Y, Zaima M. Surgical treatment of gallbladder cancer: An eight-year experience in a single center. World J Hepatol. 2020;12:641-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Jain V, Akhtar J, Priya R, Sakhuja P, Goyal S, Agarwal AK, Ghose V, Polisetty RV, Sirdeshmukh R, Siraj F, Gautam P. Tissue proteome analysis for profiling proteins associated with lymph node metastasis in gallbladder cancer. BMC Cancer. 2023;23:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 13. | Cao P, Hong H, Yu Z, Chen G, Qi S. A Novel Clinically Prognostic Stratification Based on Prognostic Nutritional Index Status and Histological Grade in Patients With Gallbladder Cancer After Radical Surgery. Front Nutr. 2022;9:850971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Mochidome N, Koga Y, Ohishi Y, Miyazaki T, Matsuda R, Yamada Y, Aishima S, Nakamura M, Oda Y. Prognostic implications of the coexisting precursor lesion types in invasive gallbladder cancer. Hum Pathol. 2021;114:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, Zhang B, Meng Q, Yu X, Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1314] [Cited by in RCA: 1311] [Article Influence: 327.8] [Reference Citation Analysis (0)] |
| 16. | Wang JX, Choi SYC, Niu X, Kang N, Xue H, Killam J, Wang Y. Lactic Acid and an Acidic Tumor Microenvironment suppress Anticancer Immunity. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 249] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 17. | Liu F, Li FY. Puzzle and Challenge in Routine Extrahepatic Bile Duct Resection for Advanced Gallbladder Carcinoma. Gut Liver. 2020;14:850-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Wu X, Li M, Wu W, Wang X, Li H, Bao R, Shu Y, Shen J, Gu J, Wang X, Gong W, Peng S, Liu Y. Hepatopancreatoduodenectomy for advanced biliary malignancies. Chin Med J (Engl). 2022;135:2851-2858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Fancellu A, Sanna V, Deiana G, Ninniri C, Turilli D, Perra T, Porcu A. Current role of hepatopancreatoduodenectomy for the management of gallbladder cancer and extrahepatic cholangiocarcinoma: A systematic review. World J Gastrointest Oncol. 2021;13:625-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Lee H, Choi DW, Park JY, Youn S, Kwon W, Heo JS, Choi SH, Jang KT. Surgical Strategy for T2 Gallbladder Cancer According to Tumor Location. Ann Surg Oncol. 2015;22:2779-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Maruyama S, Kawaida H, Hosomura N, Amemiya H, Saito R, Shimizu H, Furuya S, Akaike H, Kawaguchi Y, Sudo M, Inoue S, Kono H, Ichikawa D. Indications for extrahepatic bile duct resection due to perineural invasion in patients with gallbladder cancer. World J Surg Oncol. 2019;17:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Yamaguchi R, Nagino M, Oda K, Kamiya J, Uesaka K, Nimura Y. Perineural invasion has a negative impact on survival of patients with gallbladder carcinoma. Br J Surg. 2002;89:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 24. | de Bari O, Wang TY, Liu M, Paik CN, Portincasa P, Wang DQ. Cholesterol cholelithiasis in pregnant women: pathogenesis, prevention and treatment. Ann Hepatol. 2014;13:728-745. [PubMed] |
| 25. | Pilgrim CH, Groeschl RT, Christians KK, Gamblin TC. Modern perspectives on factors predisposing to the development of gallbladder cancer. HPB (Oxford). 2013;15:839-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Huang J, Patel HK, Boakye D, Chandrasekar VT, Koulaouzidis A, Lucero-Prisno Iii DE, Ngai CH, Pun CN, Bai Y, Lok V, Liu X, Zhang L, Yuan J, Xu W, Zheng ZJ, Wong MC. Worldwide distribution, associated factors, and trends of gallbladder cancer: A global country-level analysis. Cancer Lett. 2021;521:238-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 27. | Samuel S, Mukherjee S, Ammannagari N, Pokuri VK, Kuvshinoff B, Groman A, LeVea CM, Iyer R. Clinicopathological characteristics and outcomes of rare histologic subtypes of gallbladder cancer over two decades: A population-based study. PLoS One. 2018;13:e0198809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Goel S, Aggarwal A, Iqbal A, Talwar V, Mitra S, Singh S. Multimodality management of gallbladder cancer can lead to a better outcome: Experience from a tertiary care oncology centre in North India. World J Gastroenterol. 2021;27:7813-7830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Shen H, He M, Lin R, Zhan M, Xu S, Huang X, Xu C, Chen W, Yao Y, Mohan M, Wang J. PLEK2 promotes gallbladder cancer invasion and metastasis through EGFR/CCL2 pathway. J Exp Clin Cancer Res. 2019;38:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 30. | Lei JJ, Zhang J, Chen C, Li Q, Su JB, Zhang D, Zhang R, Jin ZC, Geng ZM. [Analysis of perineural invasion with clinicopathological factors and prognosis for curatively resected gallbladder carcinoma]. Zhonghua Wai Ke Za Zhi. 2022;60:695-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Gold DG, Miller RC, Haddock MG, Gunderson LL, Quevedo F, Donohue JH, Bhatia S, Nagorney DM. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys. 2009;75:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Li L, Ren T, Liu K, Li ML, Geng YJ, Yang Y, Li HF, Li XC, Bao RF, Shu YJ, Weng H, Gong W, Lau WY, Wu XS, Liu YB. Development and Validation of a Prognostic Nomogram Based on the Systemic Immune-Inflammation Index for Resectable Gallbladder Cancer to Predict Survival and Chemotherapy Benefit. Front Oncol. 2021;11:692647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Xie ZH, Shi X, Liu MQ, Wang J, Yu Y, Zhang JX, Chu KJ, Li W, Ge RL, Cheng QB, Jiang XQ. Development and validation of a nomogram to predict overall survival in patients with incidental gallbladder cancer: A retrospective cohort study. Front Oncol. 2022;12:1007374. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Meng FX, Zhang JX, Guo YR, Wang LJ, Zhang HZ, Shao WH, Xu J. Contrast-Enhanced CT-Based Deep Learning Radiomics Nomogram for the Survival Prediction in Gallbladder Cancer. Acad Radiol. 2024;31:2356-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Yan Y, Lin J, Zhang M, Liu H, Zhou Q, Chen R, Wen K, Wang J, Xiao Z, Mao K. A Novel Staging System to Forecast the Cancer-Specific Survival of Patients With Resected Gallbladder Cancer. Front Oncol. 2020;10:1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Geng ZM, Li Q, Zhang Z, Si SB, Cai ZQ, Zhao YL, Tang ZH. [The progress on survival prediction model of gallbladder carcinoma]. Zhonghua Wai Ke Za Zhi. 2020;58:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |