Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3224
Revised: August 12, 2024
Accepted: September 2, 2024
Published online: October 27, 2024
Processing time: 89 Days and 0.5 Hours
Gastric cancer (GC) is a relatively frequent clinical phenomenon, referring to ma
To discuss the effectiveness and safety of nab-paclitaxel in combination with oxaliplatin and S-1 (P-SOX) for the treatment of GC, and to analyze the factors that may influence its outcomes.
A total of 219 eligible patients with advanced GC, who were treated at Qinghai University Affiliated Hospital Gastrointestinal Oncology between January 2018 and March 2020, were included in the study. Among them, 149 patients received SOX regimen and 70 patients received S-1 regimen. All patients underwent both preoperative and postoperative chemotherapy consisting of 2-4 cycles each, totaling 6-8 cycles, along with parallel D2 radical surgical treatment. The patients were followed up for a period of three years or until reaching the event endpoint.
The short-term and long-term efficacy of the P-SOX group was significantly higher than that of the SOX group, and the safety was manageable. Cox multivariate analysis revealed that progression-free survival was associated with perioperative chemotherapy efficacy, tumor diameter ≤ 2cm, high differentiation, and early cTNM (T stands for invasion depth; N stands for node metastasis; M stands for distant invasion) stage.
In comparison to the SOX regimen, the P-SOX regimen demonstrates improved short-term and long-term efficacy with tolerable adverse reactions. It is anticipated that the P-SOX regimen will emerge as a first-line chemotherapy option for GC. Patients with GC who receive effective perioperative chemotherapy (Response Evaluation Criteria in Solid Tumors 1.1, Tumor Regression Grade), have a tumor diameter ≤ 2cm, exhibit high degree of differentiation, and are at an early cTNM stage show better prognosis.
Core Tip: NaB-paclitaxel combined with oxaliplatin + S-1 (P-SOX) regimen is superior to conventional SOX regimen in the treatment of gastric cancer. Progression-free survival was associated with effective perioperative chemotherapy (Response Evaluation Criteria in Solid Tumors 1.1, Tumor Regression Grade), tumor diameter ≤ 2 cm, high differentiation, and early cTNM staging.
- Citation: Wang YC, Feng L, Wang GP, Yu PJ, Guo C, Cai BJ, Song Y, Pan T, Lin BH, Li YD, Xiao JJ. Comparison of efficacy and safety of nab-paclitaxel and oxaliplatin + S-1 and standard S-1 and oxaliplatin chemotherapy regimens for treatment of gastric cancer. World J Gastrointest Surg 2024; 16(10): 3224-3238
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3224.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3224
Gastric cancer (GC) is one of the most common gastrointestinal tumors, especially in China. Surgical resection is the only possible cure for patients with GC. Most early GC can be treated by endoscopy, and the 5-year survival rate is more than 90%, while the 5-year survival rate of advanced GC is still less than 30% even after surgery-based comprehensive treat
A total of 219 eligible patients with GC, who were treated at Qinghai University Affiliated Hospital Gastrointestinal Oncology between January 2018 and March 2020, were included in the study. Among them, 149 patients received SOX regimen and 70 patients received S-1 regimen. All patients underwent both preoperative and postoperative chemothe
Inclusion criteria: (1) Inclusion of patients diagnosed with stage II-IV primary gastric adenocarcinoma, as confirmed by imaging and endoscopic biopsy according to the 8th edition of the American Cancer Consortium TNM (T stands for invasion depth; N stands for node metastasis; M stands for distant invasion) Staging Criteria of the International Union Against Cancer, and successful R0 resection (no residual tumor visible to the naked eye or under a microscope); (2) The patients underwent 2-4 cycles of preoperative and postoperative adjuvant chemotherapy, followed by a total of 6-8 cycles of chemotherapy, all in accordance with the National Comprehensive Cancer Network and Chinese Society of Clinical Oncology guidelines for surgical treatment at our hospital; (3) The size of primary tumor lesions can be measured by computed tomography and magnetic resonance imaging, with confirmation through postoperative pathological biopsy; and (4) Eastern Cooperative Oncology Group performance status ≤ 1 and able to tolerate chemotherapy; with acceptable liver, kidney, hematologic and cardiopulmonary function.
Exclusion criteria: (1) Contraindications allergic to chemotherapy drugs or related to chemotherapy, as well as the com
A total of 219 patients were included in the study, with 149 patients allocated to the P-SOX group and 70 patients to the SOX group. As depicted in Table 1, there were no statistically significant discrepancies observed in clinical characteristics such as gender, age, body mass index, degree of differentiation, anesthesia grade, tumor location, laurnen type, preope
| Variables | Total (n = 219) | P-SOX (n = 149) | SOX (n = 70) | Z | P value |
| Age | 2.674 | 0.102 | |||
| ≤ 60 | 142 (64.8) | 102 (68.5) | 40 (57.1) | ||
| > 60 | 77 (35.2) | 47 (31.5) | 30 (42.9) | ||
| Sex | 0.514 | 0.473 | |||
| Male | 184 (84) | 127 (85.2) | 57 (81.4) | ||
| Female | 35 (16) | 22 (14.8) | 13 (18.6) | ||
| BMI | 0.278 | 0.598 | |||
| 18.5–24 | 140 (63.9) | 97 (65.1) | 43 (61.4) | ||
| < 18.5 or > 24 | 79 (36.1) | 52 (34.9) | 27 (38.6) | ||
| ASA | 0.168 | 0.681 | |||
| 1+2 | 178 (81.3) | 120 (80.5) | 58 (82.9) | ||
| 3 | 41 (18.7) | 29 (19.5) | 12 (17.1) | ||
| RECIST 1.1 | 1.906 | 0.167 | |||
| Ineffective | 104 (47.5) | 66 (44.3) | 38 (54.3) | ||
| Effective | 115 (52.5) | 83 (55.7) | 32 (45.7) | ||
| TRG | 1.324 | 0.250 | |||
| Effective | 128 (58.4) | 91 (61.1) | 37 (52.9) | ||
| Ineffective | 91 (41.6) | 58 (38.9) | 33 (47.1) | ||
| Tumor location | 0.260 | 0.878 | |||
| Lower | 76 (34.7) | 51 (34.2) | 25 (35.7) | ||
| Upper | 69 (31.5) | 46 (30.9) | 23 (32.9) | ||
| Middle | 74 (33.8) | 52 (34.9) | 22 (31.4) | ||
| Differentiation | 1.883 | 0.390 | |||
| Poorly | 101 (46.1) | 64 (43) | 37 (52.9) | ||
| Moderate | 72 (32.9) | 52 (34.9) | 20 (28.6) | ||
| Well | 46 (21) | 33 (22.1) | 13 (18.6) | ||
| cT stage | 0.384 | 0.825 | |||
| 2 | 60 (27.4) | 42 (28.2) | 18 (25.7) | ||
| 3 | 117 (53.4) | 80 (53.7) | 37 (52.9) | ||
| 4 | 42 (19.2) | 27 (18.1) | 15 (21.4) | ||
| cN stage | 0.155 | 0.694 | |||
| Negative | 76 (34.7) | 53 (35.6) | 23 (32.9) | ||
| Positive | 143 (65.3) | 96 (64.4) | 47 (67.1) | ||
| cTNM1 | 1.170 | 0.557 | |||
| II | 120 (54.8) | 83 (55.7) | 37 (52.9) | ||
| III | 52 (23.7) | 37 (24.8) | 15 (21.4) | ||
| IV | 47 (21.5) | 29 (19.5) | 18 (25.7) | ||
| Tumor diameter | 2.196 | 0.333 | |||
| ≤ 2 | 55 (25.1) | 33 (22.1) | 22 (31.4) | ||
| 2-5 | 91 (41.6) | 64 (43) | 27 (38.6) | ||
| ≥ 5 | 73 (33.3) | 52 (34.9) | 21 (30) | ||
| Laurnen | 3.274 | 0.195 | |||
| Enteric | 42 (19.2) | 29 (19.5) | 13 (18.6) | ||
| Mixed type | 98 (44.7) | 72 (48.3) | 26 (37.1) | ||
| Diffuse | 79 (36.1) | 48 (32.2) | 31 (44.3) | ||
Short-term efficacy: Both groups received 2-4 cycles of preoperative chemotherapy, and the short-term efficacy between the two chemotherapy regimens was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, Tumor Regression Grade (TRG) classification. As shown in Table 1, TRG evaluation: In the P-SOX group, 91 cases were effective (grade 0, 1, 2 = 61.1%,) and 58 cases were ineffective (grade 3 = 38.9%), with an ORR of 61.1%. In the SOX group, there were 37 effective cases (grades 0, 1, 2 = 52.9%) and 33 ineffective cases (grade 3 = 47.1%), resulting in an ORR of 52.9%. There was no significant difference between the two groups (P = 0.250). According to RECIST 1.1 criteria, in the P-SOX group, there were a total of 83 effective cases [complete response (CR) + partial response (PR) = 55.7%] and 66 ineffective cases (stable disease + aggressive disease = 44.3%) with an ORR of 55 .7% (CR+PR). In contrast, in the SOX group there were a total of 32 effective patients (grade 0, 1, 2 = 45.7%) and 38 ineffective patients (grade 3 = 54.3%) with an ORR of 45.7 %. The ORRs for both P-SOX group (61.l%, 55.7%) were significantly higher than those for SOX group (52.9%, 45.7%), but there was no significant difference between them (P = 0.l67).
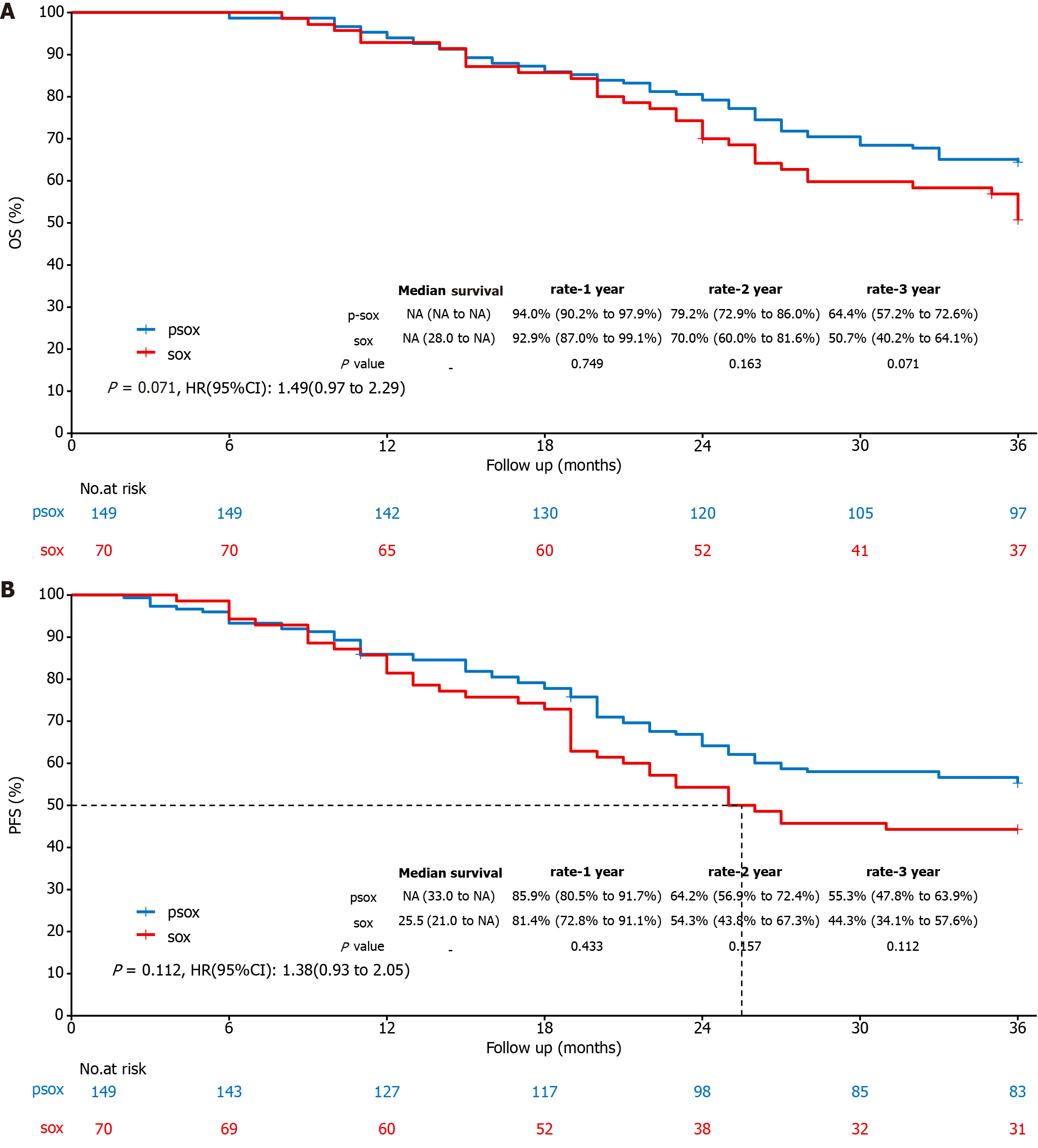
Long-term efficacy: The 1-year, 2-year and 3-year overall survival (OS) rates of the P-SOX group and the SOX group were 94.0% vs 92.9%, P = 0.749; 79.2% vs 70.0%, P = 0.163; and 64.4% vs 50.7%, P = 0.071, respectively (Figure 1A). The 1-year, 2-year and 3-year progression-free survival (PFS) rates of the P-SOX group and the SOX group were 85.9% and 81.4%, P = 0.433; 64.2% and 54.3%, P = 0.157; and 55.3% and 44.3%, P = 0.112, respectively (Figure 1B). OS and PFS in the P-SOX group were significantly greater than those in the SOX group, but there was no significant difference between the two groups (P > 0.05).
Safety: The adverse events for all participants are summarized in Table 2. Most side effects were classified as Grades 1-2, with gastrointestinal reactions (77.9% vs 78.6%), peripheral neurotoxicity (61.1% vs 48.6%), and hair loss (69.1% vs 48.6%, P = 0.013) being common in the P-SOX and SOX groups, respectively. The incidence of other adverse reactions was between 20% and 40%, and the incidence and severity of hair loss were significantly greater in the P-SOX group than in the SOX group. Except for alopecia (P = 0.013), other adverse reactions did not significantly differ between the two groups. Nevertheless, compared with that in the SOX group, the incidence of hematological toxicity above grade 2, such as neutropenia (6.1% vs 1.5%), leukopenia (5.4% vs 2.8%), thrombocytopenia (6.1% vs 4.3%), anemia (2.7% vs 0.0%), and grade 2 hepatotoxicity (7.4% vs 0.0%), was greater in the P-SOX group, which may have been caused by triple drug therapy in the P-SOX group.
| Variables | Total (n = 219) | P-SOX (n = 149) | SOX (n = 70) | P value |
| Fewer neutrophils | 0.598 | |||
| 0 | 168 (77.4) | 115 (77.2) | 53 (77.9) | |
| 1 | 37 (17.1) | 24 (16.1) | 13 (19.1) | |
| 2 | 2 (0.9) | 1 (0.7) | 1 (1.5) | |
| 3 | 6 (2.8) | 5 (3.4) | 1 (1.5) | |
| 4 | 4 (1.8) | 4 (2.7) | 0 (0) | |
| Fewer white blood cells | 0.418 | |||
| 0 | 141 (64.4) | 95 (63.8) | 46 (65.7) | |
| 1 | 50 (22.8) | 33 (22.1) | 17 (24.3) | |
| 2 | 18 (8.2) | 13 (8.7) | 5 (7.1) | |
| 3 | 9 (4.1) | 8 (5.4) | 1 (1.4) | |
| 4 | 1 (0.5) | 0 (0) | 1 (1.4) | |
| Fewer platelets | 0.985 | |||
| 0 | 158 (72.1) | 106 (71.1) | 52 (74.3) | |
| 1 | 49 (22.4) | 34 (22.8) | 15 (21.4) | |
| 2 | 7 (3.2) | 5 (3.4) | 2 (2.9) | |
| 3 | 5 (2.3) | 4 (2.7) | 1 (1.4) | |
| Anemia | 0.415 | |||
| 0 | 146 (66.7) | 102 (68.5) | 44 (62.9) | |
| 1 | 56 (25.6) | 35 (23.5) | 21 (30) | |
| 2 | 13 (5.9) | 8 (5.4) | 5 (7.1) | |
| 3 | 4 (1.8) | 4 (2.7) | 0 (0) | |
| Nausea and vomiting | 0.856 | |||
| 0 | 48 (21.9) | 33 (22.1) | 15 (21.4) | |
| 1 | 123 (56.2) | 81 (54.4) | 42 (60) | |
| 2 | 43 (19.6) | 31 (20.8) | 12 (17.1) | |
| 3 | 5 (2.3) | 4 (2.7) | 1 (1.4) | |
| Live toxicity | 0.057 | |||
| 0 | 168 (76.7) | 113 (75.8) | 55 (78.6) | |
| 1 | 40 (18.3) | 25 (16.8) | 15 (21.4) | |
| 2 | 11 (5) | 11 (7.4) | 0 (0) | |
| alopecia | 0.013 | |||
| 0 | 82 (37.4) | 46 (30.9) | 36 (51.4) | |
| 1 | 114 (52.1) | 85 (57) | 29 (41.4) | |
| 2 | 23 (10.5) | 18 (12.1) | 5 (7.1) | |
| Peripheral sensory neuropathy | 0.052 | |||
| 0 | 94 (42.9) | 58 (38.9) | 36 (51.4) | |
| 1 | 87 (39.7) | 59 (39.6) | 28 (40) | |
| 2 | 35 (16) | 30 (20.1) | 5 (7.1) | |
| 3 | 3 (1.4) | 2 (1.3) | 1 (1.4) |
Single-factor and multifactor Cox regression analysis: Univariate Cox regression analysis of baseline characteristics and short-term efficacy assessment (RECIST 1.1, TRG) in 149 patients revealed that PFS was significantly associated with RECIST 1.1, TRG, tumor diameter, degree of differentiation, lymph node metastasis, T stage, and cTNM stage (P < 0.05). The multivariate Cox regression analysis demonstrated that PFS was significantly associated with RECIST 1.1 [valid vs invalid, hazard ratio (HR): 0.507, 95%CI: 0.300–0.856, P = 0.011], TRG (invalid vs valid, HR: 1.949; 95%CI: 1.159-3.276; P = 0.012), tumor diameter (≥ 5 cm vs ≤ 2 cm, HR: 3.281; 95%CI: 1.401-7.685; P = 0.006; ≥ 5 cm vs 2-5 cm, HR: 2.503; 95%CI: 1.077-5.819; P = 0.033), differentiation degree (high vs low, HR: 0.443; 95%CI: 02000–980; P = 0044) and cTNM stage (IV vs II, HR: 3015; 95%CI: 1577–5765; P = 00001), with statistically significant differences (P < 005), as shown in Table 3 and Table 4.
| Variable | Z | HR (95%CI) | P value |
| Age | |||
| ≤ 60 | Reference | ||
| > 60 | 0.853 | 1.247 (0.751-2.069) | 0.393 |
| Sex | |||
| Male | Reference | ||
| Female | -0.350 | 0.882 (0.437-1.782) | 0.727 |
| BMI | |||
| 18.5–24 | Reference | ||
| < 18.5 or > 24 | 0.333 | 1.089 (0.659-1.799) | 0.739 |
| ASA | |||
| 1+2 | Reference | ||
| 3 | -0.192 | 0.941 (0.503-1.758) | 0.848 |
| RECIST 1.1 | |||
| Ineffective | Reference | ||
| Effective | -3.803 | 0.379 (0.230-0.625) | < 0.001 |
| TRG | |||
| Effective | Reference | ||
| Ineffective | 3.329 | 2.278 (1.403-3.698) | 0.001 |
| Tumor location | |||
| Lower | Reference | ||
| Upper | -1.622 | 0.579 (0.300-1.120) | 0.105 |
| Middle | 0.996 | 1.319 (0.765-2.277) | 0.319 |
| Differentiation | |||
| Poorly | Reference | ||
| Moderate | -0.752 | 0.819 (0.487-1.378) | 0.452 |
| Well | -2.555 | 0.365 (0.168-0.791) | 0.011 |
| cT stage | |||
| 2 | Reference | ||
| 3 | 1.105 | 1.432 (0.757-2.707) | 0.269 |
| 4 | 3.594 | 3.721 (1.817-7.618) | < 0.001 |
| cN stage | |||
| Negative | Reference | ||
| Positive | 2.097 | 1.786 (1.039-3.072) | 0.036 |
| cTNM | |||
| II | Reference | ||
| III | 2.132 | 1.935 (1.055-3.550) | 0.033 |
| IV | 4.508 | 3.724 (2.103-6.597) | < 0.001 |
| Tumor diameter | |||
| ≤ 2 | Reference | ||
| 2-5 | 2.178 | 2.503 (1.096-5.715) | 0.029 |
| ≥ 5 | 3.061 | 3.619 (1.588-8.248) | 0.002 |
| Laurnen | |||
| Enteric | Reference | ||
| Mixed type | -0.276 | 0.906 (0.447-1.833) | 0.783 |
| Diffuse | 1.506 | 1.705 (0.852-3.415) | 0.132 |
| Variable | Z | HR (95%CI) | P value |
| RECIST 1.1 | |||
| Ineffective | Reference | ||
| Effective | -2.544 | 0.507 (0.300-0.856) | 0.011 |
| TRG | |||
| Effective | Reference | ||
| Ineffective | 2.518 | 1.949 (1.159-3.276) | 0.012 |
| Differentiation | |||
| Poorly | Reference | ||
| Moderate | -1.624 | 0.628 (0.358-1.101) | 0.104 |
| Well | -2.010 | 0.443 (0.200-0.980) | 0.044 |
| cT stage | |||
| 2 | Reference | ||
| 3 | 1.263 | 1.520 (0.794-2.909) | 0.206 |
| 4 | 1.834 | 2.041 (0.952-4.376) | 0.067 |
| cN stage | |||
| Negative | Reference | ||
| Positive | 1.725 | 1.645 (0.934-2.895) | 0.085 |
| cTNM | |||
| II | Reference | ||
| III | 1.383 | 1.575 (0.827-2.997) | 0.167 |
| IV | 3.338 | 3.015 (1.577- 5.765) | 0.001 |
| Tumor diameter | |||
| ≤ 2 | Reference | ||
| 2-5 | 2.131 | 2.503 (1.077-5.819) | 0.033 |
| ≥ 5 | 2.736 | 3.281 (1.401-7.685) | 0.006 |
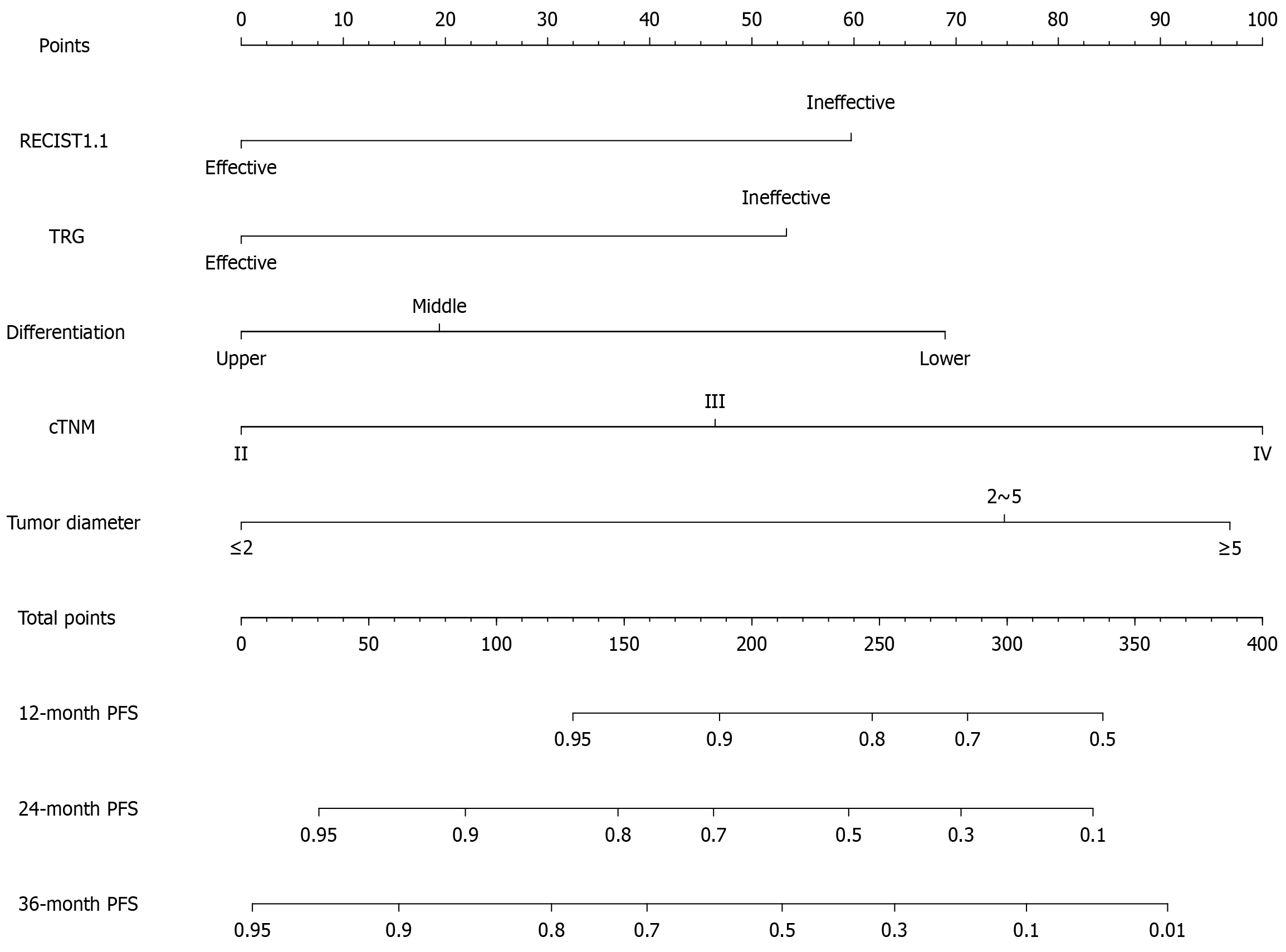
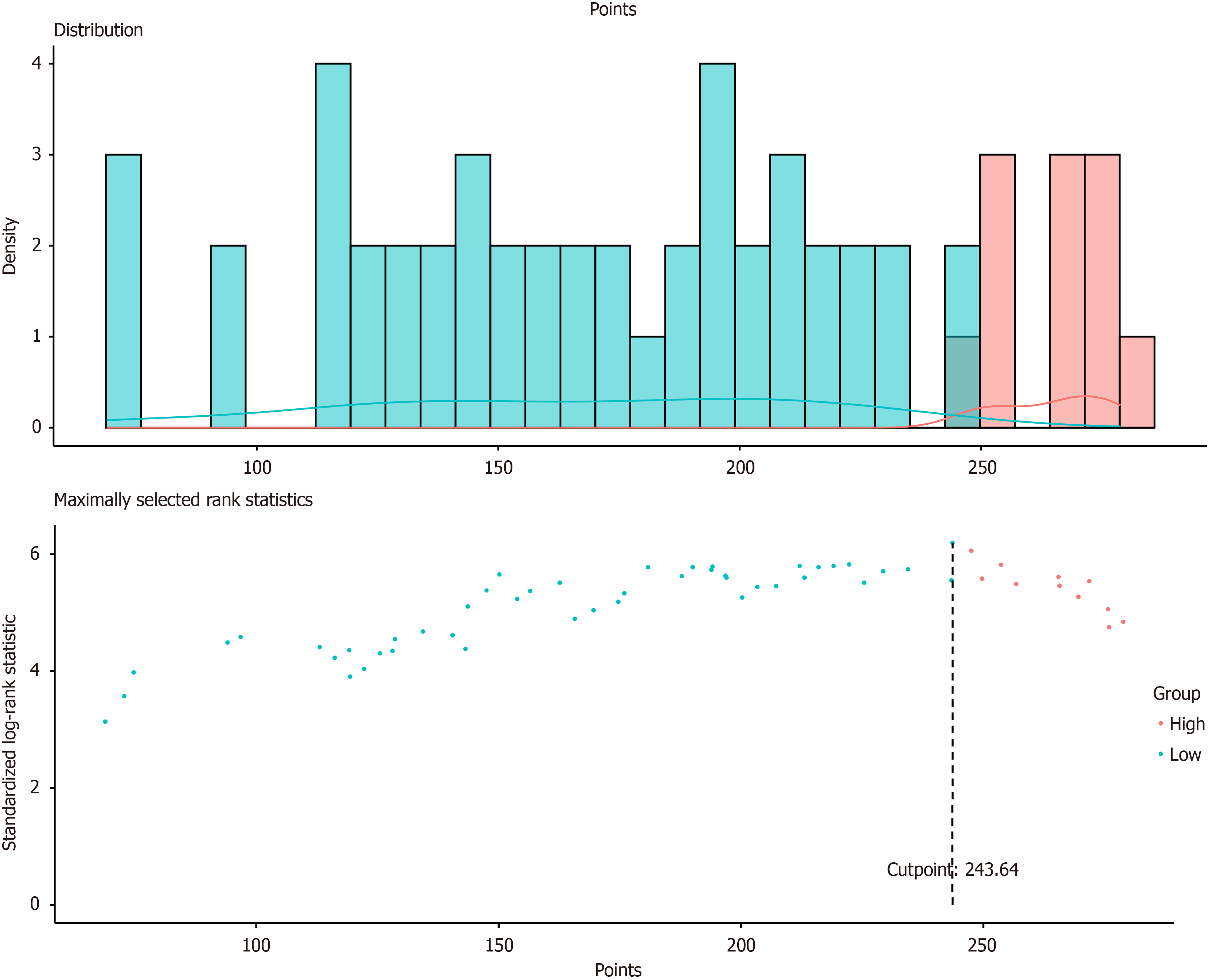
PFS model building: Based on univariate and multivariate Cox regression analyses, the final five independent risk factors screened out were used to construct a nomogram, as shown in Figure 2. Points at the top of the figure represent the score value, and the corresponding points were obtained by drawing an upward vertical line of various risk factors below. According to the sum of the corresponding factor scores, the corresponding interval of the total points below was found. According to the probability of PFS occurring at the bottom of the figure corresponding to the total score, the 1-, 2-, and 3-year PFS of the patient was estimated. In the corresponding risk column, a lower value is indicate that P-SOX chemotherapy would be more meaningful for the patient. Among these patients, those with effective perioperative chemotherapy (RECIST 1.1, TRG), a tumor diameter ≤ 2 cm, a high degree of differentiation, and cTNM Stage II gastric cancer had the highest PFS and greatest benefit.
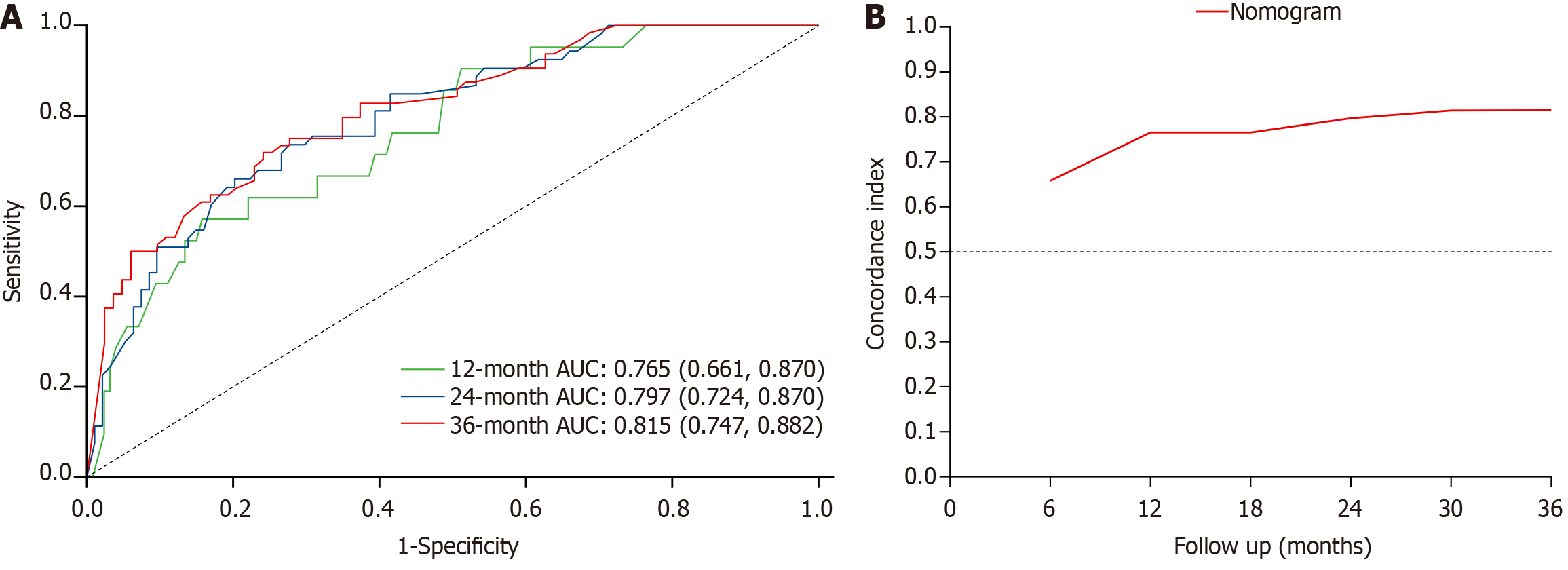
Nomogram for assessment and validation: Time-dependent receiver operating characteristic (ROC) curve analysis was used to calculate the area under the ROC curve (AUC), C-index, and other indicators for evaluating discrimination efficacy. As shown in Figure 3, the 12-month AUC was 0.765 (95%CI: 0.661-0.870), the 24-month AUC was 0.797 (95%CI: 0.724-0.870), and the 36-month AUC was 0.815 (95%CI: 0.747-0.882). The C-index of the overall model was determined to be 0.743 (95%CI: 0.687–0.799), indicating a good predictive effect of the nomogram model.
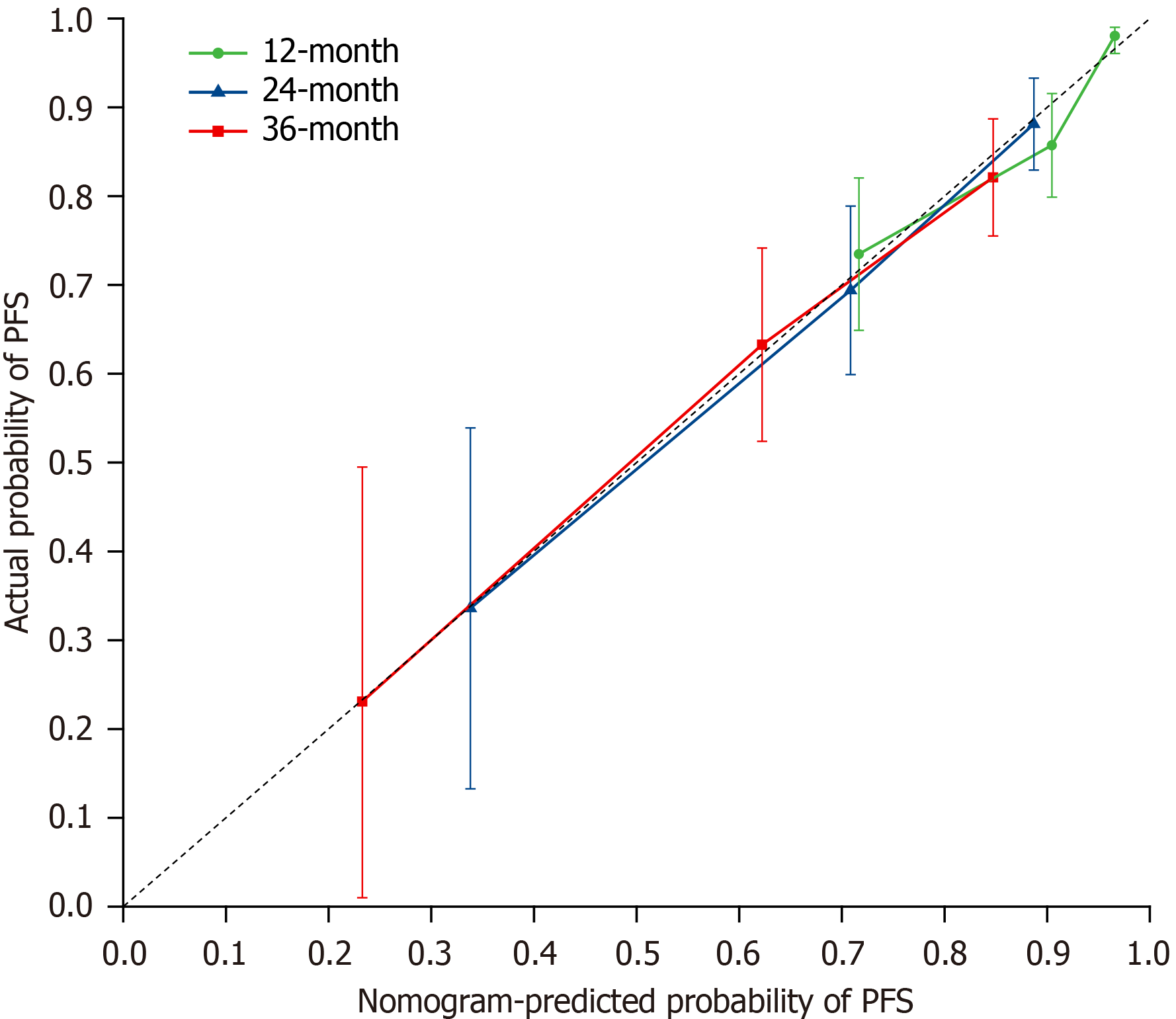
The constructed nomogram underwent bootstrap resampling verification 1000 times, and a calibration curve was generated to assess its degree of calibration, as shown in Figure 4, which demonstrated a good fit. The probability of PFS predicted by the nomogram and the actual probability of PFS in gastric cancer patients treated with P-SOX chemotherapy did not significantly differ.
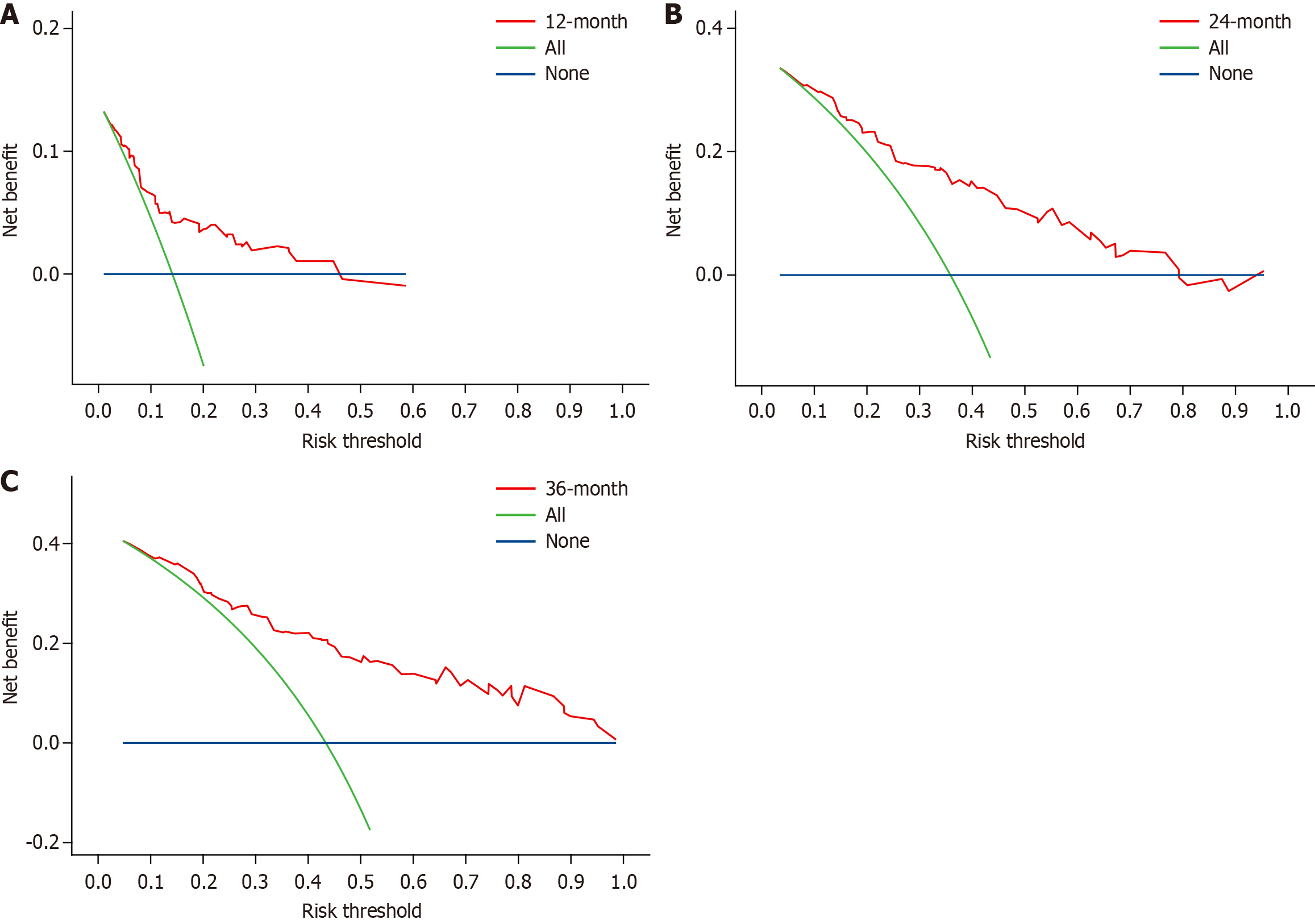
Furthermore, decision curve analysis was developed to evaluate the clinical application value of the model and quantify the net benefit within the threshold probability range. According to Figure 5, the performance of the model is good at 1 year, 2 years, and 3 years, indicating that the model has good clinical value.
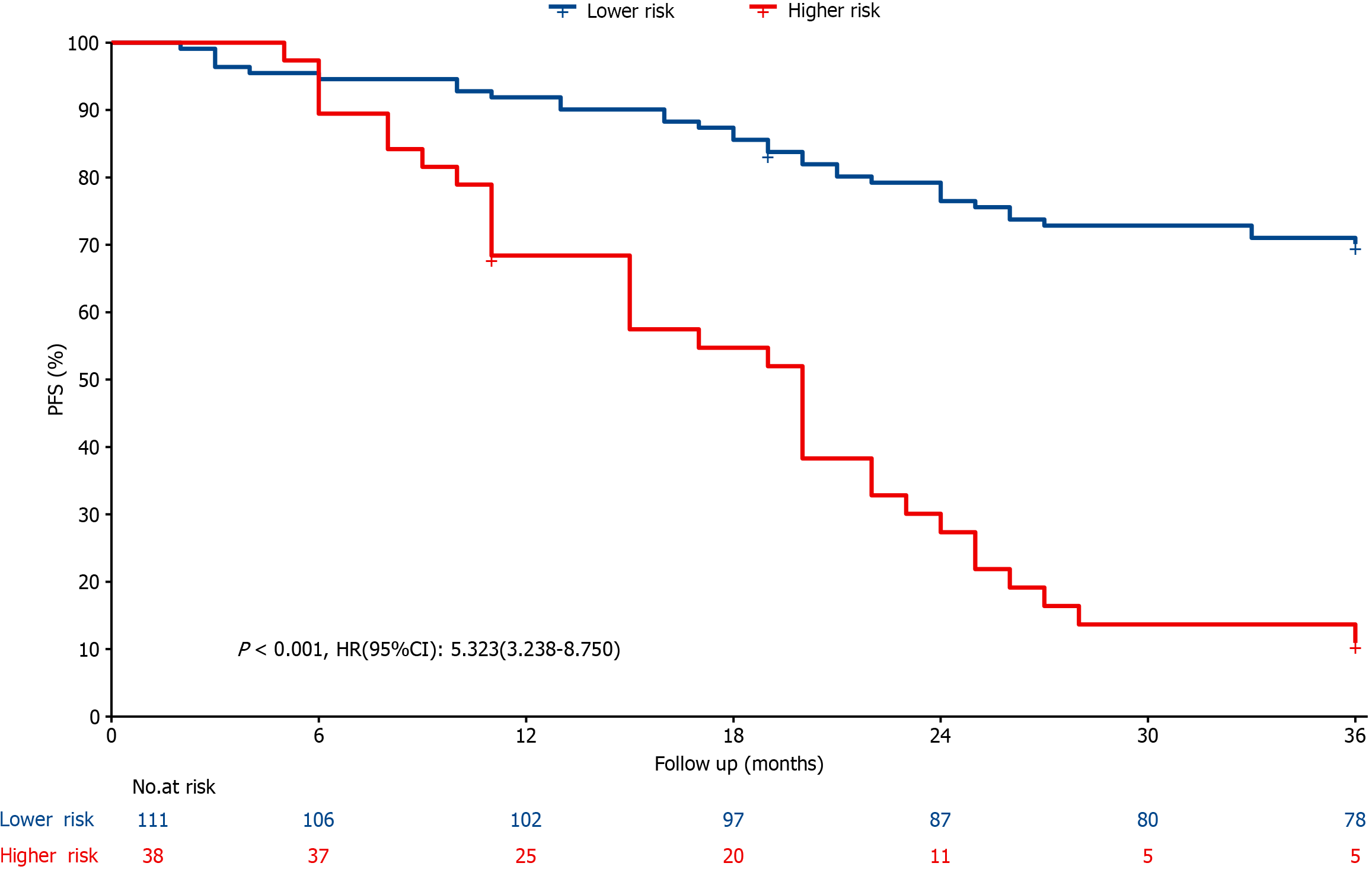
In this study, all subjects' nomogram scores were calculated according to the established model; R software was used to determine the best cutoff value of the nomogram, according to which all patients in the P-SOX group were risk stratified (low-risk and high-risk groups) on the basis of their respective nomogram scores. The results revealed that the prognosis of the high-risk group was significantly worse than that of the low-risk group at different time points (HR: 5.323, 95%CI: 3·238–8·750, P < 0001), as illustrated in Figure 6 and Figure 7.
The exploration of effective and low-toxicity chemotherapy regimens has been a hot topic in gastric cancer research, and the SOX regimen is the preferred first-line chemotherapy for the treatment of GC in Asia. Albumin-bound paclitaxel is considered a standard second-line treatment for gastric cancer. Clinical and experimental studies have demonstrated that nab-paclitaxel has a higher tumor retention rate and lower toxicity than solvent-based paclitaxel. Additionally, its antitumor activity surpasses that of the current standard chemotherapy drug oxaliplatin[5]. Triple chemotherapy has been believed to be more effective than double chemotherapy, resulting in higher tumor remission rates but also greater toxic side effects. However, these two outcomes can be achieved through adjustments in medication, dosage, and admi
Many scholars have studied the efficacy and safety of the combination of albumin-paclitaxel and Tegio in treating advanced gastric cancer and have confirmed that this regimen can improve PFS and OS to a certain extent in patients with advanced gastric cancer[1,8,9]. Furthermore, Masaki Nakamura and other Japanese researchers confirmed the good efficacy of the combination of P-SOX, and oxaliplatin in treating peritoneal metastasis of GC in a Phase 1 clinical trial[10]. The results of the Phase III PRODIGY study in South Korea suggested that the combination of paclitaxel, oxaliplatin, and S-1 had significant positive implications for the treatment of the Asian GC population. The efficacy and safety of this combination were found to be excellent, indicating its potential for widespread use[11]. This study revealed that the 3-year OS and PFS rates in the P-SOX and SOX groups were 64.4% vs 50.7% and 55.3% vs 44.3%, respectively, with no statistically significant difference observed in long-term efficacy between the two groups. However, the OS and PFS rates at 1, 2, and 3 years in the P-SOX group were greater than those in the SOX group. The data analysis results confirmed the effectiveness of the P-SOX regimen, which was found to improve patients' OS and PFS compared with the SOX regimen to a certain extent. In conclusion, we believe that the P-SOX regimen can significantly enhance both short- and long-term efficacy for gastric cancer patients compared with the SOX program. Although the P-SOX regimen has greater associated side effects than the SOX program, most patients can tolerate it.
The OS histogram of patients with gastric cancer constructed by Ma et al[12] (639 patients who underwent surgery combined with adjuvant chemotherapy) revealed that a late TNM stage was a significant prognostic factor correlated with decreased OS, and multidrug combined chemotherapy was associated with significantly greater OS than single-drug chemotherapy[12]. A retrospective analysis conducted in Japan revealed that the 5-year OS rates for patients who underwent surgical resection for GC with pathological stages IA, IB, II, IIIA, IIIB and IV GC were 91.5%, 83.6%, 70.6%, 53.6%, 34.8% and 16.4%, respectively[13]. Wang et al[14] utilized multicenter data to construct an OS histogram of patients with GC (838 patients who received neoadjuvant chemotherapy combined with surgery), and their findings indicate that patients with poor TRG regression have worse OS as the pathological T and N stage progresses[14]. Similarly, another study revealed that ypN stage (P < 0.001) and tumor pathological regression (P = 0.004) were significant risk factors for early recurrence of GC[15]. These findings collectively suggest that advanced TNM stage, ineffective perioperative che
The enlargement of a tumor indicates an increased likelihood of local or distant invasion and places a greater burden on the patient's body. In clinical practice, the T stage is closely associated with tumor size. As we all know, the TNM staging system is based on the TNM. TNM staging remains fundamental in the international consensus for assessing the prognosis and recurrence of tumor patients, with advanced stages typically indicating poor OS and PFS. Clearly, reducing tumor stage through preoperative chemotherapy can enhance patient prognosis and reduce recurrence rates, making tumor pathological or clinical response regression crucial. The degree of tumor differentiation reflects how similar tumor cells are to normal cells and serves as an important indicator for evaluating prognosis and malignant potential. It is generally believed that highly differentiated tumors have a more favorable prognosis.
An international multicenter study found that young age, high degree of differentiation, small tumor diameter, more intraoperative lymph nodes dissection, low pT stage, low pN stage, and adjuvant chemotherapy were positively corre
Moreover, several studies of the OS of patients with gastric cancer in the SEER database, including both early- and advanced-stage patients, have yielded consistent results[18-20]. The evaluation of patient prognosis based on TNM stage has limitations in terms of accuracy and precision, leaving room for improvement. Our study, which is based on preope
Of course, the results of numerous studies on the prognosis of GC may exhibit inconsistencies due to variations in data sources, data analysis and processing methods, geographical regions, and other factors[21-27]. For instance, relevant research has indicated that the prognosis of GC is also associated with age, tumor location, lymph node invasion, ASA assessment, abnormal BMI, number of lymph nodes removed during surgery, chemotherapy regimen, postoperative complications, and various other factors. Based on this situation, we expect numerous scholars to explore a convenient and recognized optimal standard with high accuracy in the future.
In comparison to the SOX regimen, the P-SOX regimen exhibits potential for enhancing both short-term and long-term efficacy while maintaining manageable tolerability of adverse reactions, thus holding promise as a prospective first-line chemotherapy protocol for GC. Patients with GC who have undergone effective perioperative chemotherapy (RECIST 1.1, TRG), exhibit tumor diameters ≤ 2cm, high degrees of differentiation, and early cTNM stages (undergoing P-SOX chemotherapy in combination with surgery) demonstrate a more favorable prognosis. The current study also has some limitations: Due to the retrospective nature of the study, some patients' case data were incomplete for various reasons and could not be included in the analysis, leading to an inability to accurately gain the rate of surgical resection after perioperative chemotherapy. As a result, the study only included patients who underwent both preoperative and postoperative chemotherapy in combination with radical surgery, which caused a certain degree of selection bias. The small sample size and single-center studies lack sufficient persuasiveness, thus warranting the need for future multi-center and large-scale phase III trials.
| 1. | Cheng X, Wu D, Xu N, Chen L, Yan Z, Chen P, Zhou L, Yu J, Cui J, Li W, Wang C, Feng W, Wei Y, Yu P, Du Y, Ying J, Xu Z, Yang L, Zhang Y. Adjuvant albumin-bound paclitaxel combined with S-1 vs. oxaliplatin combined with capecitabine after D2 gastrectomy in patients with stage III gastric adenocarcinoma: a phase III multicenter, open-label, randomized controlled clinical trial protocol. BMC Cancer. 2021;21:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hofstetter WL, Ilson DH, Keswani RN, Kleinberg LR, Korn WM, Lockhart AC, Meredith K, Mulcahy MF, Orringer MB, Posey JA, Sasson AR, Scott WJ, Strong VE, Varghese TK Jr, Warren G, Washington MK, Willett C, Wright CD, McMillian NR, Sundar H; National Comprehensive Cancer Network. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y, Kaminishi M. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O'Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794-7803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1422] [Cited by in RCA: 1487] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 5. | Zhang C, Awasthi N, Schwarz MA, Hinz S, Schwarz RE. Superior antitumor activity of nanoparticle albumin-bound paclitaxel in experimental gastric cancer. PLoS One. 2013;8:e58037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Watson S, de la Fouchardière C, Kim S, Cohen R, Bachet JB, Tournigand C, Ferraz JM, Lefevre M, Colin D, Svrcek M, Meurisse A, Louvet C. Oxaliplatin, 5-Fluorouracil and Nab-paclitaxel as perioperative regimen in patients with resectable gastric adenocarcinoma: A GERCOR phase II study (FOXAGAST). Eur J Cancer. 2019;107:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Sato S, Kunisaki C, Tanaka Y, Sato K, Miyamoto H, Yukawa N, Fujii Y, Kimura J, Takagawa R, Takahashi M, Kosaka T, Akiyama H, Saigusa Y, Taguri M, Yamanaka T, Endo I. A Phase II Study of Tri-weekly Low-dose Nab-paclitaxel Chemotherapy for Patients with Advanced Gastric Cancer. Anticancer Res. 2018;38:6911-6917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | He MM, Wang F, Jin Y, Yuan SQ, Ren C, Luo HY, Wang ZQ, Qiu MZ, Wang ZX, Zeng ZL, Li YH, Wang FH, Zhang DS, Xu RH. Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer Sci. 2018;109:3575-3582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Ma J, Xiao M, Li X, Zhao Q, Ji W, Ling Y, Yang Q. Analysis of the efficacy and safety of paclitaxel (albumin-bound) combined with S-1 and oxaliplatin combined with S-1 in the first-line treatment of advanced gastric cancer: a cohort study. J Gastrointest Oncol. 2022;13:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Nakamura M, Ojima T, Katsuda M, Hayata K, Kitadani J, Nakamori M, Yamaue H. Phase 1 Study of Combined Chemotherapy of Nab-Paclitaxel, S-1, and Oxaliplatin for Gastric Cancer with Peritoneal Metastasis (NSOX Study). Oncology. 2021;99:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, Ryu MH, Rha SY, Chung IJ, Kim IH, Oh SC, Park YS, Son T, Jung MR, Heo MH, Kim HK, Park C, Yoo CH, Choi JH, Zang DY, Jang YJ, Sul JY, Kim JG, Kim BS, Beom SH, Cho SH, Ryu SW, Kook MC, Ryoo BY, Kim HK, Yoo MW, Lee NS, Lee SH, Kim G, Lee Y, Lee JH, Noh SH. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol. 2021;39:2903-2913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 12. | Ma L, Chen G, Wang D, Zhang K, Zhao F, Tang J, Zhao J, Røe OD, He S, Liao D, Gu Y, Tao M, Shu Y, Li W, Chen X. A nomogram to predict survival probability of gastric cancer patients undergoing radical surgery and adjuvant chemotherapy. Front Oncol. 2022;12:893998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1501] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 14. | Wang G, Tan Y, Jiang Y, Liu J, Su Y, Sun Z, Liu B. Prognostic Model of D2 Radical Gastrectomy Combined with Neoadjuvant Chemotherapy for Gastric Cancer. Risk Manag Healthc Policy. 2023;16:1259-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Liu G, Zhao L, Lv M. Defining a Nomogram for Predicting Early Recurrence in Gastric Cancer Patients After Neoadjuvant Chemotherapy and Radical Gastrectomy. J Gastrointest Surg. 2023;27:1766-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Lu J, Xu BB, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Truty MJ, Huang CM. Development and External Validation of a Nomogram to Predict Recurrence-Free Survival After R0 Resection for Stage II/III Gastric Cancer: An International Multicenter Study. Front Oncol. 2020;10:574611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Fang J, Zhang F, Lu J, Deng Z, Li X, Chen X, Huang C, Chen Y, Lian L, Peng J, Chen S. Prognostic factors and the necessity of chemotherapy for stage II gastric cancer: a model based on multicenter retrospective study. Discov Oncol. 2023;14:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Ren X, Huang T, Tang X, Ma Q, Zheng Y, Hu Z, Wang Y, Zhou Y. Development and validation of nomogram models to predict radiotherapy or chemotherapy benefit in stage III/IV gastric adenocarcinoma with surgery. Front Oncol. 2023;13:1223857. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Liao F, Guo X, Lu X, Dong W. A validated survival nomogram for early-onset diffuse gastric cancer. Aging (Albany NY). 2020;12:13160-13171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Wang CY, Yang J, Zi H, Zheng ZL, Li BH, Wang Y, Ge Z, Jian GX, Lyu J, Li XD, Ren XQ. Nomogram for predicting the survival of gastric adenocarcinoma patients who receive surgery and chemotherapy. BMC Cancer. 2020;20:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Shi L, Wang Z, Wang L, Jia Y, Li J, Qin Y. A Prognostic Nomogram and Heat Map to Predict Survival in Stage II/III Gastric Cancer Patients After Curative Gastrectomy Followed by Adjuvant Chemotherapy. Cancer Manag Res. 2022;14:287-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Mo H, Li P, Jiang S. A novel nomogram based on cardia invasion and chemotherapy to predict postoperative overall survival of gastric cancer patients. World J Surg Oncol. 2021;19:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Li Z, Xiao Q, Wang Y, Wang W, Li S, Shan F, Zhou Z, Ji J. A Modified ypTNM Staging System-Development and External Validation of a Nomogram Predicting the Overall Survival of Gastric Cancer Patients Received Neoadjuvant Chemotherapy. Cancer Manag Res. 2020;12:2047-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Liu D, Quan H, Ma M, Zhou H, Yang X, Wu Z, Luo J, Xiao H, Xiao Y. Nomogram to predict overall survival of patients receiving radical gastrectomy and incomplete peri-operative adjuvant chemotherapy for stage II/III gastric cancer: a retrospective bi-center cohort study. BMC Cancer. 2024;24:344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Liu D, Xiao J, Xiang J, Liu A, Chen S, Liu J, Hu X, Peng J. Nomogram for Predicting Survival in Advanced Gastric Cancer after Neoadjuvant Chemotherapy and Radical Surgery. Gastroenterol Res Pract. 2021;2021:2923700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Park KB, Jun KH, Song KY, Chin H, Lee HH. Development of a staging system and survival prediction model for advanced gastric cancer patients without adjuvant treatment after curative gastrectomy: A retrospective multicenter cohort study. Int J Surg. 2022;101:106629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Jiang Y, Li T, Liang X, Hu Y, Huang L, Liao Z, Zhao L, Han Z, Zhu S, Wang M, Xu Y, Qi X, Liu H, Yang Y, Yu J, Liu W, Cai S, Li G. Association of Adjuvant Chemotherapy With Survival in Patients With Stage II or III Gastric Cancer. JAMA Surg. 2017;152:e171087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |