Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3211
Revised: August 28, 2024
Accepted: September 2, 2024
Published online: October 27, 2024
Processing time: 107 Days and 23.1 Hours
Early recurrence (ER) is associated with dismal outcomes in patients undergoing radical resection for pancreatic ductal adenocarcinoma (PDAC). Approaches for predicting ER will help clinicians in implementing individualized adjuvant therapies. Postoperative serum tumor markers (STMs) are indicators of tumor progression and may improve current systems for predicting ER.
To establish an improved nomogram based on postoperative STMs to predict ER in PDAC.
We retrospectively enrolled 282 patients who underwent radical resection for PDAC at our institute between 2019 and 2021. Univariate and multivariate Cox regression analyses of variables with or without postoperative STMs, were per
Postoperative carbohydrate antigen 19-9 and carcinoembryonic antigen levels, preoperative carbohydrate antigen 125 levels, perineural invasion, and pTNM stage III were independent risk factors for ER in PDAC. The postoperative STMs-based nomogram (AUC: 0.774, 95%CI: 0.713-0.835) had superior accuracy in predicting ER compared with the nomogram without postoperative STMs (AUC: 0.688, 95%CI: 0.625-0.750) (P = 0.016). Patients with a recurrence nomogram score (RNS) > 1.56 were at high risk for ER, and had significantly poorer recurrence-free survival [median: 3.08 months, interquartile range (IQR): 1.80-8.15] than those with RNS ≤ 1.56 (14.00 months, IQR: 6.67-24.80), P < 0.001).
The postoperative STMs-based nomogram improves the predictive accuracy of ER in PDAC, stratifies the risk of ER, and identifies patients at high risk of ER for tailored adjuvant therapies.
Core Tip: Patients with early recurrence (ER) of pancreatic ductal adenocarcinoma, have significantly poor survivals. Adjuvant therapy (AT) may prevent or delay ER, but the absence of AT happens to nearly 50% of patients. Predictive systems for ER remain unsatisfactory, and postoperative serum tumor markers (STMs) may change this dilemma. This study demonstrates that postoperative STMs are independent risk factors for ER. We developed a nomogram based on post
- Citation: He H, Zou CF, Yang F, Di Y, Jin C, Fu DL. Postoperative serum tumor markers-based nomogram predicting early recurrence for patients undergoing radical resections of pancreatic ductal adenocarcinoma. World J Gastrointest Surg 2024; 16(10): 3211-3223
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3211.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3211
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignancies of the alimentary system[1] and the fourth leading cause of cancer-related deaths[2]. Curative resection remains the best treatment for long-term survival in patients with PDAC[3]. However, 80% of patients who undergo radical resections experience recurrence within 2 years[4]. Notably, 50% of all recurrences occur within 12 months after surgery, which are classified as early recurrence (ER) and associated with a significantly poor prognosis[5]. The current standard treatment for PDAC is curative surgery, followed by adjuvant therapy (AT)[6], with or without neoadjuvant therapy (NAT). Unfortunately, nearly 50% of patients undergoing radical resections never receive AT, discontinue AT or undergo irregular AT, for various reasons[7,8]. Approaches for predicting ER in PDAC will help clinicians in identifying patients at high risk for ER and implementing individualized adjuvant therapies, potentially improving patients’ long-term outcomes.
Previous studies have investigated the risk factors for ER in patients with PDAC[9-14]. Several variables, including serum tumor markers (STMs), pathological parameters, and the absence of AT have been the most frequently identified risk factors, however, they remain inconclusive, and the accuracy of current predictive systems for ER based on these risk factors needs to be improved. ER after radical resection usually indicates that tumors had already spread at the time of operation. Consequently, aberrant levels of postoperative STMs might be critical indicators of ER, however, the majority of previous studies have demonstrated that preoperative carbohydrate antigen 19-9 (CA19-9) is an independent risk factor for ER[9,11,14,15], instead of postoperative STMs. It should be noted that preoperative STMs might provide risk stratification of ER for clinicians to improve their decision-making before operations[16], but the predictive accuracies of these preoperative STMs were unsatisfactory, and their inappropriate use would make patients miss the opportunity for radical surgery. Therefore, regardless of whether NAT is administered to patients, a more reliable predictive system to guide AT is essential. To the best of our knowledge, very few studies have stated that postoperative STMs play a role in predicting the ER of PDAC[10,13], and that postoperative STMs might be underestimated in current clinical practice.
In this study, we analyzed postoperative STMs, preoperative STMs, and clinicopathological parameters as predictors of ER in patients with PDAC. We attempted to explore the application of postoperative STMs in predicting ER and to establish a nomogram for ER based on postoperative STMs. This nomogram will improve the accuracy of existing systems for predicting ER and identify patients at high risk of ER for tailored adjuvant therapies.
Patients with PDAC who underwent curative surgery between 2019 and 2021 at Huashan Hospital, affiliated with Fudan University, were retrospectively enrolled in this study. All procedures were performed in accordance with the guidelines of the Institutional Ethical Committee.
The inclusion criteria were as follows: (1) Pathologically confirmed PDAC; (2) R0 resection achieved; and (3) Adjuvant chemotherapy or chemoradiotherapy performed postoperatively. The exclusion criteria were as follows: (1) Patients who died within 90 days after the operation; (2) Patients with other uncontrolled malignancies; and (3) Patients with arterial involvement, including the hepatic artery, superior mesenteric artery, and celiac axis.
Abdominal contrast-enhanced computed tomography or magnetic resonance imaging was performed preoperatively for each patient. Positron emission tomography was performed in patients at high risk of metastasis. Radical surgery included pancreaticoduodenectomy (PD), distal pancreatectomy, and total pancreatectomy (TP). Gemcitabine-based AT was initiated one month after the operation. Clinical parameters, including age, sex, smoking, drinking, diabetes mellitus, and jaundice, were recorded. Pathological parameters, including tumor site, tumor size, tumor differentiation, perineural invasion, portomesenteric vein (PV) invasion, lymphnode metastasis, and pathological stage, were retrieved. The pathological stage was determined according to the 8th edition of the American Joint Committee on Cancer guidelines. R0 resection was defined according to the International Study Group of Pancreatic Surgery criteria. Preoperative STMs including Pre_CA19-9, Pre_carcinoembryonic antigen (CEA), and Pre_ carbohydrate antigen 125 (CA125), were examined within one week before surgery, and postoperative STMs including Af_CA19-9, Af_CEA, and Af_CA125, were examined one month after surgery.
Follow-up was performed every month during the first 6 months after surgery, then every three months for the following 6 months, and every three to six months from the second year. STMs and imaging examinations were performed to evaluate the recurrence or metastasis. ER was defined as recurrence or metastasis occurring within 12 months after surgery, whereas delayed recurrence (DR) was defined as recurrence or metastasis occurring beyond 12 months. The sites of recurrence were classified as hepatic metastasis, locoregional recurrence (soft tissue around the vascular or surgical bed or remnant pancreas), lung metastasis, peritoneal or omental metastasis, other sites, or indeterminate sites.
All variables were investigated using univariate Cox regression analysis. Variables with P value < 0.05 in the above step were included in the multivariate Cox regression analysis. The backward stepwise (likelihood ratio) method was used for the multivariate Cox regression model. Independent risk factors were used to construct a nomogram for predicting ER (R package, rms). Recurrence nomogram score (RNS) was calculated using the following formula: RNS = coefficient1 × variable1 + coefficient2 × variable2 + … + coefficientN × variableN.
Receiver operating characteristic (ROC) curve analysis was performed, and the area under the curve (AUC) was calculated to evaluate the predictive accuracy of the variable. The optimal cut-off values for the variables were determined according to the Youden index. Survival analysis was performed using the Kaplan-Meier survival plot and log-rank test (R package, survival and survminer).
For this retrospective study, the sample size was determined based on the actual available cases according to the inclusion and exclusion criteria. Continuous variables with normal distribution were presented as mean ± SD, while variables with a non-normal distribution were presented as median and interquartile range (IQR). Continuous variables were categorized by a defined cut-off value, otherwise, the mean value was used. Categorical variables were presented as absolute numbers and percentages. Continuous variables were compared using Student’s t-test, while categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test, as appropriate. Two-tailed tests were considered statistically significant at P value < 0.05. Statistical analysis was performed using SPSS software (version 26.0, SPSS Inc., Chicago, IL, United States).
A total of 576 patients with pancreatic cancer underwent surgery at our center between 2019 and 2021. In this study, 282 patients were enrolled based on inclusion and exclusion criteria. The median follow-up time was 15.71 months (IQR: 9.70-25.23). A total of 169 patients with recurrence within 12 months were classified as ER. A total of 113 patients were classified as DR, including 50 with recurrence beyond 12 months and 63 with no recurrence during follow-up.
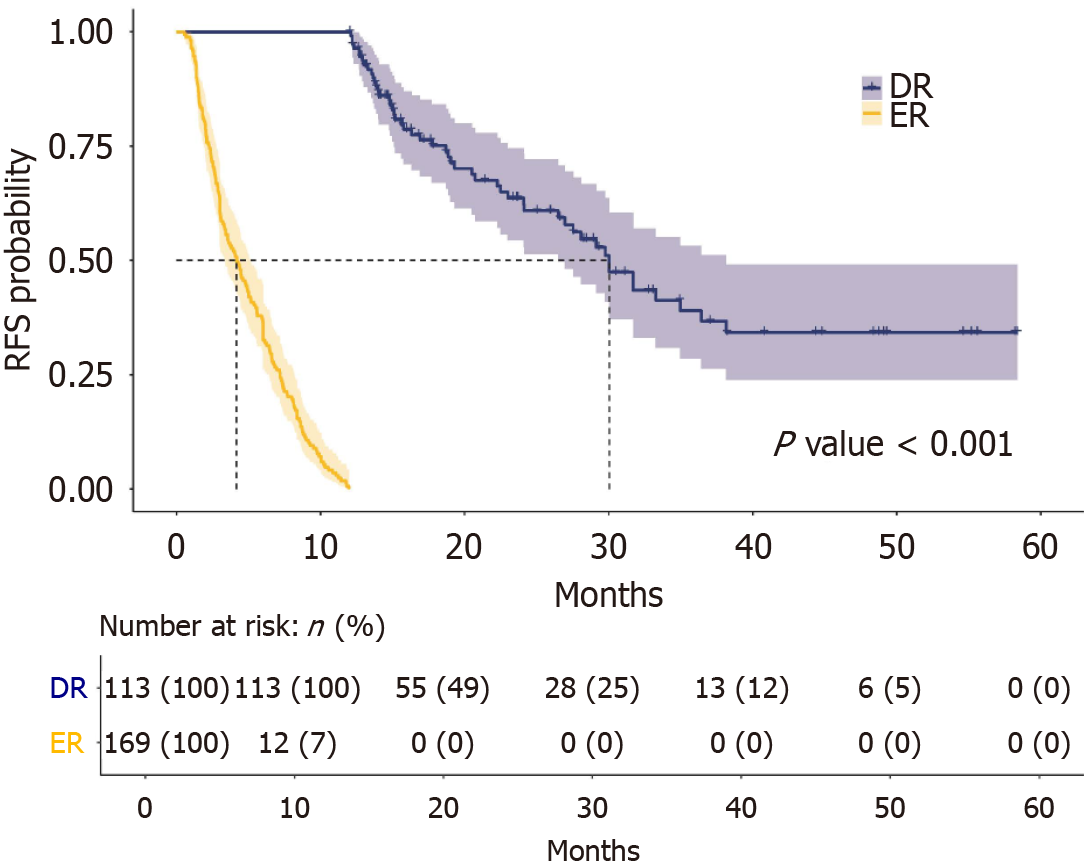
The clinicopathological characteristics of the cohort are summarized (Table 1). Patients with ER had larger tumors and more tumors with poor differentiation, perineural invasion, PV invasion, lymphnode metastasis, and pTNM stage III disease. The median recurrence-free survival (RFS) of the cohort was 8.37 months (IQR: 3.23-15.92). Patients with ER had significantly poorer RFS (median: 4.16 months, IQR: 2.26-7.20) than those with DR (median: 19.10 months, IQR: 14.60-29.77) (P < 0.001). The Kaplan-Meier survival analysis for ER and DR is presented (Figure 1). The recurrence sites are listed in Table 2. The ER group experienced hepatic metastasis more frequently (P = 0.009), whereas the DR group had a higher incidence of locoregional recurrences (P = 0.002).
| Characteristics | Total cohort (n = 282) | ER (n = 169) | DR (n = 113) | P value |
| Age (years) | 61.87 ± 8.333 | 61.91 ± 8.051 | 61.81 ± 8.775 | 0.928 |
| Gender (male) | 154 (54.6) | 92 (54.4) | 62 (54.9) | 0.943 |
| Smoking | 40 (14.2) | 19 (11.2) | 21 (18.6) | 0.083 |
| Drinking | 23 (8.2) | 12 (7.1) | 11 (9.7) | 0.415 |
| DM | 108 (38.3) | 65 (38.5) | 43 (38.1) | 0.945 |
| Jaundice | 74 (26.2) | 47 (27.8) | 27 (23.9) | 0.464 |
| Operation types (PD/TP)1 | 236 (83.7) | 147 (87.0) | 89 (78.8) | 0.067 |
| Tumor sites2 | 251 (89.0) | 152 (89.9) | 99 (87.6) | 0.540 |
| Tumor size (cm) | 3.739 ± 1.482 | 3.898 ± 1.486 | 3.501 ± 1.450 | 0.027 |
| Poor differentiation | 142 (50.4) | 95 (56.2) | 47 (41.6) | 0.021 |
| Perineural invasion | 224 (79.4) | 144 (85.2) | 80 (70.8) | 0.003 |
| PV invasion | 120 (42.6) | 82 (48.5) | 38 (33.6) | 0.013 |
| Lymphnode status (N0) | 129 (45.7) | 65 (38.5) | 64 (56.6) | 0.003 |
| Pathological stage (stage III) | 109 (38.7) | 79 (46.7) | 30 (26.5) | 0.001 |
| Median RFS (months IQR) | 8.37 (3.23-15.92) | 4.16 (2.26-7.20) | 19.10 (14.60-29.77) | < 0.001 |
| Site | ER (n = 169) | DR (n = 50) | P value |
| Liver | 70 (41.42) | 10 (20.00) | 0.009 |
| Lung | 2 (1.28) | 0 (0) | 1 |
| Locoregional recurrence | 19 (11.24) | 15 (30.00) | 0.002 |
| Peritoneal and omental metastasis | 7 (4.14) | 2 (4.00) | 1 |
| Other sites or indeterminate site | 71 (42.01) | 23 (46.00) | 0.735 |
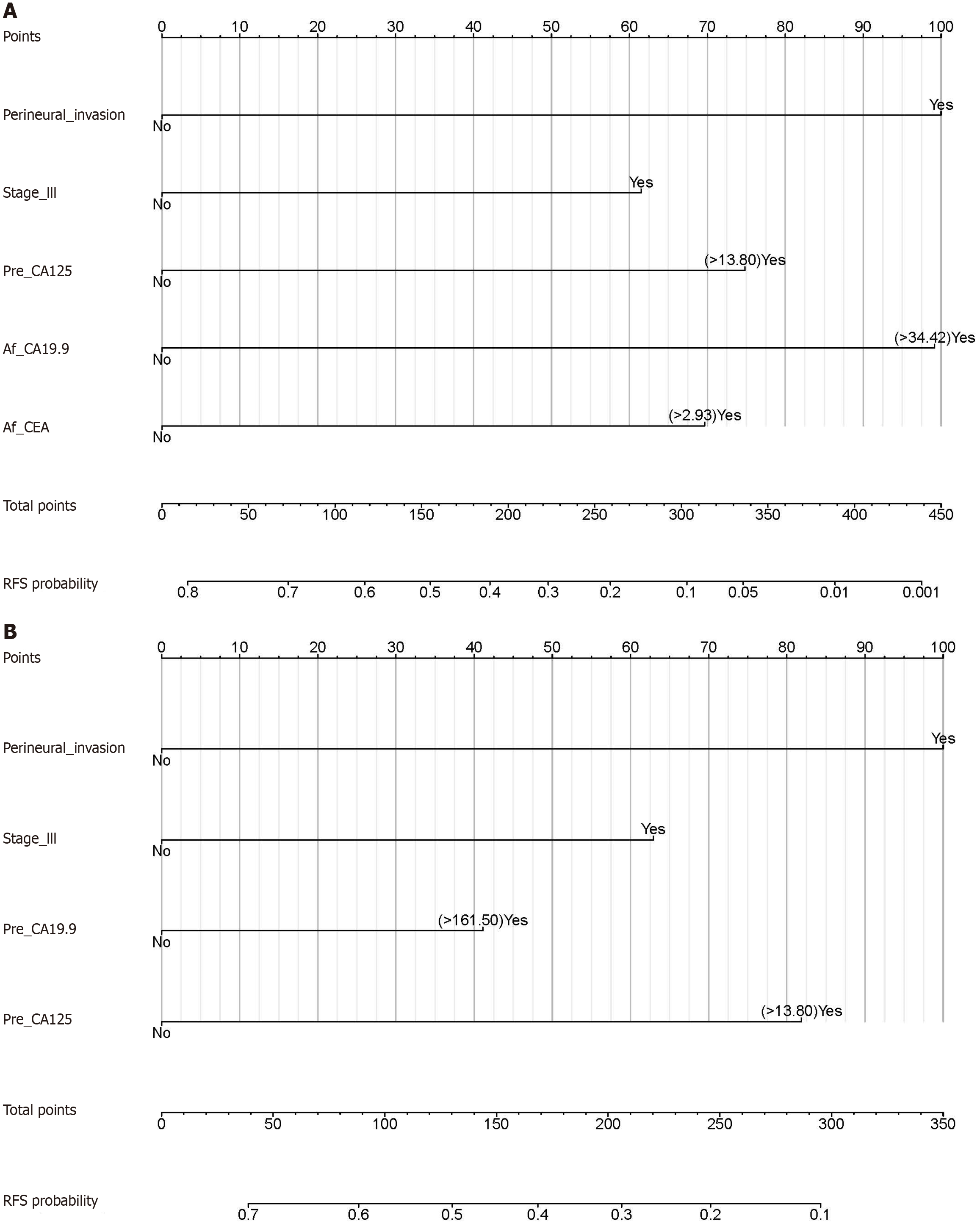
Univariate Cox regression analysis of variables with and without postoperative STMs was performed to investigate the role of pre- and postoperative STMs in predicting ER. This was followed by multivariate Cox regression analysis (Tables 3 and 4). In the absence of postoperative STMs, nine risk factors (P < 0.05) were included in the multivariate analysis, leading to the identification of four independent risk factors: Perineural invasion, pTNM stage III, Pre_CA125, and Pre_CA19-9. When postoperative STMs were added to the univariate and multivariate analyses, the three aforementioned independent risk factors (perineural invasion, pTNM stage III, and Pre_CA125) remained, and two new postoperative STMs (Af_CA19-9 and Af_CEA) were included in the multivariate model, whereas the preoperative STM Pre_CA19-9 was excluded.
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Operation types (PD/TP)1 | 1.445 (0.987-2.115) | 0.057 | ||
| Age (> 63) | 1.093 (0.836-1.428) | 0.513 | ||
| Gender (male) | 0.920 (0.706-1.200) | 0.543 | ||
| Smoking | 0.761 (0.511-1.133) | 0.179 | ||
| Drinking | 0.713 (0.421-1.207) | 0.209 | ||
| DM | 1.004 (0.765-1.318) | 0.974 | ||
| Jaundice | 0.994 (0.736-1.342) | 0.971 | ||
| Tumor_sites2 | 1.185 (0.769-1.826) | 0.440 | ||
| Tumor_size (> 3.5 cm) | 1.431 (1.097-1.866) | 0.008 | ||
| Poor_differentiation | 1.380 (1.056-1.804) | 0.018 | ||
| Perineural_invasion | 1.970 (1.364-2.845) | < 0.001 | 2.198 (1.378-3.505) | < 0.001 |
| PV_invasion | 1.510 (1.156-1.971) | 0.002 | ||
| Lymphnode_metastasis | 1.551 (1.183-2.035) | 0.001 | ||
| pTNM_stage (stage III) | 1.728 (1.321-2.259) | < 0.001 | 1.635 (1.186-2.253) | 0.002 |
| Pre_CA19-9 (> 161.50 U/mL) | 1.557 (1.189-2.038) | 0.001 | ||
| Pre_CEA (> 4.10 ng/mL) | 1.461 (1.096-1.948) | 0.009 | ||
| Pre_CA125 (> 13.80 U/mL) | 1.882 (1.431-2.475) | < 0.001 | 1.812 (1.321-2.487) | < 0.001 |
| Af_CA19-9 (> 34.42 U/mL) | 3.297 (2.400-4.528) | < 0.001 | 2.175 (1.534-3.083) | < 0.001 |
| Af_CEA (> 2.93 ng/mL) | 2.227 (1.613-3.074) | < 0.001 | 1.760 (1.248-2.483) | 0.001 |
| Af_CA125 (> 107.00 U/mL) | 1.427 (0.974-2.091) | 0.067 | ||
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Operation types (PD/TP)1 | 1.445 (0.987-2.115) | 0.057 | ||
| Age (> 63) | 1.093 (0.836-1.428) | 0.513 | ||
| Gender (male) | 0.920 (0.706-1.200) | 0.543 | ||
| Smoking | 0.761 (0.511-1.133) | 0.179 | ||
| Drinking | 0.713 (0.421-1.207) | 0.209 | ||
| DM | 1.004 (0.765-1.318) | 0.974 | ||
| Jaundice | 0.994 (0.736-1.342) | 0.971 | ||
| Tumor_sites2 | 1.185 (0.769-1.826) | 0.440 | ||
| Tumor_size (> 3.5 cm) | 1.431 (1.097-1.866) | 0.008 | ||
| Poor_differentiation | 1.380 (1.056-1.804) | 0.018 | ||
| Perineural_invasion | 1.970 (1.364-2.845) | < 0.001 | 2.001 (1.345-2.975) | < 0.001 |
| PV_invasion | 1.510 (1.156-1.971) | 0.002 | ||
| Lymphnode_metastasis | 1.551 (1.183-2.035) | 0.001 | ||
| pTNM_stage (stage III) | 1.728 (1.321-2.259) | < 0.001 | 1.595 (1.210-2.103) | < 0.001 |
| Pre_CA19-9 (> 161.50 U/mL) | 1.557 (1.189-2.038) | 0.001 | 1.350 (1.025-1.779) | 0.032 |
| Pre_CEA (> 4.10 ng/mL) | 1.461 (1.096-1.948) | 0.009 | ||
| Pre_CA125 (> 13.80 U/mL) | 1.882 (1.431-2.475) | < 0.001 | 1.824 (1.378-2.415) | < 0.001 |
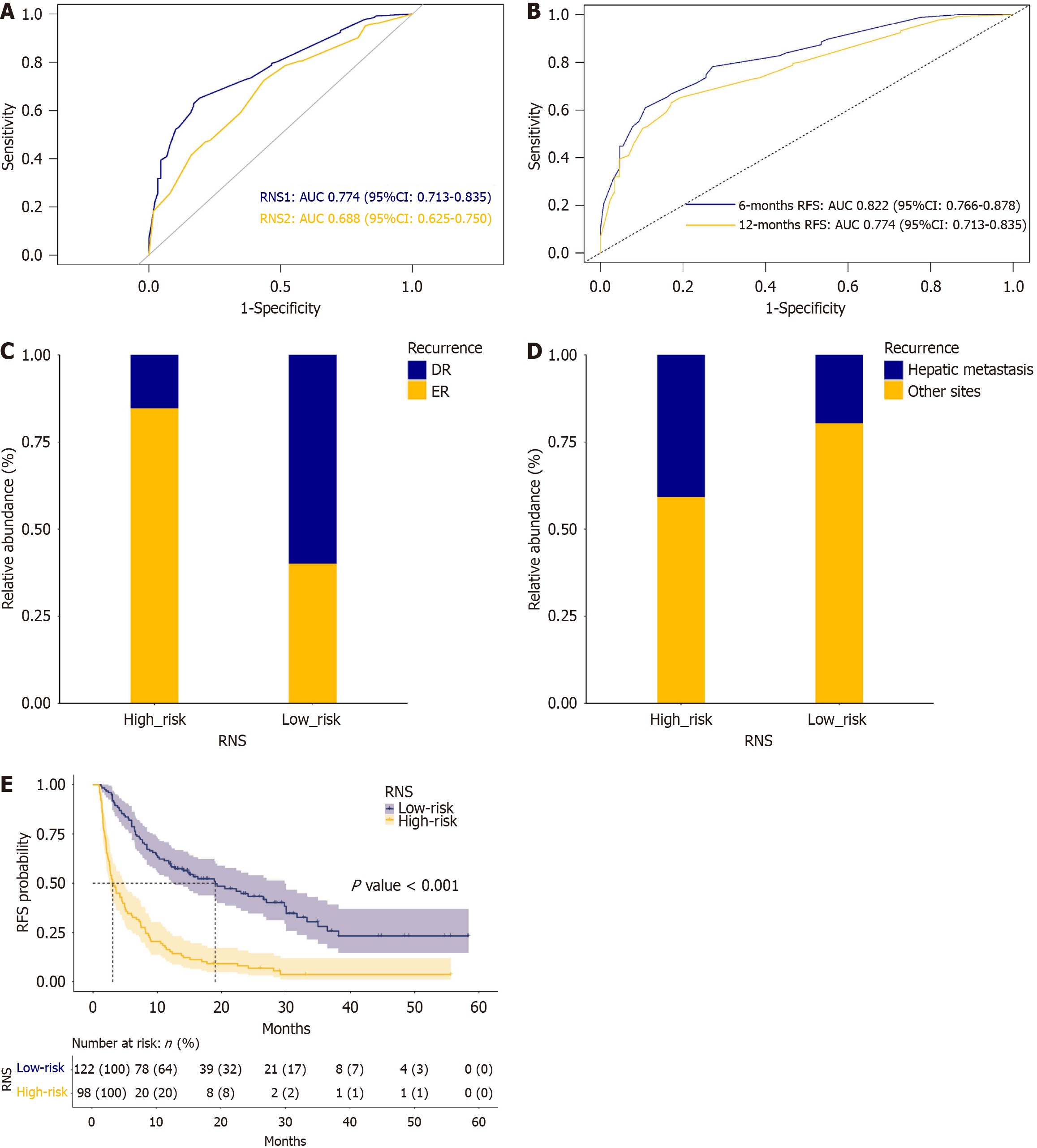
Independent risk factors for ER with and without postoperative STMs were used to construct nomograms for predicting ER (Figure 2). To further evaluate the predictive accuracy of these two nomograms, RNS1 (with postoperative STMs) and RNS2 (without postoperative STMs) were calculated according to the RNS formula, followed by the ROC curve analysis of RNS1 and RNS2. ROC curves for RNS1 and RNS2 are shown in Figure 3A. The AUC of RNS1 (AUC: 0.774, 95%CI: 0.713-0.835) was greater than the AUC of RNS2 (AUC: 0.688, 95%CI: 0.625-0.750) (P = 0.016). ROC curves for RNS1 at different time intervals are shown in Figure 3B. The AUC values decreased with time, indicating a consistent performance of the current system in predicting ER within 12 months. In this study, the nomogram with postoperative STMs was designated as the postoperative STMs-based nomogram, and RNS1 was termed RNS.
To explore the role of RNS in stratifying the risk of recurrences, the cohort was categorized into the high-risk (RNS > 1.56) and the low-risk (RNS ≤ 1.56) groups of recurrences according to the cut-off value of RNS. A stacked plot of ER and DR stratified by RNS is shown in Figure 3C. The high-risk group had significantly a higher ER ratio than the low-risk group (84.69% vs 40.16%, P < 0.001). A stacked plot of recurrence sites stratified by RNS is shown in Figure 3D. Hepatic metastasis was more frequently observed in the high-risk group than in the low-risk group (40.82% vs 19.67%, P = 0.001). The Kaplan-Meier survival analysis of RFS stratified by RNS is shown in Figure 3E. The high-risk group (median: 3.08 months, IQR: 1.80-8.15) had significantly poorer RFS compared with the low-risk group (median: 14.00 months, IQR: 6.67-24.80) (P < 0.001).
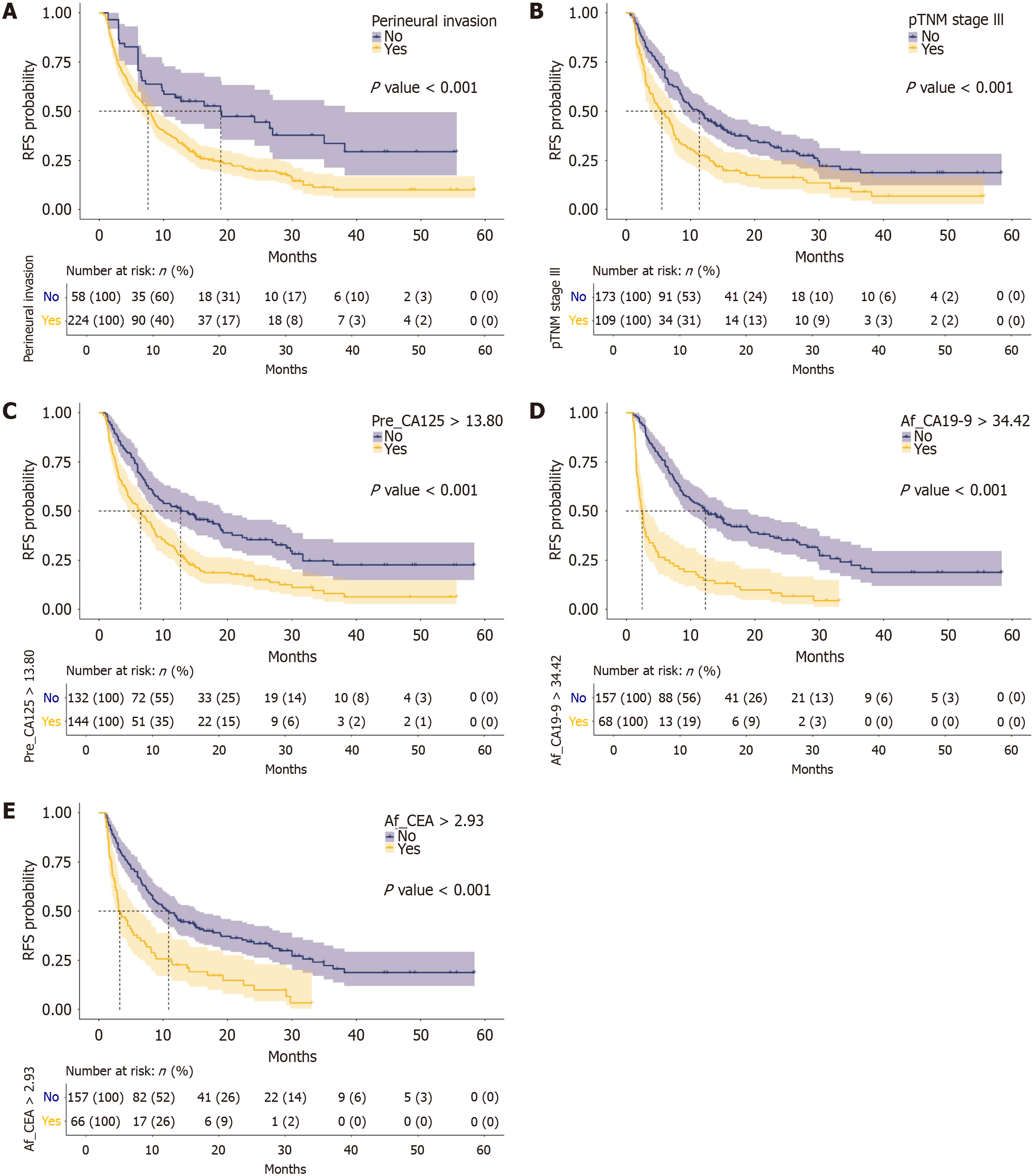
The stratified analysis of RFS by independent risk factors for ER included perineural invasion, pTNM stage III, Pre_CA125, Af_CA19-9, and Af_CEA (Figure 4). Patients with perineural invasion, stage III tumors, Pre_CA125 > 13.80U/mL, Af_CA19-9 > 34.42 U/mL, or Af_CEA > 2.93 ng/mL, had significantly poorer RFS compared with the controls.
To characterize the clinicopathological features of patients in the high-risk group for ER, we analyzed variables stratified by RNS (Table 5). Patients in the high-risk group had a higher ratio of PD or TP, and more tumors with large sizes, poor differentiation, perineural invasion, PV invasion, lymphnode metastasis, and pTNM stage III. Additionally, patients in the high-risk group were likely to have elevated levels of Pre_CA19-9 (> 161.50 U/mL), Pre_CEA (> 4.10 ng/mL), Pre_CA125 (> 13.80 U/mL), Af_CA19-9 (> 34.42 U/mL), and Af_CEA (> 2.93 ng/mL).
| Variables | RNS | P value | |
| High-risk (n = 98) | Low-risk (n = 122) | ||
| Operation types (PD/TP)1 | 90.82 | 79.51 | 0.034 |
| Age (> 63) | 45.92 | 38.52 | 0.333 |
| Gender (male) | 62.24 | 53.28 | 0.230 |
| Smoking | 12.24 | 18.85 | 0.251 |
| Drinking | 9.18 | 9.84 | 1 |
| DM | 41.84 | 33.61 | 0.265 |
| Jaundice | 28.57 | 27.05 | 0.921 |
| Tumor_sites2 | 93.88 | 86.07 | 0.096 |
| Tumor_size (> 3.5 cm) | 59.18 | 35.25 | < 0.001 |
| Poor_differentiation | 62.24 | 43.44 | 0.008 |
| Perineural_invasion | 95.92 | 68.85 | < 0.001 |
| PV_invasion | 52.04 | 36.89 | 0.034 |
| Lymphnode_metastasis | 58.16 | 45.90 | 0.094 |
| pTNM_stage (stage III) | 62.24 | 15.57 | < 0.001 |
| Pre_CA19-9 (> 161.50 U/mL) | 62.24 | 31.97 | < 0.001 |
| Pre_CEA (> 4.10 ng/mL) | 44.90 | 20.49 | < 0.001 |
| Pre_CA125 (> 13.80 U/mL) | 77.55 | 35.25 | < 0.001 |
| Af_CA19-9 (> 34.42 U/mL) | 62.24 | 4.92 | < 0.001 |
| Af_CEA (> 2.93 ng/mL) | 58.16 | 6.56 | < 0.001 |
| Af_CA125 (> 107.00 U/mL) | 18.37 | 15.57 | 0.711 |
In this study, pTNM stage III tumors and PV invasions accounted for 38.7% and 42.6% of the cohort, respectively, while they were 23.8%-34.2% and 11.1%-25.3% in previous studies[9,10,15]. Our cohort had a higher prevalence of advanced stage and more borderline resectable tumors, resulting in recurrences in 77.6% of the patients who underwent radical resections for PDAC, which is higher than those reported in previous studies (56.7%-76.9%)[9,13-15]. ER was observed in 59.9% of this cohort, and 41.4% of the ER sites were in the liver. This indicates that micro-metastases may have existed in the liver before surgical resection. NAT has been suggested for borderline resectable or resectable PDAC with a high risk of recurrence[17], however, current predictive systems for ER based on preoperative parameters remain inadequate. Additionally, as tumors might progress during NAT, peri-lesion fibrosis induced by NAT increases the difficulty of resection, and surgical tolerance worsens, it potentially deprives patients of the opportunity for resection. In contrast, the absence of AT, instead of NAT, has been proven to be an independent risk factor for ER[9,10], highlighting that AT is at least as important as NAT for patients at a high risk of ER. Moreover, although consensus has been reached regarding AT regimens of PDAC[6,18,19], the regimens and duration of AT for patients at a high risk of ER remain uncertain. Therefore, postoperative STMs might also play a role in the current predictive systems for ER and improve accuracy, aiming to serve as reliable indicators for enhanced regimens or extended periods of AT.
Preoperative STMs have been established as significant risk factors for ER[5], but the role of postoperative STMs remains unclear. Considering that CA19-9, CA125, and CEA are classic STMs and have been identified as prognostic biomarkers[20], we used these STMs for both univariate and multivariate analyses. In patients without postoperative STMs, perineural invasion, pTNM stage III, Pre_CA125, and Pre_CA19-9 were proven to be independent risk factors, consistent with previous findings[5,9,11,15]. However, when postoperative STMs were included in the analysis, perineural invasion, pTNM stage III, Pre_CA125, Af_CA19-9, and Af_CEA were identified as independent risk factors for ER. The results indicate that postoperative STMs, including Af_Ca19-9 and Af_CEA, are more important indicators for ER in PDAC, while Pre_CA19-9 is not among the most relevant factors for ER when postoperative STMs are present. The significance of Pre_CA19-9 depends on its usefulness as a preoperative biomarker.
The accuracy of previous systems for predicting ER or recurrence based on preoperative parameters has been unsatisfactory (AUC: 0.565-0.688)[9,11,12,14], which might not help clinicians in making decisions for patients with PDAC. Imamura et al[13] stated that the predictive accuracy of preoperative serum CA19-9 levels for ER is limited. Li et al[10] developed a nomogram with both preoperative and postoperative parameters, which showed improved accuracy in predicting ER in PDAC (AUC: 0.763). However, it remains unclear whether postoperative STMs can improve current predictive systems for ER. In this study, we demonstrated that the nomogram with postoperative STMs showed a significantly better performance in predicting ER than the nomogram without postoperative STMs (AUC: 0.774 vs 0.688, P = 0.016). Our findings suggest that incorporating postoperative STMs could improve the accuracy of the predictive system for ER in PDAC, but this comes at the expense of its preoperative use.
With this postoperative STMs-based nomogram, we could calculate the RNS for each patient and identify those at high risk of ER (RNS > 1.56). Clinicians should be aware that patients with RNS > 1.56 would more likely suffer from ER than those with RNS ≤ 1.56. Therefore, regular imaging examinations should be performed during the first year postopera
This study has several limitations. First, this was a retrospective study with inevitable bias. The role of NAT in predicting ER could not be evaluated in this cohort, as candidates for NAT were not assigned randomly and usually had more advanced diseases. Second, this study was conducted at a single institution and had a relatively small sample size. This limited sample size might have precluded an in-depth analysis of the model’s performance, highlighting the need for further validation of the current model in a larger cohort. Third, the regimens and durations of AT may correlate with ER in PDAC. A well-designed randomized controlled trial is required to address this question.
The role of postoperative STMs in predicting ER in PDAC has been underestimated in previous studies. We conducted both univariate and multivariate analyses of preoperative and postoperative parameters. Our findings demonstrate that postoperative STMs, including Af_CA19-9 and Af_CEA, are critical and independent risk factors for ER. By incorporating these postoperative STMs, we developed a nomogram with significantly superior accuracy for predicting ER than those without postoperative STMs. With this improved predictive system, clinicians can efficiently identify patients at high risk for ER, implement individualized follow-up protocols, and administer timely, tailored AT.
The authors want to thank Prof. Min-Rui Liang for her kind help in statistical review.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63668] [Article Influence: 15917.0] [Reference Citation Analysis (174)] |
| 2. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 4356] [Article Influence: 4356.0] [Reference Citation Analysis (3)] |
| 3. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2201] [Article Influence: 146.7] [Reference Citation Analysis (2)] |
| 4. | Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;267:936-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 482] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 5. | Groot VP, Gemenetzis G, Blair AB, Rivero-Soto RJ, Yu J, Javed AA, Burkhart RA, Rinkes IHMB, Molenaar IQ, Cameron JL, Weiss MJ, Wolfgang CL, He J. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 267] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 6. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1929] [Article Influence: 275.6] [Reference Citation Analysis (0)] |
| 7. | Sánchez-Velázquez P, Muller X, Malleo G, Park JS, Hwang HK, Napoli N, Javed AA, Inoue Y, Beghdadi N, Kalisvaart M, Vigia E, Walsh CD, Lovasik B, Busquets J, Scandavini C, Robin F, Yoshitomi H, Mackay TM, Busch OR, Hartog H, Heinrich S, Gleisner A, Perinel J, Passeri M, Lluis N, Raptis DA, Tschuor C, Oberkofler CE, DeOliveira ML, Petrowsky H, Martinie J, Asbun H, Adham M, Schulick R, Lang H, Koerkamp BG, Besselink MG, Han HS, Miyazaki M, Ferrone CR, Fernández-Del Castillo C, Lillemoe KD, Sulpice L, Boudjema K, Del Chiaro M, Fabregat J, Kooby DA, Allen P, Lavu H, Yeo CJ, Barroso E, Roberts K, Muiesan P, Sauvanet A, Saiura A, Wolfgang CL, Cameron JL, Boggi U, Yoon DS, Bassi C, Puhan MA, Clavien PA. Benchmarks in Pancreatic Surgery: A Novel Tool for Unbiased Outcome Comparisons. Ann Surg. 2019;270:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 8. | Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, Ko CY, Bentrem DJ. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 9. | Murakawa M, Kawahara S, Takahashi D, Kamioka Y, Yamamoto N, Kobayashi S, Ueno M, Morimoto M, Sawazaki S, Tamagawa H, Ohshima T, Yukawa N, Rino Y, Morinaga S. Risk factors for early recurrence in patients with pancreatic ductal adenocarcinoma who underwent curative resection. World J Surg Oncol. 2023;21:263. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Li S, Zhang G, Lu Y, Zhao T, Gao C, Liu W, Piao Y, Chen Y, Huang C, Chang A, Hao J. Perioperative Serum Scoring Systems Predict Early Recurrence and Poor Prognosis of Resectable Pancreatic Cancer. Front Oncol. 2022;12:841819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Ishido K, Kimura N, Wakiya T, Nagase H, Hara Y, Kanda T, Fujita H, Hakamada K. Development of a Biomarker-Based Scoring System Predicting Early Recurrence of Resectable Pancreatic Duct Adenocarcinoma. Ann Surg Oncol. 2022;29:1281-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Liu W, Tang B, Wang F, Qu C, Hu H, Zhuang Y, Gao H, Xie X, Tian X, Yang Y. Predicting early recurrence for resected pancreatic ductal adenocarcinoma: a multicenter retrospective study in China. Am J Cancer Res. 2021;11:3055-3069. [PubMed] |
| 13. | Imamura M, Nagayama M, Kyuno D, Ota S, Murakami T, Kimura A, Yamaguchi H, Kato T, Kimura Y, Takemasa I. Perioperative Predictors of Early Recurrence for Resectable and Borderline-Resectable Pancreatic Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Hong S, Song KB, Hwang DW, Lee JH, Lee W, Jun E, Kwon J, Park Y, Park SY, Kim N, Shin D, Kim H, Sung M, Ryu Y, Kim SC. Preoperative serum carbohydrate antigen 19-9 levels predict early recurrence after the resection of early-stage pancreatic ductal adenocarcinoma. World J Gastrointest Surg. 2021;13:1423-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Nappo G, Donisi G, Capretti G, Ridolfi C, Pagnanelli M, Nebbia M, Bozzarelli S, Petitti T, Gavazzi F, Zerbi A. Early Recurrence after Upfront Surgery for Pancreatic Ductal Adenocarcinoma. Curr Oncol. 2023;30:3708-3720. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Guo SW, Shen J, Gao JH, Shi XH, Gao SZ, Wang H, Li B, Yuan WL, Lin L, Jin G. A preoperative risk model for early recurrence after radical resection may facilitate initial treatment decisions concerning the use of neoadjuvant therapy for patients with pancreatic ductal adenocarcinoma. Surgery. 2020;168:1003-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Ghaneh P, Palmer D, Cicconi S, Jackson R, Halloran CM, Rawcliffe C, Sripadam R, Mukherjee S, Soonawalla Z, Wadsley J, Al-Mukhtar A, Dickson E, Graham J, Jiao L, Wasan HS, Tait IS, Prachalias A, Ross P, Valle JW, O'Reilly DA, Al-Sarireh B, Gwynne S, Ahmed I, Connolly K, Yim KL, Cunningham D, Armstrong T, Archer C, Roberts K, Ma YT, Springfeld C, Tjaden C, Hackert T, Büchler MW, Neoptolemos JP; European Study Group for Pancreatic Cancer. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 153] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 18. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 994] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 19. | Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, Haddock MG, Schaefer P, Willett CG, Rich TA. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 543] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 20. | Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J, Cen P, Xu J, Liu C, Long J, Guha S, Fu D, Ni Q, Jatoi A, Chari S, McCleary-Wheeler AL, Fernandez-Zapico ME, Li M, Yu X. A preoperative serum signature of CEA+/CA125+/CA19-9 ≥ 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer. 2015;136:2216-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | He H, Zhang S, Yang H, Xu P, Kutschick I, Pfeffer S, Britzen-Laurent N, Grützmann R, Fu D, Pilarsky C. Identification of Genes Associated with Liver Metastasis in Pancreatic Cancer Reveals PCSK6 as a Crucial Mediator. Cancers (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |