Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3202
Revised: August 30, 2024
Accepted: September 3, 2024
Published online: October 27, 2024
Processing time: 57 Days and 19.7 Hours
The outcome of surgical treatment for colorectal cancer (CRC) remains unsatisfactory and warrants further exploration and optimization.
To clarify the impact of chemotherapy plus cellular immunotherapy [dendritic cell-cytokine-induced killer (DC-CIK) cell immunotherapy] on patients after CRC surgery and to explore the mediating variables.
A total cohort of 121 patients who underwent CRC surgery between January 2019 and April 2022 were selected. The sample comprised a control group of 55 pa
We found a significantly higher 2-year DFS rate of treatment efficacy in the research group than in the control group, with a statistically lower incidence of adverse events. Both groups showed a reduction in serum tumor markers after treatment but there was no marked intergroup difference. After treatment, the various T-cell subgroup indicators in the control group were significantly lower than those in the research group. The indices of T-cell subsets in the research group showed no significant change from preoperative levels. Univariate analysis revealed a significant correlation between TNM staging, tumor differentiation, and the rates of nonresponse to treatment in CRC patients after surgery. Multivariate results indicated that the treatment approach significantly affected the efficacy of postoperative CRC treatment.
We concluded that XELOX + DC-CIK immunotherapy for postsurgical CRC patients offers reduced rates of treatment-induced adverse events, extended 2-year DFS, enhanced immunity, and increased physiological antitumor responses.
Core Tip: Adjuvant postoperative therapy for colorectal cancer (CRC) maximizes surgical outcomes while significantly reducing CRC-related mortality. This study analyzes the efficacy of the XELOX regimen combined with dendritic cell-cytokine-induced killer (DC-CIK) cell immunotherapy for postoperative CRC patients and the mediating variables, aiming to provide a useful clinical reference to optimize the efficacy of postoperative CRC treatment. Through a comparative analysis of the XELOX chemotherapy regimen vs XELOX regimen plus DC-CIK immunotherapy therapy across multiple outcome and safety indices, including efficacy, adverse event rates, serum tumor marker levels, and T-cell subset levels, the combination regimen is confirmed to offer greater efficacy than a chemotherapy regimen alone, enhancing patient immunity, increasing physiological antitumor activity, and providing improved treatment safety.
- Citation: Ding ZY, Piao Y, Jiang T, Chen J, Wang YN, Yu HY, Zheng ZD. Effects of postoperative treatment with chemotherapy and cellular immunotherapy on patients with colorectal cancer. World J Gastrointest Surg 2024; 16(10): 3202-3210
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3202.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3202
Colorectal cancer (CRC) is a common malignancy of the digestive system. Predisposing factors include obesity, a sedentary lifestyle, drinking, smoking, and red meat consumption[1]. In 2020, there were 1.93 million cases of CRC worldwide, with nearly 1 million deaths; and this is expected to grow to 3.2 million annual cases by 2040[2]. Although overall CRC mortality rates have shown a downward trend, confirmed cases are becoming gradually more concentrated in younger age groups, and at more advanced stages[3]. Previous studies have shown mortality rates of 13% in CRC patients at stage I/II, 47% in patients at stage III, and nearly 90% in patients at stage IV[4]. Surgery is the primary treatment for CRC, but postoperative adjuvant therapy is often required to avoid postoperative recurrence and metastasis[5-7]. Exploring adjuvant therapies for patients after CRC surgery not only optimizes the effects of surgical treatment but also provides a significant reduction in CRC-related mortality rates.
Combined postoperative adjuvant chemotherapy and radiotherapy have demonstrated beneficial effects on the elimination of tumor cells and residual lesions that improve patient survival rates. However, this approach is associated with an increased incidence of adverse events such as gastrointestinal reactions (GIRs), immune system damage, and even myelosuppression[8,9]. After surgery and adjuvant chemotherapy, patients with CRC are more severely immuno
This study analyzes the effects of XELOX + DC-CIK immunotherapy in postsurgical CRC patients and the variables that moderate these effects. We aim to provide an effective clinical reference that optimizes postoperative treatment efficacy and prognosis after CRC surgery.
A retrospective analysis was performed using data from 121 postoperative CRC patients treated at the General Hospital of Northern Theater Command, Shenyang, China, between January 2019 and April 2022. The 55 patients in the control group received postoperative chemotherapy with the XELOX regimen, while the 66 patients in the research group received XELOX + DC-CIK immunotherapy.
The inclusion criteria for this study were as follows: Meeting the diagnostic criteria for CRC[16]; pathologically confirmed as stage II or III; receiving treatment for the first time; Karnovsky Performance Scale scores ≥ 60; survival durations > 8 weeks; unilateral lesions; patients or their families having been fully informed about the condition, treatment plan, and possible adverse reactions before treatment; no contraindications to the treatment regimens used in this study.
The exclusion criteria were: Intolerance to chemotherapy; a history of allergy to biologics; long-term use of immunosuppressants; concomitant psychiatric illness; heart, lung, or kidney insufficiency; hematological diseases; immune system disorders; other malignant tumors; and pregnancy or lactation.
The control group underwent the XELOX chemotherapy regimen. The specifics of each cycle of this regimen are oxaliplatin (130 mg/m2) administered intravenously on the first day and capecitabine (1000 mg/m2, twice daily) given orally from day 1 to day 14. Depending on their condition, the patient receives 6-8 cycles of chemotherapy, with each cycle lasting 21 days.
DC-CIK cell cultures were performed 2 days before the start of chemotherapy in research group patients. The research group received the same chemotherapy regimen as the control group but initiated from day 3 of the regimen. The additional immunotherapy received by the research group was performed as described below.
First, autologous tumor cell antigens were prepared: Tumor tissue from the patient was ground into a pulp, digested by collagenase into a single-cell suspension, and then separated using a separating medium to obtain primary CRC cells. After several passages, the cells were collected and washed three times in a row. They were then lysed, and the supernatant was collected by filtration and stored for later use. Subsequently, peripheral blood was collected and centrifuged to obtain mononuclear cells, which were inoculated in a lymphocyte culture medium. The suspended cells were collected. The adherent cells were added to the medium, with granulocyte-macrophage colony-stimulating factor and interleukin-4. On day 7, autologous tumor cell antigens were added, and DCs were obtained after sensitization. Finally, the suspended cells were added to a lymphocyte culture medium containing CIK cell cytokines, and rhIL-2. Antihuman cluster of differentiation (CD)3 monoclonal antibodies were added a day later until co-culture with DCs on day 7 for the final collection of DC-CIK cells. Negative results on tests for bacteria, fungi, endotoxins, and mycoplasma were required and established before transfusion. The viability rate of the reinfused cells was > 95%, and the number of cells was ≥ 1.0 × 109 cells/time. DC-CIK cells were cultured until day 13, at which point, they were confirmed to be free of bacterial fungi and endotoxins by relevant tests. The cells were then washed three times with sterile normal saline and suspended in 200 mL of normal saline containing 2% albumin. Reinfusion was performed once a day for 3 days for a duration of 1.5 hours, with no fewer than 3 × 109 DC-CIK cells per infusion. Each patient received two cycles of DC-CIK cell therapy.
Efficacy: Patients were followed up for two years, and efficacy was evaluated by assessing 2-year disease-free survival (DFS). The DFS is the time from diagnosis to disease progression (recurrence or metastasis) and/or death.
Incidence of adverse events: Adverse reactions were evaluated per the National Cancer Institute Common Terminology Criteria for Adverse Events[17]. The adverse events observed in our cohort were diarrhea, myelosuppression, GIRs, and peripheral neuritis. In each group, we recorded the number of patients who suffered from these adverse events and calculated the incidence rate. Targeted treatment for the alleviation of these adverse events was provided in a timely manner.
Serum tumor marker levels: Before and after treatment, 5 mL of fasting venous blood was collected from the patients in both groups. Serum was extracted from the samples by centrifugation and subjected to enzyme-linked immunosorbent assays of carcinoembryonic antigen (CEA), carbohydrate antigen (CA)19-9, and CA242.
T-cell subset levels: The levels of T-cell subsets (CD3+, CD3+ CD4+, CD3+ CD8+, NK, and NK T) were determined by flow cytometry. These were considered representative of patient immune function.
Quantitative data, represented by (mean ± SD), were analyzed for intergroup differences using independent samples t-tests, while intragroup differences were determined using paired t-tests. Count data, expressed as ratios (percentages), were compared between groups using χ2 tests. The minimum sample size required per group was determined to be approximately 50. This was ascertained using the binomial proportion sample size estimation formula. The experimental data were analyzed using IBM SPSS Statistics for Windows, v. 19.0 (IBM Corp., Armonk, NY, United States) software, with P values < 0.05 considered statistically significant.
There were no significant differences between the research group and the control group in baseline characteristics. These included age, sex, onset site, pathological type, clinical stage, and tumor differentiation (P > 0.05; Table 1).
| Baseline data | Control group (n = 55) | Research group (n = 66) | χ2/t | P value |
| Age (years), mean ± SD | 57.09 ± 7.75 | 56.41 ± 11.14 | 0.382 | 0.703 |
| Sex, n (%) | 0.111 | 0.740 | ||
| Male | 30 (54.55) | 34 (51.52) | ||
| Female | 25 (45.45) | 32 (48.48) | ||
| Onset site, n (%) | 0.186 | 0.666 | ||
| Colon | 27 (49.09) | 35 (53.03) | ||
| Rectum | 28 (50.91) | 31 (46.97) | ||
| Pathological type, n (%) | 0.043 | 0.836 | ||
| Adenocarcinoma | 49 (89.09) | 58 (87.88) | ||
| Other | 6 (10.91) | 8 (12.12) | ||
| Clinical stage, n (%) | 0.029 | 0.864 | ||
| II | 35 (63.64) | 41 (62.12) | ||
| III | 20 (36.36) | 25 (37.88) | ||
| Differentiation, n (%) | 0.021 | 0.885 | ||
| Moderately- or well-differentiated | 39 (70.91) | 46 (69.70) | ||
| Poorly differentiated | 16 (29.09) | 20 (30.30) |
We evaluated the efficacy of the two groups through the two-year DFS rate. According to statistics, the two-year DFS rate of the control group was 65.45%, which was significantly lower than the 81.82% of the research group (P < 0.05).
Our univariate analysis of the factors affecting postoperative treatment efficacy in CRC patients showed treatment efficacy to be significantly correlated with clinical stage, differentiation degree, and treatment approach (P < 0.05). Multivariate analysis found only the treatment approach to significantly affect postoperative treatment efficacy (P < 0.05; Table 2, Table 3 and Table 4).
| Curative effect | Control group (n = 55) | Research group (n = 66) | χ2 | P value |
| Survival | 36 (65.45) | 54 (81.82) | ||
| Recurrence/metastasis/death | 19 (34.55) | 12 (18.18) | ||
| Two-year disease-free survival | 36 (65.45) | 54 (81.82) | 4.215 | 0.040 |
| Baseline data | Effective treatment group (n = 90) | Ineffective treatment group (n = 31) | χ2 | P value |
| Age (years) | 0.402 | 0.526 | ||
| < 60 | 58 (64.44) | 18 (58.06) | ||
| ≥ 60 | 32 (35.56) | 13 (41.94) | ||
| Sex | 0.340 | 0.560 | ||
| Male | 49 (54.44) | 15 (48.39) | ||
| Female | 41 (45.56) | 16 (51.61) | ||
| Onset site | 0.216 | 0.642 | ||
| Colon | 45 (50.00) | 17 (54.84) | ||
| Rectum | 45 (50.00) | 14 (45.16) | ||
| Pathological type | 0.072 | 0.788 | ||
| Adenocarcinoma | 80 (88.89) | 27 (87.10) | ||
| Other | 10 (11.11) | 4 (12.90) | ||
| Clinical stage | 10.363 | 0.001 | ||
| I-II | 64 (71.11) | 12 (38.71) | ||
| III | 26 (28.89) | 19 (61.29) | ||
| Differentiation | 12.550 | < 0.001 | ||
| Moderately- or well-differentiated | 71 (78.89) | 14 (45.16) | ||
| Poorly differentiated | 19 (21.11) | 17 (54.84) | ||
| Treatment approach | 4.215 | 0.040 | ||
| XELOX | 36 (40.00) | 19 (61.29) | ||
| XELOX + DC-CIK | 54 (60.00) | 12 (38.71) |
| Variable | β | SE | Wald | P value | OR | 95%CI |
| Clinical stage | 0.114 | 0.443 | 0.066 | 0.797 | 1.121 | 0.470-2.673 |
| Differentiation | −0.056 | 0.473 | 0.014 | 0.905 | 0.945 | 0.374-2.390 |
| Treatment approach | 0.867 | 0.427 | 4.115 | 0.042 | 2.379 | 1.030-5.495 |
All adverse events in both groups were of grades 0 to 2. The adverse events observed were diarrhea, myelosuppression, GIRs, and peripheral neuritis. We found a significantly lower incidence of these adverse events in the research group vs the control group (P < 0.05; Table 5).
| Adverse event | Control group (n = 55) | Research group (n = 66) | χ2 | P value |
| Diarrhea | 11 (20.00) | 5 (7.58) | 4.036 | 0.045 |
| Myelosuppression | 20 (36.36) | 10 (15.15) | 7.239 | 0.007 |
| Gastrointestinal reaction | 19 (34.55) | 9 (13.64) | 7.374 | 0.007 |
| Peripheral neuritis | 7 (12.73) | 2 (3.03) | 4.097 | 0.043 |
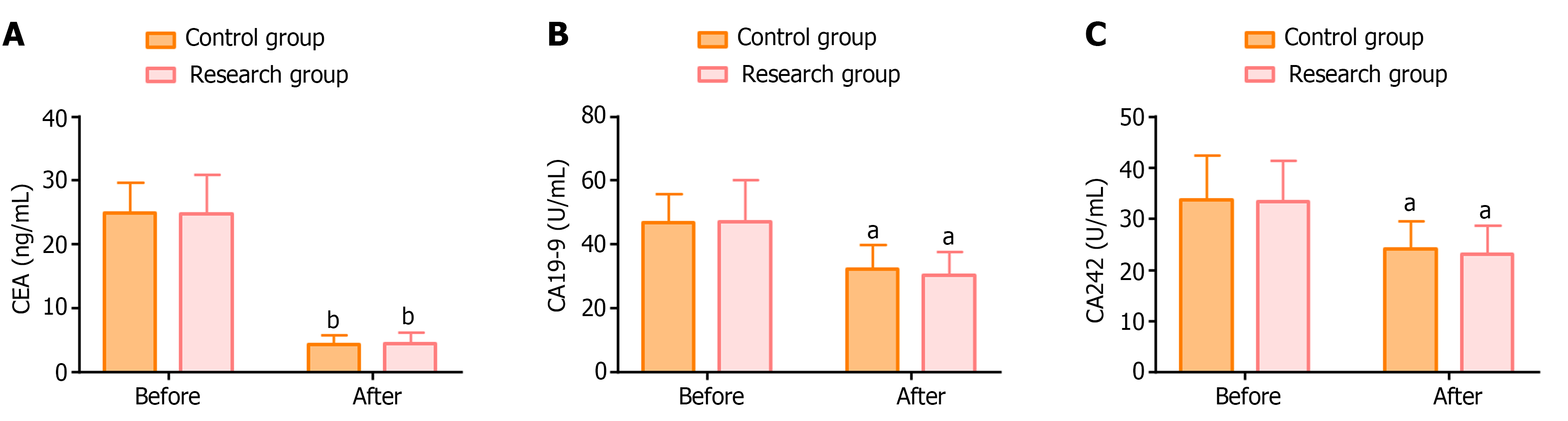
Similar levels of the serum tumor markers CEA, CA19-9, and CA242 were detected in both groups before treatment (P > 0.05). The levels of all three markers were significantly lower in both groups after treatment (P < 0.05), but with no statistically significant intergroup difference (P > 0.05; Figure 1).
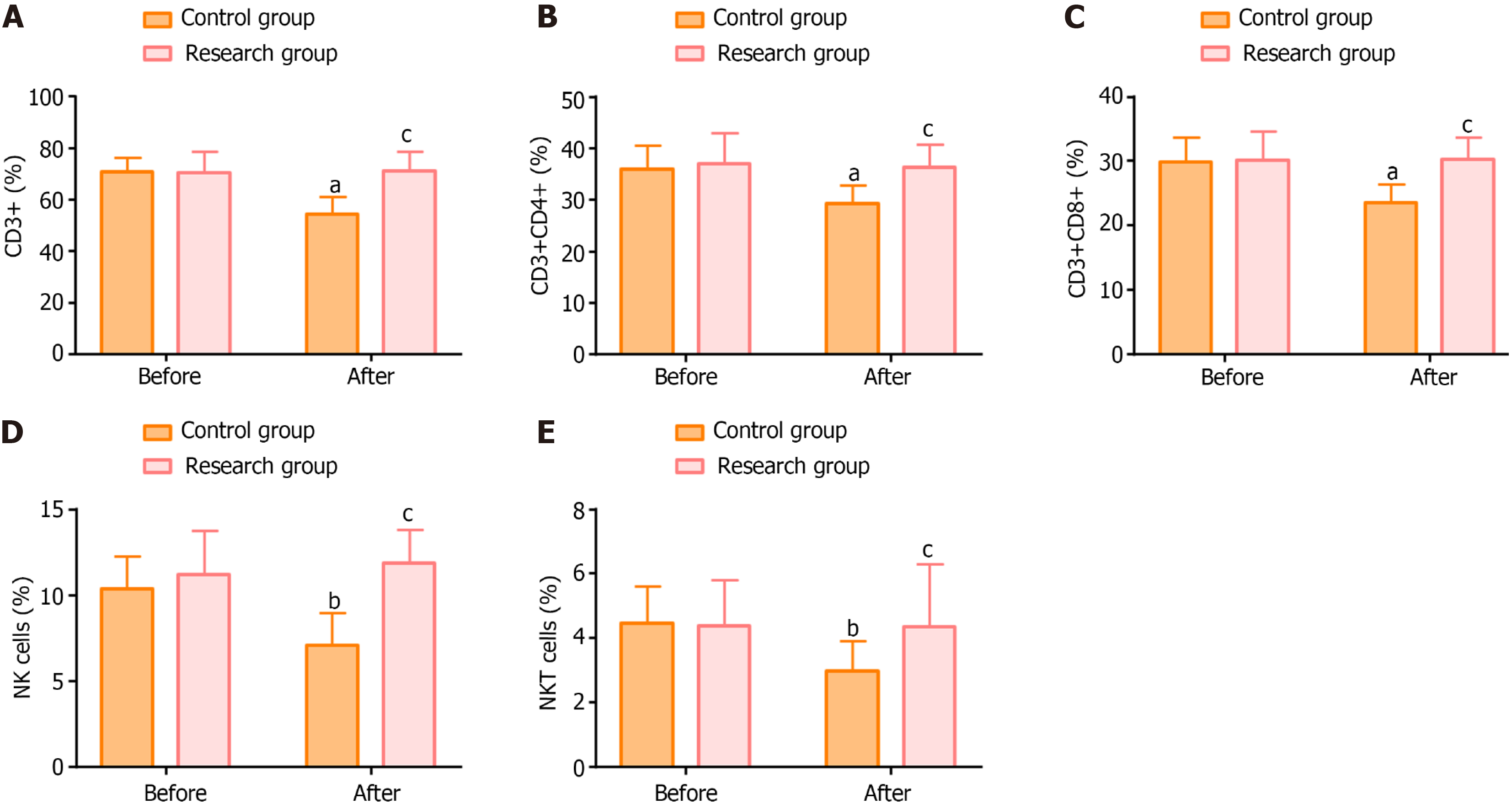
The main T-cell subsets detected in our cohort were CD3+, CD3+ CD4+, CD3+ CD8+, NK, and NK T cells. There was little difference between the two groups in the pre-treatment levels of these T-cell subsets (P > 0.05). In the research group, there was no significant difference in the levels of these indicators before and after treatment (P > 0.05); while significant posttreatment reductions were observed in the control group (P < 0.05). After treatment, T-cell subset levels were statistically lower in the control group than in the research group (P < 0.05; Figure 2).
The colorectum is not only an important component of the digestive system; it is also an important element of the immune system, mediating the body’s innate and adaptive immune responses[18]. CRC tumors are infiltrated by effector-memory lymphocytes, and further breakthroughs are needed in the immunotherapies used to treat this condition[19]. This study evaluated the outcomes of treatment with adjuvant XELOX + DC-CIK immunotherapy after CRC surgery and assessed the factors that mediate its curative effects.
We found a statistically higher 2-year DFS rate in the research group vs the XELEX-only control group (81.82% vs 65.45%), suggesting that XELOX + DC-CIK immunotherapy for postoperative CRC patients can significantly prolong patients’ 2-year DFS. This was in concordance with the findings of Wang et al., which showed that DC-CIK immunotherapy improves the overall chemotherapy response rate in CRC patients and increases the CD4 T-cell count, enhancing patient immunity[20]. Previous studies have shown that DC-CIK induces significantly greater antitumor activity than CIK alone, accelerating T-cell proliferation, enhancing tumor cell destruction, and strengthening the body’s immune responses. Together, these responses effectively inhibit tumor growth[21,22].
Our univariate analysis found clinical stage III, low differentiation, and the XELOX treatment regimen alone to be significantly correlated with treatment failure in postoperative CRC patients. Multivariate analysis confirmed that the treatment approach was a risk factor affecting the postoperative efficacy of CRC, suggesting that the addition of DC-CIK immunotherapy to XELOX chemotherapy can significantly improve treatment efficacy in postoperative CRC patients. In terms of safety, our research group showed a statistically lower incidence of adverse events (including diarrhea, myelosuppression, GIRs, and peripheral neuritis) than the control group, indicating that DC-CIK immunotherapy may offset the drug-related side effects associated with XELOX treatment. In line with our research results, a meta-analysis by Lan et al[23] found that DC-CIK immunotherapy enhanced the efficacy of chemotherapy for solid cancers, including CRC, with reasonable safety and no specific or severe side effects.
Our pathological investigation found that the serum tumor markers, CEA, CA19-9, and CA242 levels were statistically decreased by both XELOX and the combined therapy, but there were no significant intergroup differences, either before or after treatment, indicating equivalent inhibitory effects. We found marked postintervention reductions in patient levels of the T-cell subsets CD3+, CD3+ CD4+, CD3+ CD8+, NK, and NK T cells in the control group. These were statistically lower than the levels observed in the research group. There was no significant posttreatment change in the above indicators in the research group. This indicates that DC-CIK immunotherapy can significantly alleviate XELOX-related immunosuppression, with significantly improved immune function seen in postsurgical CRC patients. Xu et al[24] found that combining chemotherapy with DC-CIK immunotherapy statistically increased the percentages of CD3+, CD8+, NK, and NKT cells in CRC patients compared with simple adjuvant chemotherapy and palliative treatment. Supporting our findings, they also observed good maintenance effects on patient immune function. Pan et al[25] found that CIK immunotherapy combined with first-line chemotherapy effectively prolonged overall survival and progression-free survival in patients with metastatic CRC. Li et al[26] also demonstrated that the XELOX + DC-CIK immunotherapy regimen helps to prolong the survival of patients after CRC surgery. They identified a correlation between the primary tumor expression of SLAMF7 and treatment efficacy.
This study had some limitations that should be addressed in future studies. First, there was no follow-up. Data on patient recurrence and overall survival would have enabled evaluation of the long-term effects and sustainability of treatment benefits. Second, detailed information on the severity of adverse events and specific management measures were not provided. Such information needs to be provided in future research to facilitate our understanding of the safety of DC-CIK immunotherapy and related interventions. Finally, this study was subject to the limitations inherent in retrospective designs. Future clinical trials would strengthen the validity of our results.
We found XELOX + DC-CIK immunotherapy for postoperative CRC patients to demonstrate significantly greater clinical efficacy and safety than XELOX alone. The adoption of this treatment approach may provide statistical improvement in the 2-year DFS rate, reduce the risk of adverse events, and enhance patients’ immune response to the disease. To ensure the therapeutic effectiveness of postoperative treatment, CRC patients should be screened, and those at clinical stage II and those with medium to high differentiation favored for treatment with the XELOX + DC-CIK immunotherapy regimen due to their particular responsiveness to this approach. However, our findings are generalizable to the broader population of CRC patients beyond the study cohort and can be applied in clinical practice.
| 1. | Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz KE. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 2. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1356] [Article Influence: 339.0] [Reference Citation Analysis (5)] |
| 3. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1567] [Reference Citation Analysis (3)] |
| 4. | Koi M, Carethers JM. The colorectal cancer immune microenvironment and approach to immunotherapies. Future Oncol. 2017;13:1633-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Kotani D, Oki E, Nakamura Y, Yukami H, Mishima S, Bando H, Shirasu H, Yamazaki K, Watanabe J, Kotaka M, Hirata K, Akazawa N, Kataoka K, Sharma S, Aushev VN, Aleshin A, Misumi T, Taniguchi H, Takemasa I, Kato T, Mori M, Yoshino T. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med. 2023;29:127-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 277] [Article Influence: 138.5] [Reference Citation Analysis (0)] |
| 6. | Zhang C, Stampfl-Mattersberger M, Ruckser R, Sebesta C. [Colorectal cancer]. Wien Med Wochenschr. 2023;173:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 7. | Alam Z, Shang X, Effat K, Kanwal F, He X, Li Y, Xu C, Niu W, War AR, Zhang Y. The potential role of prebiotics, probiotics, and synbiotics in adjuvant cancer therapy especially colorectal cancer. J Food Biochem. 2022;46:e14302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 8. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3029] [Article Influence: 504.8] [Reference Citation Analysis (3)] |
| 9. | Peng H, Yao M, Fan H, Song L, Sun J, Zhou Z, Du Y, Lu K, Li T, Yin A, Xu J, Wei S. Effects of Autologous Cytokine-Induced Killer Cells Infusion in Colorectal Cancer Patients: A Prospective Study. Cancer Biother Radiopharm. 2017;32:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Mezheyeuski A, Micke P, Martín-Bernabé A, Backman M, Hrynchyk I, Hammarström K, Ström S, Ekström J, Edqvist PH, Sundström M, Ponten F, Leandersson K, Glimelius B, Sjöblom T. The Immune Landscape of Colorectal Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Weng J, Li S, Zhu Z, Liu Q, Zhang R, Yang Y, Li X. Exploring immunotherapy in colorectal cancer. J Hematol Oncol. 2022;15:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 198] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 12. | Fan J, Shang D, Han B, Song J, Chen H, Yang JM. Adoptive Cell Transfer: Is it a Promising Immunotherapy for Colorectal Cancer? Theranostics. 2018;8:5784-5800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Fiorino E, Merlini A, D'Ambrosio L, Cerviere I, Berrino E, Marchiò C, Giraudo L, Basiricò M, Massa A, Donini C, Leuci V, Rotolo R, Galvagno F, Vitali L, Proment A, Ferrone S, Pisacane A, Pignochino Y, Aglietta M, Grignani G, Mesiano G, Sangiolo D. Integrated Antitumor Activities of Cellular Immunotherapy with CIK Lymphocytes and Interferons against KIT/PDGFRA Wild Type GIST. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Liu Y, Zhang Z, Tian Y, Wang D, Liu S, Li L, Hao N, Qin G, Zhao X, Yang S, Huang J, Shen C, Lei Q, Wang L, Zhang Y. Long-term clinical efficacy of cytokine-induced killer cell-based immunotherapy in early-stage esophageal squamous cell carcinoma. Cytotherapy. 2022;24:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Lu D, Li T, Yang Z, Zhao X, Su Y, Nian L. DC-CIK combined with chemotherapy on the efficacy, immune function, and life quality in colorectal cancer patients after radical resection. Am J Transl Res. 2023;15:2793-2801. [PubMed] |
| 16. | Mahmoud NN. Colorectal Cancer: Preoperative Evaluation and Staging. Surg Oncol Clin N Am. 2022;31:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 17. | Villa A, Vollemans M, De Moraes A, Sonis S. Concordance of the WHO, RTOG, and CTCAE v4.0 grading scales for the evaluation of oral mucositis associated with chemoradiation therapy for the treatment of oral and oropharyngeal cancers. Support Care Cancer. 2021;29:6061-6068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Chen Y, Zheng X, Wu C. The Role of the Tumor Microenvironment and Treatment Strategies in Colorectal Cancer. Front Immunol. 2021;12:792691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Fidelle M, Yonekura S, Picard M, Cogdill A, Hollebecque A, Roberti MP, Zitvogel L. Resolving the Paradox of Colon Cancer Through the Integration of Genetics, Immunology, and the Microbiota. Front Immunol. 2020;11:600886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Wang ZX, Cao JX, Liu ZP, Cui YX, Li CY, Li D, Zhang XY, Liu JL, Li JL. Combination of chemotherapy and immunotherapy for colon cancer in China: a meta-analysis. World J Gastroenterol. 2014;20:1095-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Wang QJ, Wang H, Pan K, Li YQ, Huang LX, Chen SP, He J, Ke ML, Zhao JJ, Li JJ, Sun JC, Liang XT, Ma HQ, Chen YB, Xia JC. Comparative study on anti-tumor immune response of autologous cytokine-induced killer (CIK) cells, dendritic cells-CIK (DC-CIK), and semi-allogeneic DC-CIK. Chin J Cancer. 2010;29:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Zhou L, Chen Q, Chen H, Wang L, Zhang J. Enhanced Inhibitory Effect of DC-CIK Cells on Lung Adenocarcinoma via Anti-Tim-3 Antibody and Antiprogrammed Cell Death-1 Antibody and Possible Mechanism. Evid Based Complement Alternat Med. 2022;2022:4097576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Lan XP, Chen YG, Wang Z, Yuan CW, Wang GG, Lu GL, Mao SW, Jin XB, Xia QH. Immunotherapy of DC-CIK cells enhances the efficacy of chemotherapy for solid cancer: a meta-analysis of randomized controlled trials in Chinese patients. J Zhejiang Univ Sci B. 2015;16:743-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Xu H, Qin W, Feng H, Song D, Yang X, Zhang J. Analysis of the Clinical Efficacy of Dendritic Cell -cytokine Induced Killer Cell-based Adoptive Immunotherapy for Colorectal Cancer. Immunol Invest. 2021;50:622-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Pan QZ, Gu JM, Zhao JJ, Tang Y, Wang QJ, Zhu Q, Song MJ, Li YQ, He J, Chen SP, Weng DS, Xia JC. Retrospective analysis of the efficacy of cytokine-induced killer cell immunotherapy combined with first-line chemotherapy in patients with metastatic colorectal cancer. Clin Transl Immunology. 2020;9:e1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Li X, Zhou H, Huang W, Wang X, Meng M, Hou Z, Liao L, Tang W, Xie Y, Wang R, Yu H, Wang L, Zhu H, Wang W, Tan J, Li R. Retrospective analysis of the efficacy of adjuvant cytokine-induced killer cell immunotherapy combined with chemotherapy in colorectal cancer patients after surgery. Clin Transl Immunology. 2022;11:e1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |