Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3171
Revised: August 17, 2024
Accepted: August 29, 2024
Published online: October 27, 2024
Processing time: 140 Days and 3.5 Hours
The benefit of adjuvant chemotherapy (ACT) for patients with no evidence of disease after pulmonary metastasis resection (PM) from colorectal cancer (CRC) remains controversial.
To assess the efficacy of ACT in patients after PM resection for CRC.
This study included 96 patients who underwent pulmonary metastasectomy for CRC at a single institution between April 2008 and July 2023. The primary end
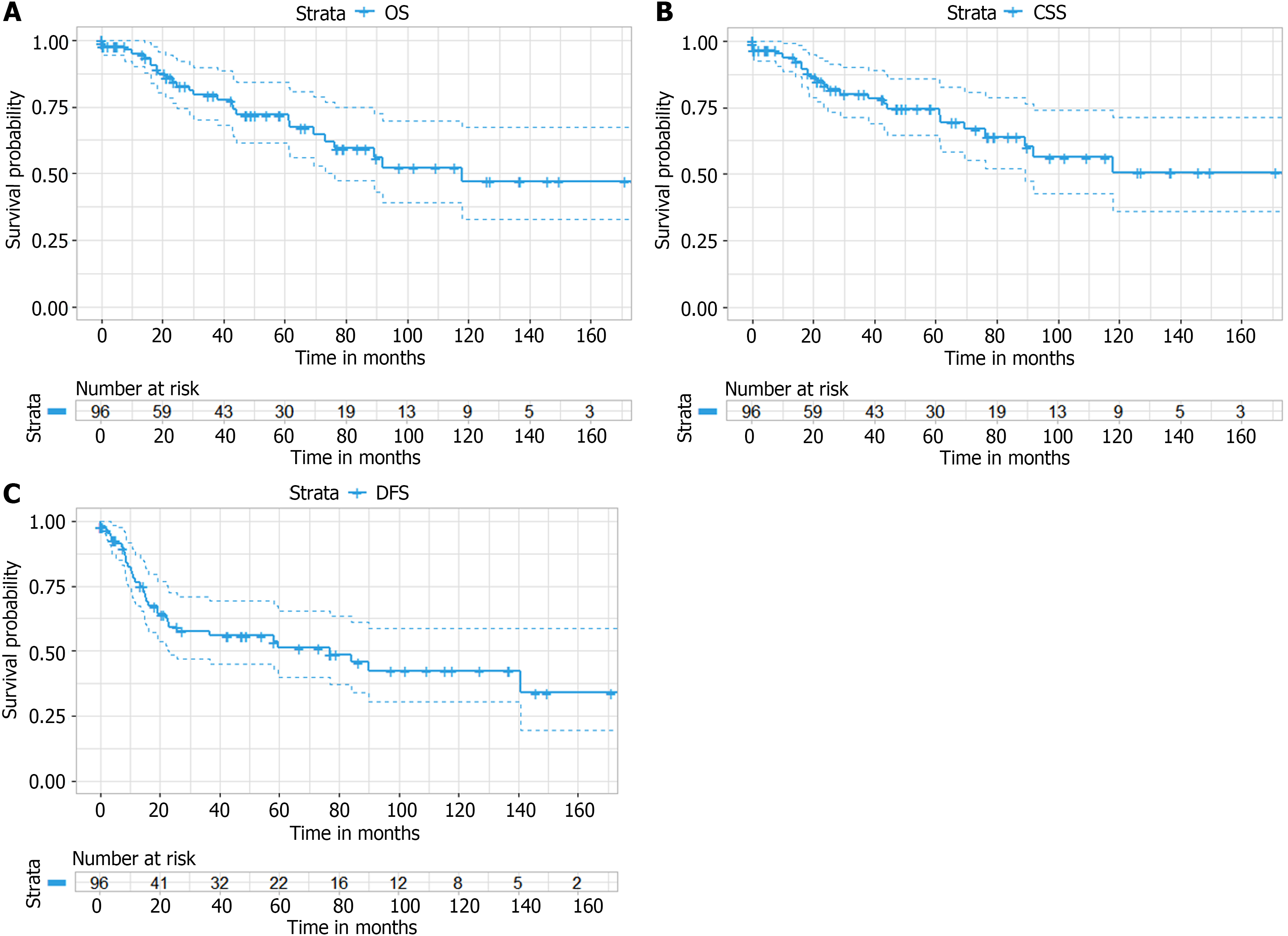
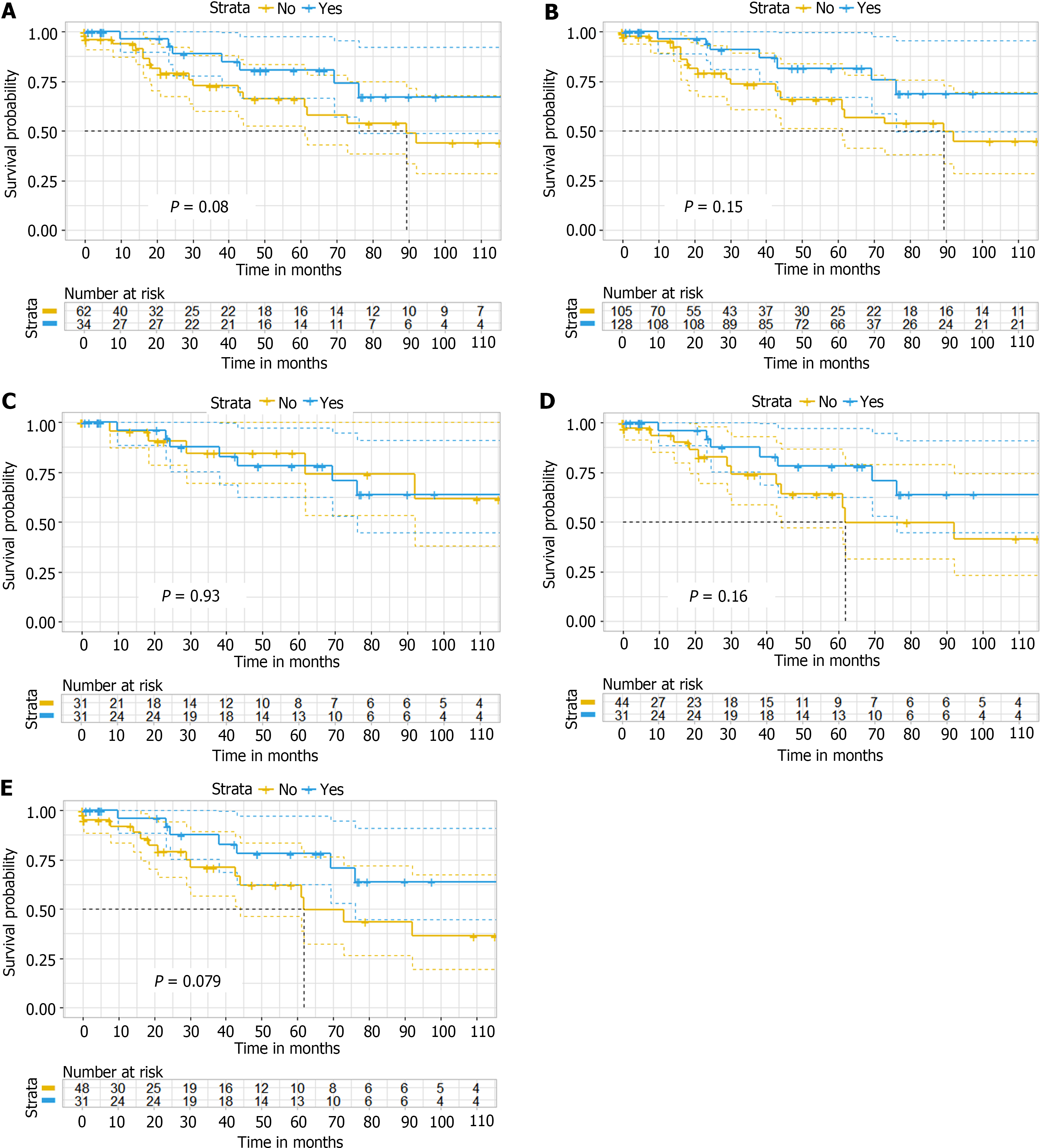
With a median follow-up of 27.5 months (range, 18.3-50.4 months), the 5-year OS, CSS and DFS were 72.0%, 74.4% and 51.3%, respectively. ACT had no significant effect on OS after PM resection from CRC [original cohort: P = 0.08; IPTW: P = 0.15]. No differences were observed for CSS (P = 0.12) and DFS (P = 0.68) between the ACT and non-ACT groups. Multivariate analysis showed no association of ACT with better survival, while sublobar resection (HR = 0.45; 95%CI: 0.20-1.00, P = 0.049) and longer disease-free interval (HR = 0.45; 95%CI: 0.20-0.98, P = 0.044) were associated with improved survival.
ACT does not improve survival after PM resection for CRC. Further well-designed randomized controlled trials are needed to determine the optimal ACT regimen and duration.
Core Tip: It remains controversial whether patients who have reached no evidence disease after resection of pulmonary metastasis of colorectal cancer (CRC) can benefit from adjuvant chemotherapy (ACT). We aimed to evaluate the efficacy of ACT in patients after resection of pulmonary metastasis resection from CRC. Due to the lack of randomized prospective trials and high level evidence, our study may support valuable data support for individual participant data meta-analysis and help further research on this type of disease.
- Citation: Gao Z, Wu SK, Zhang SJ, Wang X, Wu YC, Jin X. Adjuvant chemotherapy for isolated resectable colorectal lung metastasis: A retrospective study using inverse probability treatment weighting propensity analysis. World J Gastrointest Surg 2024; 16(10): 3171-3184
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3171.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3171
In 2022, colorectal cancer (CRC) became the third most common malignancy worldwide and the second leading cause of cancer-related deaths[1]. Metastasis of CRC is the primary cause of death in CRC[2,3], with a 5-year survival rate of approximately 56% for non-metastatic cases, which drops significantly once metastasis occurs[4,5]. Unlike many other cancers, metastatic CRC is often amenable to surgical intervention[6]. The lungs, following the liver, are the second most common site for CRC metastasis, accounting for 10%-15% of cases[7]. Although research on lung metastasis is limited compared to liver metastasis, patients with lung metastases generally have a better prognosis[8]. Key prognostic factors for prolonged survival include disease-free interval (DFI), tumor diameter, preoperative carcinoembryonic antigen (CEA) levels, and the number of lung metastases[9]. Despite surgery being the primary treatment for lung metastasis, with a 5-year overall survival (OS) rate of 50%, the recurrence rate remains high at 68%, predominantly in the remaining lung tissue[10].
Metastasis is the main determinant of long-term survival in CRC, responsible for 90% of tumor-related deaths[11,12]. Adjuvant therapy is intended to eliminate micro-metastases following surgery[13]; however, the role of adjuvant chemotherapy (ACT) in improving survival after resection of pulmonary metastases (PM) remains controversial[14-16]. Previous studies and meta-analyses have provided conflicting evidence regarding the benefit of ACT and perioperative chemotherapy in this setting[17,18]. This study aimed to clarify the role of ACT after lung metastasectomy in patients with CRC at our center.
The medical records were retrospectively queried to identify patients who underwent pulmonary metastasectomy for CRC at Peking University First Hospital between April 2008 to June 2023. Patients with an index pulmonary metastasectomy at an outside facility were excluded. Inclusion criteria were: (1) Histopathologically confirmed adenocarcinoma of CRC, radically resected with no signs of local recurrence; and (2) If ACT was administered after surgery for the primary lesion, the interval between the last ACT and the radical resection of lung metastases was greater than three months. Exclusion criteria were: (1) Synchronous lung metastases; and (2) Extrapulmonary metastases or multiple bilateral lung metastases that could not be resected using R0 criteria. Isolated lung metastasis was defined as a CRC lung metastasis without extrapulmonary involved. Ten patients (10.4%) underwent surgery for extrathoracic metastases (mainly liver metastases), with the last treatment for extrathoracic metastases occurring at least 3 months after the discovery of isolated lung metastases. Surgical methods for lung metastases included lobectomy and sublobar resection (wedge resection and segmentectomy). This study was approved by the Ethics Committee of the Peking University First Hospital.
Follow-up data were obtained through hospital record reviews and telephone contact. The final follow-up period was January 2024. OS was the primary outcome, calculated from the date of lung surgery to the date of death from any cause, with censored cases defined by the last available follow-up. Cancer-specific survival (CSS) and disease-free survival (DFS) were assessed monthly from the surgery date until tumor progression or death.
Analyses were performed with R statistical software version 4.3.2, with significance set at P ≤ 0.05. Continuous variables were compared using Student’s t-test, while categorical variables were assessed with the χ2 test. Survival analysis was conducted using the Kaplan-Meier method and multivariate Cox regression. Inverse probability of treatment weighting (IPTW) was used to adjust for differences between the ACT and non-ACT groups, with weights set as the inverse of the propensity score for those receiving ACT and the inverse of (1-propensity score) for those not receiving ACT. Stan
To address potential biases, three sensitivity analyses were conducted. First, comparisons were made between data before and after the missing-value interpolation. Second, a complete case analysis was used to replace imputed laboratory va
During the study period, 96 patients met the inclusion criteria, with a majority being men (n = 58; 60.4%), and a median age of 62.6 years (Table 1). Among these patients, 28 (29.2%) reported a history of tobacco use. All patients (100%) were diagnosed with lung metastases during follow-up, with a median DFI of 28.5 months. Ten patients (10.4%) had pre
| Factors | Total, n (%) |
| Gender | |
| Male | 58 (60.4) |
| Female | 38 (39.6) |
| Age at CRC diagnosis | |
| Median (IQR) | 59.7 (49.8-69.6) |
| Age at time of pulmonary surgery | |
| Median (IQR) | 62.6 (53-72.2) |
| Access | |
| Open | 20 (20.8) |
| VATS | 76 (79.2) |
| Type of resection | |
| Sublobar resection | 54 (56.2) |
| Lobectomy | 42 (43.8) |
| Adjuvant chemotherapy for PM | |
| No | 62 (64.6) |
| Yes | 34 (35.4) |
| Primary tumor T stage | |
| T1 or T2 | 8 (11.8) |
| T3 or T4 | 60 (88.2) |
| Primary tumor N stage | |
| N0 | 28 (39.4) |
| N1 or N2 | 43 (60.6) |
| Primary tumor location | |
| Left colon | 29 (33.0) |
| Right colon | 17 (19.3) |
| Rectum | 42(47.7) |
| Adjuvant chemotherapy for CRC | |
| No | 30 (31.2) |
| Yes | 66 (68.8) |
| CEA levels | |
| ≤ 5 ng/mL | 47 (60.3) |
| > 5 ng/mL | 31 (39.7) |
| Number of metastatic lesions | |
| 1 | 76 (79.2) |
| > 1 | 20 (20.8) |
| Tumor size (cm) | |
| ≤ 2 cm | 51 (54.8) |
| > 2 cm | 42 (45.2) |
| Prior extra-thoracic metastasis | |
| No | 86 (89.6) |
| Yes | 10 (10.4) |
| CRC differentiation | |
| Well/well to moderate | 8 (11.8) |
| Moderate | 57 (83.8) |
| Moderate to poor/poor | 3 (4.4) |
| Smoking history | |
| No | 68 (70.8) |
| Yes | 28 (29.2) |
| RAS | |
| Wild type | 10 (62.5) |
| mutant type | 6 (37.5) |
| Bilateral pulmonary nodules | |
| No | 91 (94.8) |
| Yes | 5 (5.2) |
| LN sampling at PM | |
| No | 51 (53.1) |
| Yes | 45 (46.9) |
| Positive LN at PM | |
| No | 90 (93.8) |
| Yes | 6 (6.2) |
In the entire cohort, 62 patients did not receive ACT, while 34 did. The groups did not show significant differences in sex, resection type, number of metastatic lesions, tumor size, bilateral pulmonary nodules, lymph node (LN) sampling during PM, or positive LN at PM when stratified by ACT. However, age at primary cancer diagnosis (P = 0.038), age at lung surgery (P = 0.014), and smoking history (P = 0.038) did differ (Table 2).
| Factors | Levels | Surgery alone (n = 62) | Adjuvant chemotherapy (n = 34) | P value |
| Gender | Male | 39 (62.9) | 19 (55.9) | 0.649 |
| Female | 23 (37.1) | 15 (44.1) | ||
| Age at CRC diagnosis | mean ± SD | 61.3 ± 9.9 | 56.9 ± 9.4 | 0.038 |
| Age at time of pulmonary surgery | mean ± SD | 64.4 ± 9.6 | 59.4 ± 9.0 | 0.014 |
| Smoking history | No | 39 (62.9) | 29 (85.3) | 0.038 |
| Yes | 23 (37.1) | 5 (14.7) | ||
| Prior extra-thoracic metastasis | No | 55 (88.7) | 31 (91.2) | 0.977 |
| Yes | 7 (11.3) | 3 (8.8) | ||
| Access | Open | 16 (25.8) | 4 (11.8) | 0.175 |
| VATS | 46 (74.2) | 30 (88.2) | ||
| Type of resection | Lobe | 30 (48.4) | 12 (35.3) | 0.307 |
| Segmental wedge | 32 (51.6) | 22 (64.7) | ||
| Number of metastatic lesions | 1 | 50 (80.6) | 26 (76.5) | 0.827 |
| > 1 | 12 (19.4) | 8 (23.5) | ||
| Tumor size (cm) | ≤ 2cm | 33 (53.2) | 20 (58.8) | 0.754 |
| > 2 cm | 29 (46.8) | 14 (41.2) | ||
| Bilateral pulmonary nodules | No | 59 (95.2) | 32 (94.1) | 1.000 |
| Yes | 3 (4.8) | 2 (5.9) | ||
| LN sampling at PM | No | 31 (50) | 20 (58.8) | 0.539 |
| Yes | 31 (50) | 14 (41.2) | ||
| Positive LN at PM | No | 59 (95.2) | 31 (91.2) | 0.741 |
| Yes | 3 (4.8) | 3 (8.8) | ||
| CEA | ≤ 5 ng/mL | 40 (64.5) | 21 (61.8) | 0.963 |
| > 5 ng/mL | 22 (35.5) | 13 (38.2) | ||
| DFI | mean ± SD | 1070.1 ± 754.2 | 946.0 ± 620.6 | 0.415 |
| Primary tumor location | Left colon | 18 (29) | 11 (32.4) | 0.340 |
| Rectal | 29 (46.8) | 19 (55.9) | ||
| Right colon | 15 (24.2) | 4 (11.8) |
The median follow-up was 27.5 months, with 26 patients having died by analysis. The OS rates at 1, 2, and 5 years were 94.8% [95% confidence interval (CI): 90.0%-99.9%], 99.9% (95%CI: 76.3%-93.4%), and 72.0% (95%CI: 61.6%-84.1%), respec
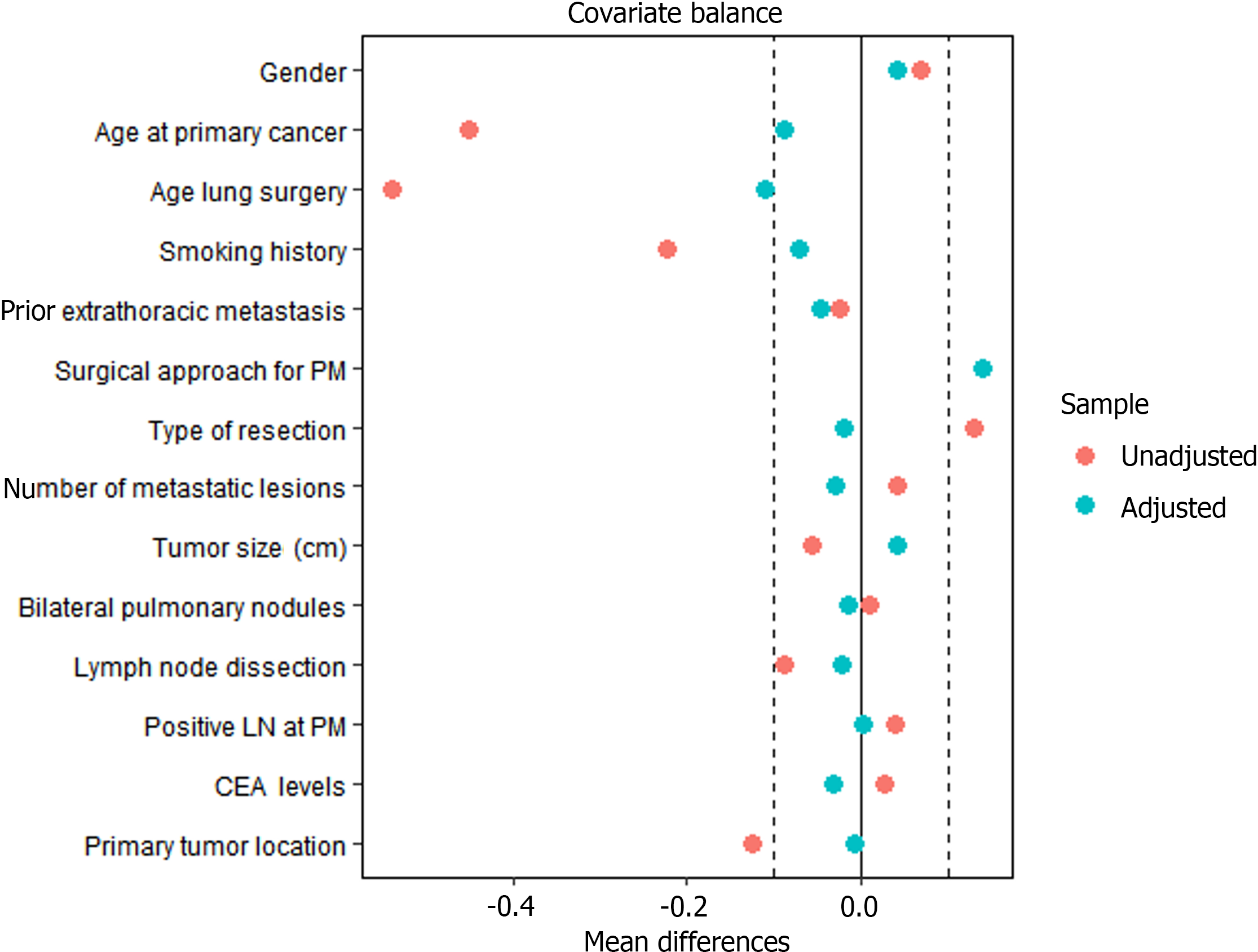
After applying the IPTW method, the effective sample size was modestly altered, with data from 128 to 105 patients analyzed in the ACT after PM resection and PM resection alone groups. Kaplan-Meier analysis and the log-rank test showed no significant difference in time to death, the primary outcome, between the ACT after PM resection and PM resection alone groups, with OS favoring ACT after resection of PM (P = 0.08 before IPTW analysis; P = 0.15 after IPTW analysis) (Figure 2A-B). The P value of the stabilized IPTW analysis is 0.17. Meanwhile, after applying weights, the variables between the two groups maintained a substantial balance at the baseline level (Figure 3).
Table 3, Table 4, Table 5, and Table 6 summarize survival data according to risk factors before and after IPTW. CEA levels (P = 0.038) and prior extra-thoracic metastasis (P = 0.049) were significant predictors of survival. In multivariate analysis, prior extra-thoracic metastasis of PM [Hazard ratio (HR): 4.97; 95%CI: 1.03-24.08; P = 0.046] was associated with improved survival before and after IPTW, while the type of resection (HR: 0.45; 95%CI: 0.20-1.00; P = 0.049) and DFI (HR: 0.45; 95%CI: 0.20-0.98; P = 0.044) were confirmed as predictors OS in the original cohort.
| Factors | HR (univariable) | |
| Gender | M | |
| F | 0.8556 (0.3201-2.287, P = 0.756) | |
| Age at primary cancer | ≤ 60 years | |
| > 60 years | 1.1914 (0.4402-3.224, P = 0.730) | |
| Smoking history | No | |
| Yes | 1.07988 (0.3514-3.319, P = 0.893) | |
| Age lung surgery | ≤ 60 years | |
| > 60 years | 0.6498 (0.2495-1.692, P = 0.377) | |
| Surgical approach for PM | Open | |
| VATS | 0.5052 (0.1863-1.37, P = 0.18) | |
| Type of resection | Lobectomy | |
| Sublobar resection | 0.6032 (0.2248-1.619, P = 0.065) | |
| Lymph node dissection | No | |
| Yes | 1.2383 (0.4682-2.728, P = 0.667) | |
| Positive LN at PM | No | |
| Yes | 0.9268 (0.1181-7.271, P = 0.942) | |
| Adjuvant chemotherapy | No | |
| Yes | 0.4204 (0.1632-1.083, P = 0.0726) | |
| Primary tumor location | Left colon | |
| Right colon | 0.4590 (0.1093-1.928, P = 0.288) | |
| Rectum | 0.6267 (0.2328-1.687, P = 0.355) | |
| CEA levels | ≤ 5 ng/mL | |
| > 5 ng/mL | 2.7261 (1.075-6.913, P = 0.0347) | |
| Number of metastatic lesions | 1 | |
| > 1 | 2.1928 (0.8496-5.66, P = 0.105) | |
| Bilateral pulmonary nodules | No | |
| Yes | 2.478 (0.9703-6.329, P = 0.0578) | |
| Tumor size (cm) | ≤ 2 cm | |
| > 2 cm | 0.7971 (0.2961-2.146, P = 0.653) | |
| Prior extra-thoracic metastasis | No | |
| Yes | 4.627 (1.01-21.2, P = 0.0485) | |
| Survival | HR | 95%CI | P value | |
| Adjuvant chemotherapy | No | |||
| Yes | 0.43 | 0.16-1.18 | 0.10 | |
| Number of metastatic lesions | 1 | |||
| ≥ 2 | 1.95 | 0.64-5.93 | 0.24 | |
| Prior extra-thoracic metastasis | No | |||
| Yes | 4.97 | 1.03-24.08 | 0.046 | |
| Bilateral pulmonary nodules | No | |||
| Yes | 1.84 | 0.36-9.43 | 0.47 | |
| CEA | ≤ 5 ng/mL | |||
| > 5 ng/mL | 2.00 | 0.76-5.28 | 0.16 | |
| Type of resection | Lobectomy | |||
| Sublobar resection | 0.53 | 0.21-1.35 | 0.19 |
| Factors | HR (univariable) | |
| Gender | M | |
| F | 0.78 (0.35-1.77, P = 0.555) | |
| Age at primary cancer | ≤ 60 years | |
| > 60 years | 1.41 (0.65-3.05, P = 0.381) | |
| Smoking history | No | |
| Yes | 1.11 (0.48-2.55, P = 0.814) | |
| Age lung surgery | ≤ 60 years | |
| > 60 years | 0.98 (0.45-2.12, P = 0.956) | |
| Surgical approach for PM | Open | |
| VATS | 0.77 (0.32-1.84, P = 0.556) | |
| Type of resection | Lobectomy | |
| Sublobar resection | 0.47 (0.21-1.04, P = 0.062) | |
| Lymph node dissection | No | |
| Yes | 1.18 (0.55-2.55, P = 0.674) | |
| Positive LN at PM | No | |
| Yes | 0.62 (0.08-4.60, P = 0.642) | |
| Adjuvant chemotherapy | No | |
| Yes | 0.47 (0.20-1.12, P = 0.087) | |
| Primary tumor location | Left colon | |
| Right colon | 0.91 (0.32-2.63, P = 0.863) | |
| Rectum | 0.60 (0.24-1.53, P = 0.288) | |
| CEA levels | ≤ 5 ng/mL | |
| > 5 ng/mL | 1.48 (0.66-3.31, P = 0.335) | |
| Number of metastatic lesions | 1 | |
| > 1 | 1.75 (0.73-4.23, P = 0.211) | |
| Bilateral pulmonary nodules | No | |
| Yes | 1.88 (0.44-8.08, P = 0.398) | |
| Tumor size (cm) | ≤ 2 cm | |
| > 2 cm | 0.67 (0.29-1.53, P = 0.337) | |
| Prior extra-thoracic metastasis | No | |
| Yes | 1.94 (0.58-6.50, P = 0.283) | |
| DFI | ≤ 600 | |
| > 600 | 0.48 (0.22-1.04, P = 0.063) | |
| Survival | HR | 95%CI | P value | |
| Adjuvant chemotherapy | No | |||
| Yes | 0.50 | 0.21-1.18 | 0.114 | |
| Type of resection | Lobectomy | |||
| Sublobar resection | 0.45 | 0.20-1.00 | 0.049 | |
| DFI | ≤ 600 | |||
| > 600 | 0.45 | 0.20-0.98 | 0.044 |
Sensitivity analyses, encompassing data before and after imputation, complete case analysis, and propensity score-adjusted analysis, did not significantly change the results (Table 6 and Table 7; Figure 2C-E).
| Factors | Levels | Post-imputation (n = 96) | Pre-imputation (n = 96) | P value |
| Gender | Male | 58 (60.4) | 58 (60.4) | 1.000 |
| Female | 38 (39.6) | 38 (39.6) | ||
| Age at CRC diagnosis | mean ± SD | 59.7 ± 9.9 | 59.7 ± 9.9 | 1.000 |
| Age at time of pulmonary surgery | mean ± SD | 62.6 ± 9.6 | 62.6 ± 9.6 | 1.000 |
| Smoking history | No | 68 (70.8) | 68 (70.8) | 1.000 |
| Yes | 28 (29.2) | 28 (29.2) | ||
| Adjuvant chemotherapy for CRC | No | 30 (31.2) | 30 (31.2) | 1.000 |
| Yes | 66 (68.8) | 66 (68.8) | ||
| CRC differentiation | Moderate | 84 (87.5) | 57 (83.8) | 0.760 |
| Moderate to poor | 4 (4.2) | 3 (4.4) | ||
| Well to moderate | 8 (8.3) | 8 (11.8) | ||
| Primary tumor T stage | T1 or T2 | 10 (10.4) | 8 (11.8) | 0.985 |
| T3 or T4 | 86 (89.6) | 60 (88.2) | ||
| Primary tumor N stage | N0 | 39 (40.6) | 28 (39.4) | 1.000 |
| N1 or N2 | 57 (59.4) | 43 (60.6) | ||
| Prior extra-thoracic metastasis | No | 86 (89.6) | 86 (89.6) | 1.000 |
| Yes | 10 (10.4) | 10 (10.4) | ||
| Access | Open | 20 (20.8) | 20 (20.8) | 1.000 |
| VATS | 76 (79.2) | 76 (79.2) | ||
| Type of resection | Lobe | 42 (43.8) | 42 (43.8) | 1.000 |
| Segmental wedge | 54 (56.2) | 54 (56.2) | ||
| Number of metastatic lesions | 1 | 76 (79.2) | 76 (79.2) | 1.000 |
| > 1 | 20 (20.8) | 20 (20.8) | ||
| Tumor size (cm) | ≤ 2 | 53 (55.2) | 51 (54.8) | 1.000 |
| > 2 | 43 (44.8) | 42 (45.2) | ||
| Bilateral pulmonary nodules | No | 91 (94.8) | 91 (94.8) | 1.000 |
| Yes | 5 (5.2) | 5 (5.2) | ||
| LN sampling at PM | No | 51 (53.1) | 51 (53.1) | 1.000 |
| Yes | 45 (46.9) | 45 (46.9) | ||
| Positive LN at PM | No | 90 (93.8) | 90 (93.8) | 1.000 |
| Yes | 6 (6.2) | 6 (6.2) | ||
| CEA levels | ≤ 5 ng/mL | 61 (63.5) | 47 (60.3) | 0.774 |
| > 5 ng/mL | 35 (36.5) | 31 (39.7) | ||
| CRC LVI | No | 81 (84.4) | 45 (81.8) | 0.858 |
| Yes | 15 (15.6) | 10 (18.2) | ||
| CRC PNI | No | 77 (80.2) | 41 (74.5) | 0.545 |
| Yes | 19 (19.8) | 14 (25.5) | ||
| Adjuvant chemotherapy | No | 62 (64.6) | 62 (64.6) | 1.000 |
| Yes | 34 (35.4) | 34 (35.4) | ||
| DFI | mean ± SD | 1026.2 ± 708.9 | 1026.2 ± 708.9 | 1.000 |
| Primary tumor location | Left colon | 29 (30.2) | 29 (33) | 0.921 |
| Rectal | 48 (50) | 42 (47.7) | ||
| Right colon | 19 (19.8) | 17 (19.3) |
Although surgery for CRC metastasis to the lungs can improve patient prognosis, it remains far from ideal[23]. Current research is focused on improving outcomes for patients with CRC with lung metastases who undergo surgery[24]. Although patients with liver metastases from CRC can benefit from postoperative chemotherapy[25], it remains unclear whether patients with PM derive similar benefits from perioperative chemotherapy. The benefit of ACT for patients achieving no evidence of disease (NED) after PM resection is still debated. For example, a meta-analysis by Zhang et al[18] found no improvement in prognosis with postoperative ACT for CRC lung metastasis, whereas a meta-analysis by Li and Qin[17] suggested that perioperative chemotherapy could enhance outcomes. These two meta-analyses, which investigated the impact of chemotherapy on the prognosis of patients undergoing resection of CRC lung metastases, reached different conclusions. As a result, it remains unclear whether the mode of chemotherapy-whether neoadjuvant, adjuvant, or both-affects the prognosis of these patients. Supporting this uncertainty, Pagès et al[26] found that neoad
In this study, we retrospectively analyzed the need for ACT in patients who underwent lung metastasectomy for CRC at our center. The HR estimation indicated that patients did not benefit from ACT after lung metastasis resection (HR: 0.50; 95%CI: 0.21-1.18; P = 0.114). Multivariate analysis adjusting for other survival-influencing variables confirmed that postoperative ACT did not significantly alter OS, CSS, or DFS, consistent with previous meta-studies[18].
The role of a history of liver metastasis as a prognostic factor in patients undergoing pulmonary metastasectomy has been controversial[27]. Although some studies dismiss liver metastasis history as a significant survival factor, others have reached the opposite conclusion[23]. Indeed we identified it as an independent adverse prognostic factor for OS (P = 0.046) after inverse probability of treatment-weighting. Additionally, preoperative CEA levels were significantly asso
The DFI between CRC resection and the development of lung metastasis has consistently been shown to correlate with treatment outcomes, though studies vary in defining the cutoff for a prolonged DFI[29-32]. A pooled analysis indicated that a DFI of less than 36 months is a poor prognostic factor for OS[29]. In this study, the optimal DFI cutoff, calculated using the Youden index, was 20 months, with shorter DFI’s predicting worse outcomes. Interestingly, while previous studies have identified the number of lung metastases as a key adverse prognostic factor, our study did not find a sig
There is ongoing debate about whether open thoracic surgery offers superior outcomes compared to video-assisted thoracic surgery (VATS) for CRC lung metastasis. Open surgery allows for the palpation of the lungs to identify occult metastases not visible on imaging, traditionally making it the gold standard[35]. However, recent studies, including a multi-institutional retrospective analysis from Japan, found no significant difference in survival rates between open surgery and VATS after propensity score adjustment[36]. Similarly, our study found comparable survival outcomes between patients undergoing VATS and those undergoing open surgery.
This study had several limitations. First, it is a retrospective, single-center study which may introduce selection bias. Second, the diverse chemotherapy regimens, doses, and cycles may have influenced the conclusions. The study only included carefully selected patients excluding many with CRC lung metastases. Furthermore, the decision to administer ACT was influenced by clinical decision-making factors specific to a single institution, potentially causing imbalances. Notably patients who received postoperative ACT were younger at CRC diagnosis, had earlier lung metastases, and shorter median DFI than those who did not receive ACT. These patients might have had more aggressive tumors but also better to responses to cytotoxic therapy. These factors must be considered when interpreting the study’s results.
In conclusion, our findings indicate that ACT does not confer survival benefits for patients who reach NED after PM resection. However, this conclusion is limited by the retrospective nature of the analysis, underscoring the need for randomized controlled trials focused on ACT in this specific patient subgroup.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8187] [Article Influence: 8187.0] [Reference Citation Analysis (2)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15319] [Article Influence: 3063.8] [Reference Citation Analysis (4)] |
| 3. | Kow AWC. Hepatic metastasis from colorectal cancer. J Gastrointest Oncol. 2019;10:1274-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Davini F, Ricciardi S, Zirafa CC, Romano G, Alì G, Fontanini G, Melfi FMA. Lung metastasectomy after colorectal cancer: prognostic impact of resection margin on long term survival, a retrospective cohort study. Int J Colorectal Dis. 2020;35:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Panahi MH, Panahi H, Mahdavi Hezaveh A, Mansournia MA, Bidhendi Yarandi R. Survival rate of colon and rectum cancer in Iran: A systematic review and meta-analysis. Neoplasma. 2019;66:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 6. | Pinto F, Pangrazio MD, Martinino A, Todeschini L, Toti F, Cristin L, Caimano M, Mattia A, Bianco G, Spoletini G, Giovinazzo F. Laparoscopic versus open liver resection for colorectal liver metastasis: an umbrella review. Front Oncol. 2024;14:1340430. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Parnaby CN, Bailey W, Balasingam A, Beckert L, Eglinton T, Fife J, Frizelle FA, Jeffery M, Watson AJ. Pulmonary staging in colorectal cancer: a review. Colorectal Dis. 2012;14:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Zhang GQ, Taylor JP, Stem M, Almaazmi H, Efron JE, Atallah C, Safar B. Aggressive Multimodal Treatment and Metastatic Colorectal Cancer Survival. J Am Coll Surg. 2020;230:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Li J, Yuan Y, Yang F, Wang Y, Zhu X, Wang Z, Zheng S, Wan D, He J, Wang J, Ba Y, Bai C, Bai L, Bai W, Bi F, Cai K, Cai M, Cai S, Chen G, Chen K, Chen L, Chen P, Chi P, Dai G, Deng Y, Ding K, Fan Q, Fang W, Fang X, Feng F, Fu C, Fu Q, Gu Y, He Y, Jia B, Jiang K, Lai M, Lan P, Li E, Li D, Li J, Li L, Li M, Li S, Li Y, Li Y, Li Z, Liang X, Liang Z, Lin F, Lin G, Liu H, Liu J, Liu T, Liu Y, Pan H, Pan Z, Pei H, Qiu M, Qu X, Ren L, Shen Z, Sheng W, Song C, Song L, Sun J, Sun L, Sun Y, Tang Y, Tao M, Wang C, Wang H, Wang J, Wang S, Wang X, Wang X, Wang Z, Wu A, Wu N, Xia L, Xiao Y, Xing B, Xiong B, Xu J, Xu J, Xu N, Xu R, Xu Z, Yang Y, Yao H, Ye Y, Yu Y, Yu Y, Yue J, Zhang J, Zhang J, Zhang S, Zhang W, Zhang Y, Zhang Z, Zhang Z, Zhao L, Zhao R, Zhou F, Zhou J, Jin J, Gu J, Shen L. Expert consensus on multidisciplinary therapy of colorectal cancer with lung metastases (2019 edition). J Hematol Oncol. 2019;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, Nakagawa K, Fukuhara K, Maeda H, Takeda S, Minami M, Ohno Y, Matsuda H; Thoracic Surgery Study Group of Osaka University. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2004;78:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Bhullar DS, Barriuso J, Mullamitha S, Saunders MP, O'Dwyer ST, Aziz O. Biomarker concordance between primary colorectal cancer and its metastases. EBioMedicine. 2019;40:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3059] [Cited by in RCA: 3622] [Article Influence: 258.7] [Reference Citation Analysis (0)] |
| 13. | Kosmider S, Lipton L. Adjuvant therapies for colorectal cancer. World J Gastroenterol. 2007;13:3799-3805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Okazaki Y, Shibutani M, Wang E, Nagahara H, Fukuoka T, Iseki Y, Maeda K, Hirakawa K, Ohira M. Efficacy of adjuvant chemotherapy after complete resection of pulmonary metastasis from colorectal cancer. Mol Clin Oncol. 2021;15:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Karim S, Nanji S, Brennan K, Pramesh CS, Booth CM. Chemotherapy for resected colorectal cancer pulmonary metastases: Utilization and outcomes in routine clinical practice. Eur J Surg Oncol. 2017;43:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Park S, Kang BW, Lee SJ, Yoon S, Chae YS, Kim JG, Lee KH, Koh SA, Song HS, Park KU, Kim JY, Heo MH, Ryoo HM, Cho YY, Jo J, Lee JL, Lee SA. Clinical significance of systemic chemotherapy after curative resection of metachronous pulmonary metastases from colorectal cancer. Cancer Chemother Pharmacol. 2017;80:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Li Y, Qin Y. Peri-operative chemotherapy for resectable colorectal lung metastasis: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2020;146:545-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Zhang C, Tan Y, Xu H. Does adjuvant chemotherapy improve the prognosis of patients after resection of pulmonary metastasis from colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis. 2019;34:1661-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388-3414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 926] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 20. | Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 578] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 21. | Buuren SV, Groothuis-Oudshoorn K. Mice: Multivariate Imputation by Chained Equations inR. J Stat Soft. 2011;45:1-67. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3242] [Cited by in RCA: 3278] [Article Influence: 234.1] [Reference Citation Analysis (0)] |
| 22. | Omar R. Clinical Prediction Models: A Practical Approach to Development, Validation and Updating by STEYERBERG, E. W. Biometrics. 2010;66:661-662. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Gkikas A, Kakos C, Lampridis S, Godolphin PJ, Patrini D. Preoperative prognostic factors for 5-year survival following pulmonary metastasectomy from colorectal cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2023;63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Rapicetta C, Lococo F, Davini F, Carleo F, Kauppi J, Di Stefano TS, Ricciardi S, Di Martino M, Räsänen J, Paci M, Melfi F, Cardillo G. Is Adjuvant Chemotherapy Worthwhile After Radical Resection for Single Lung Metastasis From Colorectal Cancer? A Multicentric Analysis Evaluating the Risk of Recurrence. Front Oncol. 2019;9:763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Kawashima J, Chatzipanagiotou OP, Tsilimigras DI, Khan MMM, Catalano G, Rashid Z, Khalil M, Altaf A, Munir MM, Endo Y, Woldesenbet S, Guglielmi A, Ruzzenente A, Aldrighetti L, Alexandrescu S, Kitago M, Poultsides G, Sasaki K, Aucejo F, Endo I, Pawlik TM. Preoperative and postoperative predictive models of early recurrence for colorectal liver metastases following chemotherapy and curative-intent one-stage hepatectomy. Eur J Surg Oncol. 2024;50:108532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 26. | Pagès PB, Serayssol C, Brioude G, Falcoz PE, Brouchet L, Le Pimpec-Barthes F, Thomas PA, Bernard A. Risk factors for survival and recurrence after lung metastasectomy. J Surg Res. 2016;203:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Zabaleta J, Iida T, Falcoz PE, Salah S, Jarabo JR, Correa AM, Zampino MG, Matsui T, Cho S, Ardissone F, Watanabe K, Gonzalez M, Gervaz P, Emparanza JI, Abraira V. Individual data meta-analysis for the study of survival after pulmonary metastasectomy in colorectal cancer patients: A history of resected liver metastases worsens the prognosis. Eur J Surg Oncol. 2018;44:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Huang J, Zang Q, Wen Y, Pan Z, Yao Z, Huang M, Huang J, Chen J, Wang R. Prognostic value of KRAS mutation in patients undergoing pulmonary metastasectomy for colorectal cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;160:103308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Salah S, Watanabe K, Welter S, Park JS, Park JW, Zabaleta J, Ardissone F, Kim J, Riquet M, Nojiri K, Gisabella M, Kim SY, Tanaka K, Al-Haj Ali B. Colorectal cancer pulmonary oligometastases: pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann Oncol. 2012;23:2649-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Park HS, Jung M, Shin SJ, Heo SJ, Kim CG, Lee MG, Beom SH, Lee CY, Lee JG, Kim DJ, Ahn JB. Benefit of Adjuvant Chemotherapy After Curative Resection of Lung Metastasis in Colorectal Cancer. Ann Surg Oncol. 2016;23:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Zabaleta J, Aguinagalde B, Fuentes MG, Bazterargui N, Izquierdo JM, Hernández CJ, Enriquez-Navascués JM, Emparanza JI. Survival after lung metastasectomy for colorectal cancer: importance of previous liver metastasis as a prognostic factor. Eur J Surg Oncol. 2011;37:786-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Lin BR, Chang TC, Lee YC, Lee PH, Chang KJ, Liang JT. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol. 2009;16:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Onaitis MW, Petersen RP, Haney JC, Saltz L, Park B, Flores R, Rizk N, Bains MS, Dycoco J, D'Amico TA, Harpole DH, Kemeny N, Rusch VW, Downey R. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg. 2009;87:1684-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Welter S, Jacobs J, Krbek T, Poettgen C, Stamatis G. Prognostic impact of lymph node involvement in pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg. 2007;31:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Eckardt J, Licht PB. Thoracoscopic versus open pulmonary metastasectomy: a prospective, sequentially controlled study. Chest. 2012;142:1598-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Murakawa T, Sato H, Okumura S, Nakajima J, Horio H, Ozeki Y, Asamura H, Ikeda N, Otsuka H, Matsuguma H, Yoshino I, Chida M, Nakayama M, Iizasa T, Okumura M, Shiono S, Kato R, Iida T, Matsutani N, Kawamura M, Sakao Y, Funai K, Furuyashiki G, Akiyama H, Sugiyama S, Kanauchi N, Shiraishi Y; Metastatic Lung Tumor Study Group of Japan. Thoracoscopic surgery versus open surgery for lung metastases of colorectal cancer: a multi-institutional retrospective analysis using propensity score adjustment†. Eur J Cardiothorac Surg. 2017;51:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |