Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3142
Revised: August 8, 2024
Accepted: August 28, 2024
Published online: October 27, 2024
Processing time: 155 Days and 19.6 Hours
Anastomotic leakage (AL) is one of the severest complications after laparoscopic surgery for middle/low rectal cancer, significantly impacting patient outcomes. Identifying reliable predictive factors for AL remains a clinical challenge. Serum nutritional biomarkers have been implicated in surgical outcomes but are un
To determine the predictive value of preoperative serum nutritional biomarkers for rectal cancer AL following laparoscopic surgery.
In the retrospective cohort study carried out at a tertiary cancer center, we examined 560 individuals who underwent laparoscopic procedures for rectal cancer from 2018 to 2022. Preoperative serum levels of PA, ALB, and TRF were measured. We employed multivariate logistic regression to determine the independent risk factors for AL, and a predictive model was constructed and evaluated using receiver operating characteristic curve analysis.
AL occurred in 11.96% of cases, affecting 67 out of 560 patients. Multivariate analysis identified PA, ALB, and TRF as the independent risk factor, each with an odds ratio of 2.621 [95% confidence interval (CI): 1.582-3.812, P = 0.012], 3.982 (95%CI: 1.927-4.887, P = 0.024), and 2.109 (95%CI: 1.162-2.981, P = 0.031), respectively. Tumor location (< 7 cm from anal verge) and intraoperative bleeding ≥ 300 mL also increased AL risk. The predictive model demonstrated an excellent accuracy, achieving an area under the receiver operating characteristic curve of 0.942, a sensitivity of 0.844, and a specificity of 0.922, demonstrating an excellent ability to discriminate.
Preoperative serum nutritional biomarkers, combined with surgical factors, reliably predict anastomotic leakage risk after rectal cancer surgery, highlighting their importance in preoperative assessment.
Core Tip: This study establishes a robust predictive model for anastomotic leakage in middle/low rectal cancer patients undergoing laparoscopic surgery, leveraging preoperative serum levels of prealbumin, albumin, and transferrin. Demon
- Citation: Shayimu P, Awula M, Wang CY, Jiapaer R, Pan YP, Wu ZM, Chen Y, Zhao ZL. Serum nutritional predictive biomarkers and risk assessment for anastomotic leakage after laparoscopic surgery in rectal cancer patients. World J Gastrointest Surg 2024; 16(10): 3142-3154
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3142.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3142
Colorectal cancer (CRC) ranks among the most prevalent malignancies globally, posing a significant public health threat. According to World Health Organization reports, CRC is the third most common cancer worldwide and the second leading cause of cancer-related deaths[1,2]. Annually, more than 1.9 million new cases of CRC are diagnosed, leading to nearly 935000 deaths[3]. CRC not only imposes a substantial burden on patients both physically and psychologically but also exerts considerable economic pressure on the global health system. With advancements in medical technology, laparoscopic surgery has become an essential approach for treating middle and low rectal cancer. Compared to tra
AL is defined as the leakage of luminal contents from a surgical join through an unnatural passage into the abdominal cavity following abdominal surgery, especially following rectal cancer resection. The incidence of AL in laparoscopic surgery for rectal cancer is reported to vary between 3% to 15%, depending on surgical skill, individual patient dif
Serum nutritional biomarkers such as albumin (ALB), prealbumin (PA), and transferrin (TRF) are widely utilized to assess the nutritional status of patients and its effect on surgical outcomes[11]. These biomarkers are clinically significant due to their sensitivity and specificity for the early diagnosis of malnutrition and monitoring of treatment response. PA[12], a protein with a short half-life, reflects short-term changes in nutritional status, while ALB[13] and TRF[14] indicate mid- to long-term nutritional reserves. Studies have shown that low levels of these serum nutritional biomarkers are associated with lots of adverse clinical outcomes, including an increased risk of postoperative complications, extended hospital stays, and elevated mortality rates[15]. In the context of surgical operations, especially abdominal surgeries like resection for rectal cancer, nutritional status is crucial for patients’ postoperative recovery. Malnutrition not only affects wound healing but also lowers immune response, increases the risk of infection, and thereby promotes the occurrence of postoperative complications, including AL[16]. AL not only prolongs the recovery process but may also lead to the need for intensive care, reoperation, or even death. Therefore, preoperative assessment of serum nutritional biomarkers en
Here, we conducted a retrospective analysis of 560 patients receiving laparoscopic radical procedures for middle and low rectal cancer, including 67 cases with postoperative AL and 493 without. By comparing the serum nutritional bio
This study included 560 patients with middle/low rectal cancer who received laparoscopic radical surgery at the Affiliated Cancer Hospital of Xinjiang Medical University from December 2018 to December 2022. Inclusion criteria were: (1) Patients diagnosed with middle/low rectal cancer; (2) Age over 18 years; (3) Patients undergoing laparoscopic rectal cancer radical surgery; and (4) Patients with complete preoperative data and examinations. Exclusion criteria comprised: (1) Patients with a prior history of distant metastasis; (2) Patients who underwent radiotherapy or chemotherapy prior to surgery; (3) Patients with other major complications before surgery; and (4) Patients whose procedure was converted to open surgery. This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Xinjiang Medical University (Approval No. G-2021005), and all included patients were thoroughly briefed and signed consent forms.
The collected clinical data of patients mainly included basic information [gender, age, body mass index (BMI), comorbid conditions like diabetes and hypertension, smoking or drinking history], surgery-related factors (preoperative intestinal obstruction, preventive stoma, surgical time, intraoperative bleeding, and neoadjuvant treatment), tumor-related factors [tumor size, tumor location, and tumor node metastasis (TNM) stage], and preoperative hematological indicators [white blood cell (WBC), platelet (PLT), hemoglobin (Hb), C-reactive protein (CRP), PA, ALB, and TRF]. Smoking history was defined as a binary variable, indicating whether a patient had ever smoked at any point in their life, without quantifying the exposure in terms of pack-years or other indices. Preoperative hematological values were measured from fasting peripheral venous blood taken within 24 hours before surgery to accurately assess the nutritional status of middle/low rectal cancer patients undergoing laparoscopic radical procedures. Tumor location was determined by measuring the distance from the edge of the anus to the bottom of the tumor. Tumors were categorized into two groups: Those located less than 7 cm from the anal verge and those located 7 cm or more from the anal verge. This categorization was used to evaluate the impact of tumor location on the risk of AL.
We categorized patients into the AL group (n = 67) and the non-AL group (n = 493) based on the occurrence of AL within 2 weeks postoperatively. Diagnosis of AL was based on[26]: (1) Clinical presentation: Postoperative unexplained abdominal pain and distension, unexplained persistent fever, with or without signs of peritoneal irritation; (2) Drainage characteristics: A significant increase in the volume of pelvic drainage fluid, which may contain gas, pus, feces, etc.; (3) Digital rectal examination: The leakage site at the anastomosis can be palpated; and (4) Imaging examination: Ex
We performed statistical analysis by using SPSS 26.0 (IBM Corp, Armonk, NY, United States). All variable data which is continuous are presented as mean ± SD. Comparisons among three or more groups were conducted using the χ2 test, and the independent samples t-test was used to compare two groups. The risk factors for postoperative AL were analyzed using univariate and multivariate logistic regression models. Receiver operating characteristic (ROC) curves were used to analyze the predictive value of serum nutritional indicators for postoperative AL. The predictive model was built in R platform, and the “rms” package created nomograms[28,29]. The evaluation of serum nutritional indicators and pre
A total of 560 patients undergoing laparoscopic surgery for the middle and low rectal cancer were included, with 67 patients experiencing postoperative AL, resulting in an incidence rate of 11.96%. Table 1 compares the clinical and pathological characteristics between AL patients (n = 67) and non-AL patients (n = 493). Statistical analysis revealed no significant differences between the two groups regarding gender (P = 0.488) and age (P = 0.180), indicating these factors are not primary influencers of AL occurrence. A significant correlation was found between smoking history and the occurrence of AL (P = 0.002), suggesting smoking may increase the risk of postoperative AL. However, patients with comorbidities such as hypertension and diabetes did not show significant statistical differences (P = 0.140 and P = 0.958, respectively), implying these conditions have a limited impact on AL. The proportion of patients with preoperative intestinal obstruction in the AL group was much higher (P = 0.027), suggesting the presence of preoperative intestinal obstruction may require more cautious surgical strategies. Surgical time and intraoperative bleeding were also significant factors affecting the occurrence of AL, with surgery lasting over 180 minutes (P < 0.001) and intraoperative blood loss over 300 mL (P = 0.041) significantly increasing the risk of AL. Other clinical parameters, such as BMI, drinking history, neoadjuvant treatment, tumor size, TNM stage, tumor location, WBC, PLT, and CRP, revealed no statistical differences between AL and non-AL groups, indicating these factors have a minor effect on AL occurrence.
| Characteristics | AL (n = 67) | Non-AL (n = 493) | χ2 | P value |
| Gender | ||||
| Male | 37 | 250 | 0.481 | 0.488 |
| Female | 30 | 243 | ||
| Age | ||||
| < 60 | 19 | 181 | 1.794 | 0.180 |
| ≥ 60 | 48 | 312 | ||
| BMI (kg/m2) | ||||
| < 24 | 57 | 395 | 0.930 | 0.335 |
| ≥ 24 | 10 | 98 | ||
| Hypertension | ||||
| Yes | 21 | 114 | 2.178 | 0.140 |
| No | 46 | 379 | ||
| Diabetes | ||||
| Yes | 12 | 87 | 0.003 | 0.958 |
| No | 55 | 406 | ||
| Smoking history | ||||
| Yes | 19 | 68 | 9.536 | 0.002 |
| No | 48 | 425 | ||
| Drinking history | ||||
| Yes | 23 | 178 | 0.081 | 0.776 |
| No | 44 | 315 | ||
| Preoperative intestinal obstruction | ||||
| Yes | 11 | 40 | 4.914 | 0.027 |
| No | 56 | 453 | ||
| Preventive stoma | ||||
| Yes | 7 | 32 | 1.425 | 0.233 |
| No | 60 | 461 | ||
| Surgical time (minutes) | ||||
| < 180 | 39 | 417 | 27.133 | < 0.001 |
| ≥ 180 | 28 | 76 | ||
| Intraoperative bleeding (mL) | ||||
| < 300 | 58 | 461 | 4.189 | 0.041 |
| ≥ 300 | 9 | 32 | ||
| Neoadjuvant treatment | ||||
| Yes | 5 | 43 | 0.119 | 0.730 |
| No | 62 | 450 | ||
| Tumor size (cm) | ||||
| < 5 | 61 | 439 | 0.246 | 0.620 |
| ≥ 5 | 6 | 54 | ||
| TNM stage | ||||
| I | 50 | 397 | 1.454 | 0.483 |
| II | 12 | 72 | ||
| III | 5 | 24 | ||
| Tumor location (cm) | ||||
| < 7 | 30 | 159 | 4.138 | 0.042 |
| ≥ 7 | 37 | 334 | ||
| WBC (× 109/L) | ||||
| < 10 | 50 | 395 | 1.091 | 0.296 |
| ≥ 10 | 17 | 98 | ||
| Hb (g/L) | ||||
| < 130 (male) or 120 (female) | 8 | 56 | 0.033 | 0.855 |
| ≥ 130 (male) or 120 (female) | 59 | 437 | ||
| PLT (× 109/L) | ||||
| < 400 | 57 | 381 | 2.102 | 0.147 |
| ≥ 400 | 10 | 112 | ||
| CRP (mg/L) | ||||
| < 10 | 48 | 385 | 1.400 | 0.237 |
| ≥ 10 | 19 | 108 |
We compared preoperative serum nutritional indicators between AL (n = 67) and non-AL (n = 493) middle/rectal cancer patients after laparoscopic surgery. Results in Table 2 show that levels of PA, ALB, and TRF were significantly lower in the AL group, at 245.74 ± 55.18 mg/L vs 331.83 ± 67.12 mg/L, 32.19 ± 4.87 g/L vs 38.74 ± 5.62 g/L, and 2.24 ± 0.32 g/L vs 2.89 ± 0.54 g/L, respectively. T-tests confirmed these differences were statistically significant (P < 0.001), indicating a significant association between preoperative nutritional status and the occurrence of AL.
| Group | n | Serum nutritional factors (mean ± SD) | ||
| PA (mg/L) | ALB (g/L) | TRF (g/L) | ||
| AL | 67 | 245.74 ± 55.18 | 32.19 ± 4.87 | 2.24 ± 0.32 |
| Non-AL | 493 | 331.83 ± 67.12 | 38.74 ± 5.62 | 2.89 ± 0.54 |
| t | 10.045 | 9.086 | 9.621 | |
| P value | < 0.001 | < 0.001 | < 0.001 | |
In the study comparing preoperative serum nutritional indicators across different severity grades of AL in patients, we found that levels of PA, ALB, and TRF significantly decreased as the severity of AL increased, as shown in Table 3. The average levels of PA, ALB, and TRF for Grade A (minor leakage) patients were 312.04 mg/L, 36.81 g/L, and 2.76 g/L, respectively, while those for Grade C (severe leakage) patients dropped to 207.18 mg/L, 22.60 g/L, and 1.98 g/L. Statistical analysis showed these differences were highly significant (P < 0.001), revealing a significant correlation between the status of preoperative nutrition and the severity of AL, emphasizing the importance of preoperative nutritional assessment in predicting AL risk.
| Grade | n | Serum nutritional factors (mean ± SD) | ||
| PA (mg/L) | ALB (g/L) | TRF (g/L) | ||
| Grade A | 30 | 312.04 ± 56.61 | 36.81 ± 5.29 | 2.76 ± 0.41 |
| Grade B | 26 | 243.19 ± 54.28 | 30.72 ± 4.18 | 2.27 ± 0.34 |
| Grade C | 11 | 207.18 ± 43.66 | 22.60 ± 3.76 | 1.98 ± 0.27 |
| F | 19.801 | 39.323 | 23.173 | |
| P value | < 0.001 | < 0.001 | < 0.001 | |
In this study, univariate and multivariate logistic regression analyses were conducted to explore the risk factors for AL. As shown in Table 4, univariate analysis identified six factors significantly associated with an increased risk of AL. Specifically, the odds ratios (ORs) for PA, ALB, TRF, smoking history, preoperative intestinal obstruction, intraoperative bleeding ≥ 300 mL, and tumor location < 7 cm were 1.721 [95% confidence interval (CI): 1.271-2.198, P = 0.021], 1.594 (95%CI: 1.165-2.063, P = 0.017), 1.982 (95%CI: 1.366-2.598, P = 0.030), 1.369 (95%CI: 1.064-1.517, P = 0.032), 2.083 (95%CI: 1.695-3.206, P = 0.028), 1.436 (95%CI: 1.102-1.763, P = 0.021), and 1.498 (95%CI: 1.073-2.019, P = 0.012), respectively. However, after multivariate logistic regression analysis, only PA, ALB, TRF, intraoperative bleeding ≥ 300 mL, and tumor location < 7 cm were significantly associated with an increased risk of AL, with adjusted OR values of 2.621 (95%CI: 1.582-3.812, P = 0.012), 3.982 (95%CI: 1.927-4.887, P = 0.024), 2.109 (95%CI: 1.162-2.981, P = 0.031), 4.182 (95%CI: 2.108-5.482, P = 0.009), and 3.124 (95%CI: 1.779-4.215, P = 0.016), respectively. Notably, the significance of smoking history and preoperative intestinal obstruction was not maintained in the multivariate analysis, suggesting their effects may be modulated by other variables. Additionally, factors such as gender, age, BMI, hypertension, diabetes, drinking history, preventive stoma, surgical time, neoadjuvant treatment, tumor size, TNM stage, WBC, PLT, and CRP showed no sig
| Factors | Reference | Univariate | Multivariate | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||
| PA | Continuous variable | 1.721 | 1.271-2.198 | 0.021 | 2.621 | 1.582-3.812 | 0.012 |
| ALB | Continuous variable | 1.594 | 1.165-2.063 | 0.017 | 3.982 | 1.927-4.887 | 0.024 |
| TRF | Continuous variable | 1.982 | 1.366-2.598 | 0.030 | 2.109 | 1.162-2.981 | 0.031 |
| Gender | 0 = male, 1 = female | 0.982 | 0.764-1.175 | 0.281 | / | / | / |
| Age | 0 = < 60, 1 = ≥ 60 | 1.021 | 0.835-1.284 | 0.473 | / | / | / |
| BMI | 0 = < 24 kg/m2, 1 = ≥ 24 kg/m2 | 0.894 | 0.712-1.092 | 0.501 | / | / | / |
| Hypertension | 0 = No, 1 = Yes | 1.123 | 0.902-1.351 | 0.124 | / | / | / |
| Diabetes | 0 = No, 1 = Yes | 1.076 | 0.863-1.376 | 0.374 | / | / | / |
| Smoking history | 0 = No, 1 = Yes | 1.369 | 1.064-1.517 | 0.032 | 1.170 | 1.086-1.375 | 0.088 |
| Drinking history | 0 = No, 1 = Yes | 0.994 | 0.704-1.328 | 0.584 | / | / | / |
| Preoperative intestinal obstruction | 0 = No, 1 = Yes | 2.083 | 1.695-3.206 | 0.028 | 0.927 | 0.786-1.159 | 0.617 |
| Preventive stoma | 0 = No, 1 = Yes | 1.046 | 0.903-1.254 | 0.438 | / | / | / |
| Surgical time | 0 = < 180 minutes, 1 = ≥ 180 minutes | 1.098 | 0.916-1.328 | 0.330 | / | / | / |
| Intraoperative bleeding | 0 = < 300 mL, 1 = ≥ 300 mL | 1.436 | 1.102-1.763 | 0.021 | 4.182 | 2.108-5.482 | 0.009 |
| Neoadjuvant treatment | 0 = No, 1 = Yes | 1.121 | 0.921-1.328 | 0.287 | / | / | / |
| Tumor size | 0 = < 5 cm, 1 = ≥ 5 cm | 0.899 | 0.711-1.197 | 0.319 | / | / | / |
| TNM stage | 0 = I, 1 = II or III | 0.876 | 0.702-1.354 | 0.437 | / | / | / |
| Tumor location | 0 = ≥ 7 cm, 1 = < 7 cm | 1.498 | 1.073-2.019 | 0.012 | 3.124 | 1.779-4.215 | 0.016 |
| WBC | 0 = < 10 × 109/L, 1 = ≥ 10 × 109/L | 0.926 | 0.804-1.154 | 0.572 | / | / | / |
| PLT | 0 = < 400 × 109/L, 1 = ≥ 400 × 109/L | 0.957 | 0.726-1.368 | 0.439 | / | / | / |
| CRP | 0 = < 10 mg/L, 1 = ≥ 10 mg/L | 1.051 | 0.804-1.328 | 0.265 | / | / | / |
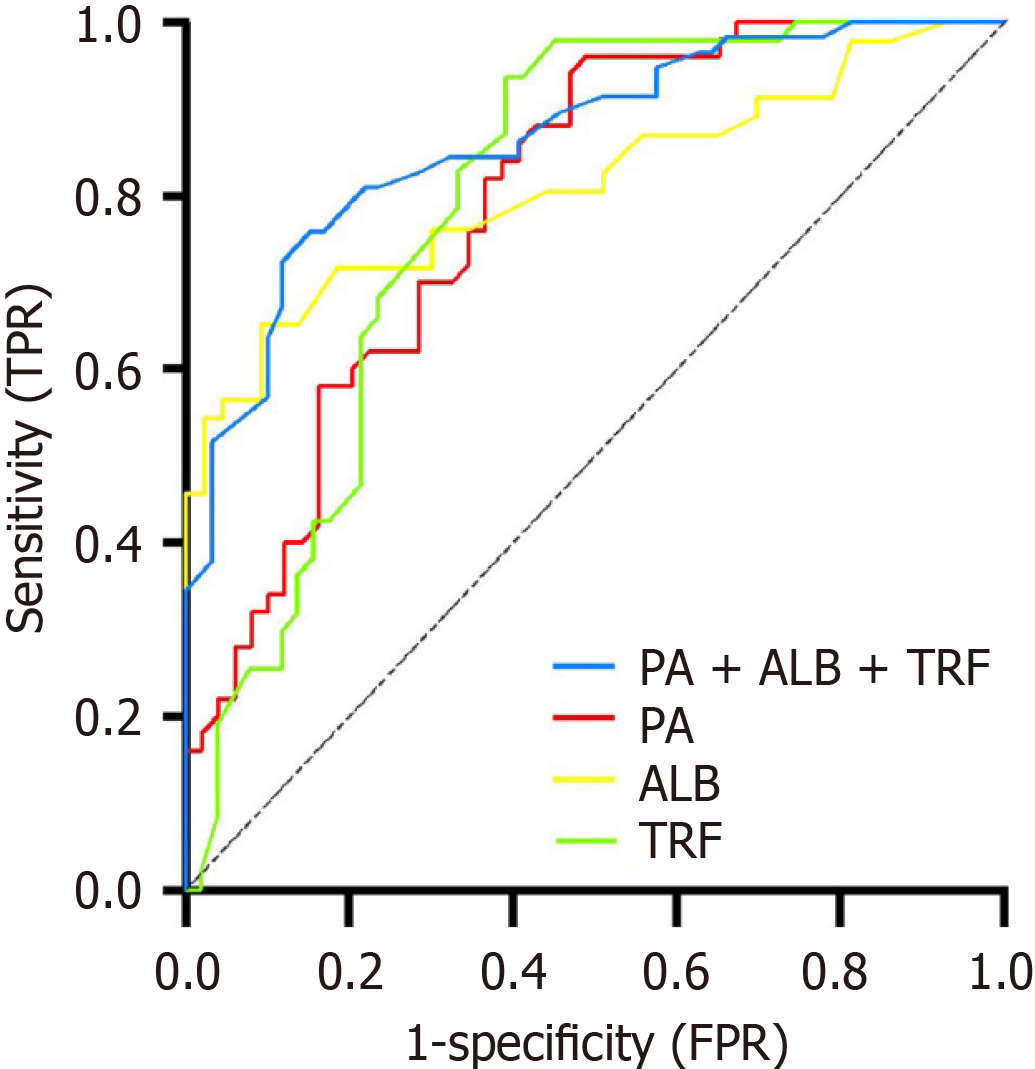
The diagnostic levels of the three preoperative serum nutritional biomarkers (PA, ALB, TRF) for AL occurrence, either independently or combined, were explored. ROC curve analysis showed that the optimal cutoff value for preoperative serum PA in predicting AL after laparoscopic surgery for middle and low rectal cancer was 288.50 mg/L, with an AUC of 0.790 (95%CI: 0.702-0.879), and corresponding sensitivity and specificity of 70.00% and 71.43%, respectively. The optimal cutoff value for preoperative serum ALB was 33.30 g/L, with an AUC of 0.812 (95%CI: 0.722-0.902), and corresponding sensitivity and specificity of 71.74% and 72.09%, respectively. The optimal cutoff value for preoperative serum TRF was 2.550 g/L, with an AUC of 0.793 (95%CI: 0.702-0.884), and corresponding sensitivity and specificity of 70.21% and 74.51%, respectively. The combined prediction of preoperative serum nutritional indicators (PA + ALB + TRF) for AL occurrence showed an AUC of 0.864 (95%CI: 0.800-0.929), with corresponding sensitivity and specificity of 81.03% and 77.97%, respectively. The value of combining preoperative serum PA, ALB, TRF for predicting postoperative AL exceeds that of any single indicator, which is shown in Figure 1.
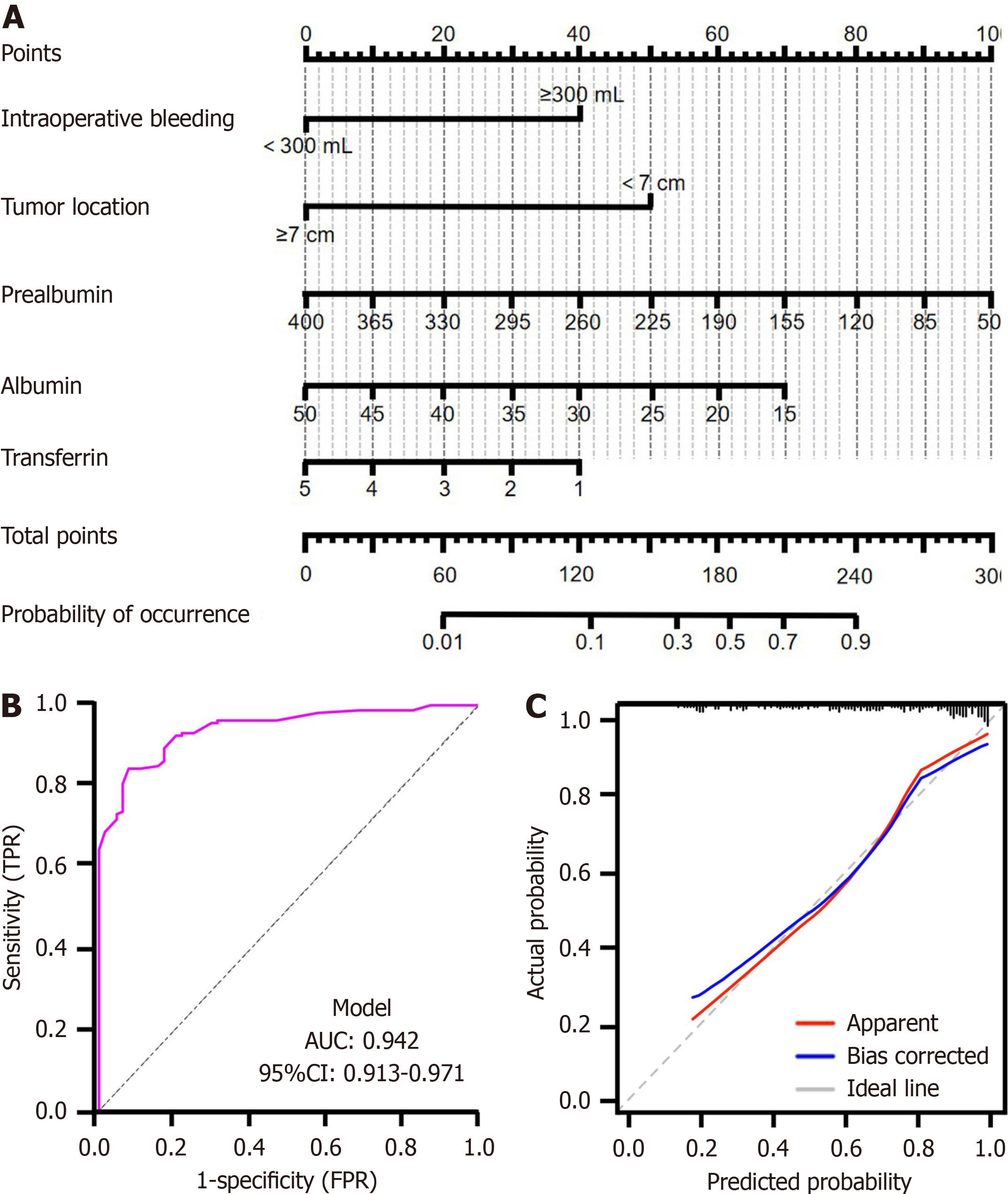
Based on the five independent risk factors for AL identified through multivariate logistic regression analysis (PA, ALB, TRF, intraoperative bleeding, and tumor location), we constructed a predictive model for rectal cancer AL risk following laparoscopic surgery, as shown in Figure 2A. The model demonstrated good predictive performance, established by the ROC curve analysis. The AUC of the model reached 0.942 (95%CI: 0.913-0.971), with a sensitivity of 0.844 and a specificity of 0.922, indicating high discriminative ability (Figure 2B). The calibration performance of the model was assessed through calibration plots. The calibration plot (Figure 2C) shows the calibration curve closely aligning with the 45-degree diagonal line, with a concordance index of 0.838 (95%CI: 0.780-0.897, P < 0.001), indicating a high degree of consistency between predicted probabilities and observed rates of AL. Furthermore, the Hosmer-Lemeshow test yielded a P value of 0.328, suggesting no significant discrepancy between model predictions and actual observations, thereby validating the model’s good calibration.
In this study, we conducted a statistical analysis on the incidence of AL following laparoscopic surgery in 560 middle/low rectal cancer patients. Although our study is retrospective, efforts were made to ensure group homogeneity by applying consistent inclusion criteria and standardizing surgical procedures across facilities. Additional analyses confirmed that demographic and clinical characteristics were comparable between AL and non-AL groups, with some differences observed in smoking history and intraoperative factors. No significant clustering of AL cases was found among specific surgeons or facilities. Out of 560 patients, 67 patients experienced AL postoperatively, marking an incidence rate of 11.96%. This rate is comparable to the previous report[30] in a cohort of 5398 rectal cancer patients (10.20%) and by Li et al[31] in 497 rectal cancer patients (10.26%), yet it is lower than the incidence reported by Peltrini et al[32] in 367 rectal cancer patients (17.4%). The incidence of AL in our study (11.96%) is comparable to the range reported in similar studies. Factors such as tumor location, intraoperative blood loss, and patient comorbidities contribute to this rate, aligning with established risk profiles for AL in laparoscopic rectal surgery. These findings suggest that the laparoscopic approach to rectal surgery does not elevate the risk of AL postoperatively in comparison to open surgery. Variations in these results may also be attributable to different inclusion criteria and patient populations. AL following radical resection for rectal cancer arises from numerous factors such as characteristics, tumor status, as well as surgical factors. Our results indicate that the tumor location, the volume of surgical blood loss, and preoperative hematological nutritional biomarkers have an influence on the occurrence of AL.
The influence of tumor location on the incidence of AL in middle and low rectal cancer has reached a consensus both domestically and internationally[33], and the risk of developing AL increases as the tumor’s proximity to the anal margin decreases[34-38]. Our study arrived at the same conclusion. This could be due to the larger wound created during resection when the tumor is nearer to the anus. Additionally, the damage to tissues and blood vessels caused by electrocoagulation during surgery can lead to exudation and bleeding, thereby reducing the blood supply to the anastomotic site and increasing the risk of postoperative AL[39]. Hb is associated with perfusion and oxygenation at the edges of the anastomosis, which are critical factors for the healing of the anastomosis. Hence, postoperative anemia due to excessive intraoperative blood loss has been described as a risk factor for leakage[40]. Multiple studies have found[41-43] that Hb levels below 110 g/L increase the AL risk after rectal cancer surgery, which can be attributed to the decreased capacity to transport oxygen to tissues and the subsequent risk of ischemia[44,45]. Both surgical blood loss and blood transfusion are independently associated with an increased risk of anastomotic failure[46]. Blood loss may lead to ischemia at the anastomotic site, impairing healing. Transfusion may induce immunosuppression, thereby increasing the risk of in
From a pathophysiological perspective, malnutrition can increase the risk of AL through various mechanisms, including impaired wound healing, diminished immune response, and elevated infection risk. Thus, nutritional status not only indirectly affects the speed of postoperative recovery and long-term prognosis but also directly impacts the occurrence of postoperative complications. PA and ALB are among the most thoroughly investigated nutritional bio
Beyond identifying the diagnostic potential of preoperative serum nutritional biomarkers for the risk of AL, our study meticulously developed and validated a predictive model based on these markers to forecast the risk of AL in patients with low and mid-rectal cancer undergoing laparoscopic surgery. The cornerstone of our discovery is the model’s robust performance, highlighted by an AUC of 0.942, demonstrating exceptional sensitivity and specificity. The ROC-AUC analysis was chosen not only due to its favorable results but because it is the most appropriate tool for evaluating the discriminative ability of our predictive model in the clinical context. Additionally, supplementary statistical evaluations (Hosmer-Lemeshow and NRI) confirmed the robustness of our findings. This achievement underscores the model’s capability in accurately differentiating between high-risk and low-risk patients for AL. Incorporating preoperative serum nutritional biomarkers (PA, ALB, and TRF) along with intraoperative blood loss and tumor location as predictive factors not only enhanced the model’s predictive accuracy but also aligned with the evolving understanding of AL’s mul
In this retrospective analysis, we leveraged clinical data from 560 middle/low rectal cancer patients undergoing laparoscopic surgery, focusing on preoperative serum nutritional biomarkers. We developed a predictive model that identifies patients at increased risk of developing AL based on PA, ALB, and TRF, achieving an AUC of 0.942. Highlighting the significant impact of nutritional status on AL risk, our study not only deepens the understanding of factors influencing surgical outcomes but also emphasizes the essential role of integrating nutritional assessments into preoperative planning. This strategy is poised to significantly improve patient care by enhancing postoperative recovery and po
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Wang Q, Huang X, Zhou S, Ding Y, Wang H, Jiang W, Xu M. IL1RN and PRRX1 as a Prognostic Biomarker Correlated with Immune Infiltrates in Colorectal Cancer: Evidence from Bioinformatic Analysis. Int J Genomics. 2022;2022:2723264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Yang L, Wang Q, He L, Sun X. The critical role of tumor microbiome in cancer immunotherapy. Cancer Biol Ther. 2024;25:2301801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 4. | Sawada N, Akagi T, Shimomura M, Todate Y, Nagakari K, Takeshita H, Maruyama S, Takata M, Ichikawa N, Hida K, Iijima H, Yamaguchi S, Taketomi A, Naitoh T; EnSSURE Study Group Collaboratives in Japan Society of Laparoscopic Colorectal Surgery. Evaluation of the advantage of surgeons certified by the endoscopic surgical skill qualification system participating in laparoscopic low anterior rectal resection. Ann Gastroenterol Surg. 2024;8:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Jiang WZ, Xu JM, Xing JD, Qiu HZ, Wang ZQ, Kang L, Deng HJ, Chen WP, Zhang QT, Du XH, Yang CK, Guo YC, Zhong M, Ye K, You J, Xu DB, Li XX, Xiong ZG, Tao KX, Ding KF, Zang WD, Feng Y, Pan ZZ, Wu AW, Huang F, Huang Y, Wei Y, Su XQ, Chi P; LASRE trial investigators. Short-term Outcomes of Laparoscopy-Assisted vs Open Surgery for Patients With Low Rectal Cancer: The LASRE Randomized Clinical Trial. JAMA Oncol. 2022;8:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Re AD, Tooza S, Diab J, Karam C, Sarofim M, Ooi K, Turner C, Kozman D, Blomberg D, Morgan M. Outcomes following anastomotic leak from rectal resections, including bowel function and quality of life. Ann Coloproctol. 2023;39:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Chen X, Jiang X, Wang H, Wang C, Wang C, Pan C, Zhou F, Tian J, Niu X, Nie Z, Chen W, Huang X, Pu J, Li C. DNA methylation-regulated SNX20 overexpression correlates with poor prognosis, immune cell infiltration, and low-grade glioma progression. Aging (Albany NY). 2022;14:5211-5222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 682] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 9. | Branagan G, Finnis D; Wessex Colorectal Cancer Audit Working Group. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum. 2005;48:1021-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 343] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 10. | Wu X, Lu W, Xu C, Jiang C, Zhuo Z, Wang R, Zhang D, Cui Y, Chang L, Zuo X, Wang Y, Mei H, Zhang W, Zhang M, Li C. Macrophages Phenotype Regulated by IL-6 Are Associated with the Prognosis of Platinum-Resistant Serous Ovarian Cancer: Integrated Analysis of Clinical Trial and Omics. J Immunol Res. 2023;2023:6455704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 11. | Keller U. Nutritional Laboratory Markers in Malnutrition. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 415] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 12. | Liang Y, Guo H, Man Q, Chang S, Wang E, Gao S. Prognostic nutritional score based on pretreatment lymphocyte, platelet, and prealbumin predicts prognosis in patients with pancreatic cancer. J Surg Oncol. 2023;128:831-843. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Yu Y, Huang Y, Li C, Ou S, Xu C, Kang Z. Clinical value of M1 macrophage-related genes identification in bladder urothelial carcinoma and in vitro validation. Front Genet. 2022;13:1047004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Ivanova TI, Klabukov ID, Krikunova LI, Poluektova MV, Sychenkova NI, Khorokhorina VA, Vorobyev NV, Gaas MY, Baranovskii DS, Goryainova OS, Sachko AM, Shegay PV, Kaprin AD, Tillib SV. Prognostic Value of Serum Transferrin Analysis in Patients with Ovarian Cancer and Cancer-Related Functional Iron Deficiency: A Retrospective Case-Control Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Cheng Z, Luo Y, Zhang Y, Wang Y, Chen Y, Xu Y, Peng H, Zhang G. A novel NAP1L4/NUTM1 fusion arising from translocation t(11;15)(p15;q12) in a myeloid neoplasm with eosinophilia and rearrangement of PDGFRA highlights an unusual clinical feature and therapeutic reaction. Ann Hematol. 2020;99:1561-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Peters EG, Smeets BJ, Dekkers M, Buise MD, de Jonge WJ, Slooter GD, Reilingh TS, Wegdam JA, Nieuwenhuijzen GA, Rutten HJ, de Hingh IH, Hiligsmann M, Buurman WA, Luyer MD. The effects of stimulation of the autonomic nervous system via perioperative nutrition on postoperative ileus and anastomotic leakage following colorectal surgery (SANICS II trial): a study protocol for a double-blind randomized controlled trial. Trials. 2015;16:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Yin L, Xie S, Chen Y, Li W, Jiang X, Li H, Li J, Wu Z, Xiao X, Zhang G, Cheng Z, Peng H. Novel germline mutation KMT2A G3131S confers genetic susceptibility to familial myeloproliferative neoplasms. Ann Hematol. 2021;100:2229-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Reference Citation Analysis (0)] |
| 18. | Kang B, Zhao ZQ, Liu XY, Cheng YX, Tao W, Wei ZQ, Peng D. Effect of hypoalbuminemia on short-term outcomes after colorectal cancer surgery: A propensity score matching analysis. Front Nutr. 2022;9:925086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Wang H, Jiang J, Cao X, Liu Q. Early decrease in postoperative serum albumin predicts severe complications in patients with colorectal cancer after curative laparoscopic surgery. World J Surg Oncol. 2018;16:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Chen Y, Li C, Wang N, Wu Z, Zhang J, Yan J, Wei Y, Peng Q, Qi J. Identification of LINC00654-NINL Regulatory Axis in Diffuse Large B-Cell Lymphoma In Silico Analysis. Front Oncol. 2022;12:883301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Zhang Z, Yu X, Zhou B, Zhang J, Chang J. Circular RNA circ_0026359 Enhances Cisplatin Resistance in Gastric Cancer via Targeting miR-1200/POLD4 Pathway. Biomed Res Int. 2020;2020:5103272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Chen Y, Zhang Y, Wang Z, Wang Y, Luo Y, Sun N, Zheng S, Yan W, Xiao X, Liu S, Li J, Peng H, Xu Y, Hu G, Cheng Z, Zhang G. CHST15 gene germline mutation is associated with the development of familial myeloproliferative neoplasms and higher transformation risk. Cell Death Dis. 2022;13:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Messias BA, Botelho RV, Saad SS, Mocchetti ER, Turke KC, Waisberg J. Serum C-reactive protein is a useful marker to exclude anastomotic leakage after colorectal surgery. Sci Rep. 2020;10:1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Wang XT, Kong FB, Mai W, Li L, He CG. Monitoring perioperative serum albumin can identify anastomotic leakage in colorectal cancer patients with curative intent. Asian J Surg. 2019;42:472-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Venn ML, Hooper RL, Pampiglione T, Morton DG, Nepogodiev D, Knowles CH. Systematic review of preoperative and intraoperative colorectal Anastomotic Leak Prediction Scores (ALPS). BMJ Open. 2023;13:e073085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Zhang H, Shi Y, Ying J, Chen Y, Guo R, Zhao X, Jia L, Xiong J, Jiang F. A bibliometric and visualized research on global trends of immune checkpoint inhibitors related complications in melanoma, 2011-2021. Front Endocrinol (Lausanne). 2023;14:1164692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Cong ZJ, Hu LH, Bian ZQ, Ye GY, Yu MH, Gao YH, Li ZS, Yu ED, Zhong M. Systematic review of anastomotic leakage rate according to an international grading system following anterior resection for rectal cancer. PLoS One. 2013;8:e75519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Degiuli M, Elmore U, De Luca R, De Nardi P, Tomatis M, Biondi A, Persiani R, Solaini L, Rizzo G, Soriero D, Cianflocca D, Milone M, Turri G, Rega D, Delrio P, Pedrazzani C, De Palma GD, Borghi F, Scabini S, Coco C, Cavaliere D, Simone M, Rosati R, Reddavid R; collaborators from the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Risk factors for anastomotic leakage after anterior resection for rectal cancer (RALAR study): A nationwide retrospective study of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Colorectal Dis. 2022;24:264-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 29. | Qin Q, Huang B, Wu A, Gao J, Liu X, Cao W, Ma T, Kuang Y, Guo J, Wu Q, Shao B, Guan Q, Yao H, Zhang X, Wang H; Chinese Radiation Intestinal Injury Research Group. Development and Validation of a Post-Radiotherapy Prediction Model for Bowel Dysfunction After Rectal Cancer Resection. Gastroenterology. 2023;165:1430-1442.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Liu Y, Chen Y, Wang F, Lin J, Tan X, Chen C, Wu LL, Zhang X, Wang Y, Shi Y, Yan X, Zhao K. Caveolin-1 promotes glioma progression and maintains its mitochondrial inhibition resistance. Discov Oncol. 2023;14:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Li HY, Zhou JT, Wang YN, Zhang N, Wu SF. Establishment and application of three predictive models of anastomotic leakage after rectal cancer sphincter-preserving surgery. World J Gastrointest Surg. 2023;15:2201-2210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Peltrini R, Carannante F, Costa G, Bianco G, Garbarino GM, Canali G, Mercantini P, Bracale U, Corcione F, Caricato M, Capolupo GT. Oncological outcomes of rectal cancer patients with anastomotic leakage: A multicenter case-control study. Front Surg. 2022;9:993650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 33. | Kim CW, Baek SJ, Hur H, Min BS, Baik SH, Kim NK. Anastomotic Leakage After Low Anterior Resection for Rectal Cancer Is Different Between Minimally Invasive Surgery and Open Surgery. Ann Surg. 2016;263:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Dong Y, Wu X, Xu C, Hameed Y, Abdel-Maksoud MA, Almanaa TN, Kotob MH, Al-Qahtani WH, Mahmoud AM, Cho WC, Li C. Prognostic model development and molecular subtypes identification in bladder urothelial cancer by oxidative stress signatures. Aging (Albany NY). 2024;16:2591-2616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Zhou S, Pei W, Li Z, Zhou H, Liang J, Liu Q, Zhou Z, Wang X. Evaluating the predictive factors for anastomotic leakage after total laparoscopic resection with transrectal natural orifice specimen extraction for colorectal cancer. Asia Pac J Clin Oncol. 2020;16:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Liu CA, Zhang Q, Ruan GT, Shen LY, Xie HL, Liu T, Tang M, Zhang X, Yang M, Hu CL, Zhang KP, Liu XY, Shi HP. Novel Diagnostic and Prognostic Tools for Lung Cancer Cachexia: Based on Nutritional and Inflammatory Status. Front Oncol. 2022;12:890745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 37. | Liu CA, Liu T, Li HC, Song MM, Ge YZ, Ruan GT, Deng L, Zhang Q, Xie HL, Lin SQ, Shi JY, Shi HP. Nutrition impact symptoms: Noteworthy prognostic indicators for lung cancer. Clin Nutr. 2023;42:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 38. | Tang T, Sun J, Li C. The role of Phafin proteins in cell signaling pathways and diseases. Open Life Sci. 2024;19:20220896. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Zheng H, Wu Z, Wu Y, Mo S, Dai W, Liu F, Xu Y, Cai S. Laparoscopic surgery may decrease the risk of clinical anastomotic leakage and a nomogram to predict anastomotic leakage after anterior resection for rectal cancer. Int J Colorectal Dis. 2019;34:319-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Mazza M, Crippa S, Pecorelli N, Tamburino D, Partelli S, Castoldi R, Balzano G, Falconi M. Duodeno-jejunal or gastro-enteric leakage after pancreatic resection: a case-control study. Updates Surg. 2019;71:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Mäkelä JT, Kiviniemi H, Laitinen S. Risk factors for anastomotic leakage after left-sided colorectal resection with rectal anastomosis. Dis Colon Rectum. 2003;46:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 42. | Liu Y, Zhao S, Chen Y, Ma W, Lu S, He L, Chen J, Chen X, Zhang X, Shi Y, Jiang X, Zhao K. Vimentin promotes glioma progression and maintains glioma cell resistance to oxidative phosphorylation inhibition. Cell Oncol (Dordr). 2023;46:1791-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 43. | Hayden DM, Mora Pinzon MC, Francescatti AB, Saclarides TJ. Patient factors may predict anastomotic complications after rectal cancer surgery: Anastomotic complications in rectal cancer. Ann Med Surg (Lond). 2015;4:11-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Lai R, Lu Y, Li Q, Guo J, Chen G, Zeng W. Risk factors for anastomotic leakage following anterior resection for colorectal cancer: the effect of epidural analgesia on occurrence. Int J Colorectal Dis. 2013;28:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Gu W, Li C, Shen T, Tong L, Yuan W, Zheng X, Wang T, Wang S, Zhu B, Zhang C, Zhang C. NAT1 inhibits liver metastasis of colorectal cancer by regulating EMT and glycolysis. Aging (Albany NY). 2024;16:10546-10562. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Jannasch O, Klinge T, Otto R, Chiapponi C, Udelnow A, Lippert H, Bruns CJ, Mroczkowski P. Risk factors, short and long term outcome of anastomotic leaks in rectal cancer. Oncotarget. 2015;6:36884-36893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Krarup PM, Jorgensen LN, Andreasen AH, Harling H; Danish Colorectal Cancer Group. A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal Dis. 2012;14:e661-e667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 48. | Kang CY, Halabi WJ, Chaudhry OO, Nguyen V, Pigazzi A, Carmichael JC, Mills S, Stamos MJ. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013;148:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 283] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 49. | Zhang F, Wu Z, Sun S, Fu Y, Chen Y, Liu J. POEMS syndrome in the 21st century: A bibliometric analysis. Heliyon. 2023;9:e20612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Li J, Zhu N, Wang C, You L, Guo W, Yuan Z, Qi S, Zhao H, Yu J, Huang Y. Preoperative albumin-to-globulin ratio and prognostic nutritional index predict the prognosis of colorectal cancer: a retrospective study. Sci Rep. 2023;13:17272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 51. | Telem DA, Chin EH, Nguyen SQ, Divino CM. Risk factors for anastomotic leak following colorectal surgery: a case-control study. Arch Surg. 2010;145:371-6; discussion 376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 52. | Li J, Wu Z, Pan Y, Chen Y, Chu J, Cong Y, Fang Q. GNL3L exhibits pro-tumor activities via NF-κB pathway as a poor prognostic factor in acute myeloid leukemia. J Cancer. 2024;15:4072-4080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 53. | Tsalikidis C, Mitsala A, Mentonis VI, Romanidis K, Pappas-Gogos G, Tsaroucha AK, Pitiakoudis M. Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going? Curr Oncol. 2023;30:3111-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 54. | Frasson M, Flor-Lorente B, Rodríguez JL, Granero-Castro P, Hervás D, Alvarez Rico MA, Brao MJ, Sánchez González JM, Garcia-Granero E; ANACO Study Group. Risk Factors for Anastomotic Leak After Colon Resection for Cancer: Multivariate Analysis and Nomogram From a Multicentric, Prospective, National Study With 3193 Patients. Ann Surg. 2015;262:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 55. | Herrinton LJ, Friedman GD, Baer D, Selby JV. Transferrin saturation and risk of cancer. Am J Epidemiol. 1995;142:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Sawayama H, Miyamoto Y, Hiyoshi Y, Shimokawa M, Kato R, Akiyama T, Sakamoto Y, Daitoku N, Yoshida N, Baba H. Preoperative transferrin level is a novel prognostic marker for colorectal cancer. Ann Gastroenterol Surg. 2021;5:243-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Ochiai T, Nishimura K, Watanabe T, Kitajima M, Nakatani A, Sato T, Kishine K, Futagawa S, Mashiko S, Nagaoka I. Mechanism underlying the transient increase of serum iron during FOLFOX/FOLFIRI therapy. Mol Clin Oncol. 2014;2:968-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Furukawa K, Onda S, Taniai T, Hamura R, Kumamoto T, Shirai Y, Yasuda J, Haruki K, Shiozaki H, Gocho T, Ikegami T. Transferrin predicts outcome in patients who underwent liver resection for colorectal liver metastases. Jpn J Clin Oncol. 2021;51:1400-1406. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 59. | Saeki H, Masuda T, Okada S, Ando K, Sugiyama M, Yoshinaga K, Endo K, Sadanaga N, Emi Y, Kakeji Y, Morita M, Yamashita N, Maehara Y. Impact of perioperative peripheral blood values on postoperative complications after esophageal surgery. Surg Today. 2010;40:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Kuvshinoff BW, Brodish RJ, McFadden DW, Fischer JE. Serum transferrin as a prognostic indicator of spontaneous closure and mortality in gastrointestinal cutaneous fistulas. Ann Surg. 1993;217:615-22; discussion 622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |