Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.29
Peer-review started: September 29, 2023
First decision: November 21, 2023
Revised: November 28, 2023
Accepted: December 19, 2023
Article in press: December 19, 2023
Published online: January 27, 2024
Processing time: 118 Days and 0.7 Hours
Due to the prolonged life expectancy and increased risk of colorectal cancer (CRC) among patients with human immunodeficiency virus (HIV) infection, the prognosis and pathological features of CRC in HIV-positive patients require examination.
To compare the differences in oncological features, surgical safety, and prognosis between patients with and without HIV infection who have CRC at the same tumor stage and site.
In this retrospective study, we collected data from HIV-positive and -negative patients who underwent radical resection for CRC. Using random stratified sampling, 24 HIV-positive and 363 HIV-negative patients with colorectal adenocarcinoma after radical resection were selected. Using propensity score matching, we selected 72 patients, matched 1:2 (HIV-positive:negative = 24:48). Differences in basic characteristics, HIV acquisition, perioperative serological indicators, surgical safety, oncological features, and long-term prognosis were compared between the two groups.
Fewer patients with HIV infection underwent chemotherapy compared to patients without. HIV-positive patients had fewer preoperative and postoperative leukocytes, fewer preoperative lymphocytes, lower carcinoembryonic antigen levels, more intraoperative blood loss, more metastatic lymph nodes, higher node stage, higher tumor node metastasis stage, shorter overall survival, and shorter progression-free survival compared to patients who were HIV-negative.
Compared with CRC patients who are HIV-negative, patients with HIV infection have more metastatic lymph nodes and worse long-term survival after surgery. Standard treatment options for HIV-positive patients with CRC should be explored.
Core Tip: This study aimed to compare the differences in oncological features, surgical safety, and prognosis between colorectal cancer (CRC) patients with and without human immunodeficiency virus (HIV) infection. HIV-positive patients with CRC had more metastatic lymph nodes and worse long-term survival compared to patients without HIV infection; however, the risk of surgery was not increased. To our knowledge, our series of 24 postoperative patients represents the largest reported study of HIV-positive patients with CRC.
- Citation: Yang FY, He F, Chen DF, Tang CL, Woraikat S, Li Y, Qian K. Oncological features and prognosis of colorectal cancer in human immunodeficiency virus-positive patients: A retrospective study. World J Gastrointest Surg 2024; 16(1): 29-39
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/29.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.29
Since the widespread application of highly active antiretroviral therapy (HAART) starting in 1996, the survival period for patients with Acquired Immune Deficiency Syndrome (AIDS) has significantly increased, and the incidence rates of AIDS-defining cancers, Kaposi’s sarcoma, non-Hodgkin lymphoma, and cervical cancer have significantly decreased[1-5]. However, the incidence rates of non-AIDS-defining cancers, such as colorectal cancer (CRC), liver cancer, lung cancer, anal cancer, and Hodgkin’s disease, have increased[6,7], in a manner related to the prolonged life expectancy of patients with AIDS. These non-AIDS-defining cancers account for an increasing number of deaths among carriers of human immunodeficiency virus (HIV). According to GLOBOCAN 2020[8] data, approximately 1.93 million new cases of CRC were recorded worldwide in 2020, ranking third in malignant tumors (10.0%), after only breast (11.7%) and lung cancer (11.4%). In addition, approximately 930,000 (9.4%) people died from CRC, ranking it as the second most fatal malignant tumor, after only lung cancer (18.0%). The global number of new cases and deaths from CRC is increasing yearly. Compared to the general population, patients with AIDS have an increased incidence rate of CRC and earlier age of invasion, and are diagnosed at more advanced stages of disease[9].
During routine performance of CRC resection in our institute, our team discovered more suspicious positive lymph nodes in CRC patients with HIV infection. However, reports considering differences in oncological features and prognoses between CRC patients with the same tumor stage and tumor site with and without HIV infection are rare. However, given the prolonged life expectancy and increased risk of CRC among HIV-positive patients, it is important to understand the prognosis and pathological features of CRC in HIV-positive patients. Therefore, in the present study, we aimed to compare the differences in oncological features, surgical safety, and prognoses between patients with and without HIV infection who had CRC at the same tumor stage and tumor site.
We extracted the clinical data of patients who were diagnosed with CRC complicated with HIV infection and underwent radical CRC resection between January 1, 2012, and March 31, 2022 at our institute. Twenty-four cases were retrieved. After analysis, we observed that the pathological classification of all HIV-positive patients with CRC was adenocarcinoma, and no preoperative neoadjuvant chemotherapy or radiotherapy was administered. However, because our hospital conducts more than 1000 radical CRC operations every year, to control the sample size, we used random stratified sampling to collect the data of 363 HIV-negative colorectal adenocarcinoma patients who had not received preoperative neoadjuvant chemotherapy or radiotherapy, and had undergone radical CRC resection. We collected data on demographic characteristics, basic preoperative profile, preoperative HIV treatment, perioperative serological indicators, surgical outcomes, oncological characteristics, and patient survival. The authors did not utilize any artificial intelligence tools.
Propensity score matching (PSM) analysis is widely used to minimize intervention or patient selection bias in non-randomized controlled studies and observational studies[10]. Herein, we used PSM to pair HIV-positive and -negative patients to reduce the impact of differences in baseline data between patients with and without HIV infection on the results, especially the effect on the number of metastatic lymph nodes. Before matching, we identified a cohort of patients with nearly 15 times as many patients without HIV infection as patients with HIV infection. However, matching at 1:1 would have resulted in substantial data loss and reduced the statistical power. Therefore, we applied 1:2 matching. Baseline data and variables that may affect the number of peri-intestinal lymph node metastases were applied to construct propensity scores, including age, sex, tumor site, degree of tumor differentiation, and tumor stage. The matching package was used to match the data for propensity scores, and 1:2 matching was adopted, with a caliper width limit of 0.1 SD of the logarithmic score. The matched groups were considered balanced if the standardized mean difference between them after matching was less than 0.1[11]. Categorical variables are expressed as frequencies (%), and continuous variables are expressed as medians (P25, P75). Categorical variables were analyzed using Fisher’s exact or Chi-square tests, and continuous variables were analyzed using the Mann-Whitney U test. Statistical significance was set at P < 0.05. The Kaplan-Meier method was used to compare the overall survival and progression-free survival between the two groups, and the log-rank test was used to determine whether the differences were significant. Calculation of propensity scores and selection of the matched cohort were performed using R version 4.0.2 (R Foundation for Statistical Computing, 2020) with the MatchIt package. Other statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, United States). A biomedical statistician performed a statistical review of the study.
Twenty-four patients with HIV and 363 patients without HIV infection were initially enrolled, of whom a total of 72 were matched by PSM (HIV-positive:negative = 24:48, Table 1). Although the differences in all variables were non-significant both before and after matching, the differences in baseline data, such as tumor site, degree of differentiation, tumor stage, and age, decreased after matching.
| Before PSM | After PSM | |||||
| HIV-negative (n = 363) | HIV-positive (n = 24) | P value | HIV-negative (n = 48) | HIV-positive (n = 24) | P value | |
| Sex | 1 | 1 | ||||
| Male | 211 (58.1) | 14 (58.3) | 29 (60.4) | 14 (58.3) | ||
| Female | 152 (41.9) | 10 (41.7) | 19 (39.6) | 10 (41.7) | ||
| Age (yr) | 66.3 ± 10.6 | 63.6 ± 12.0 | 0.283 | 64.3 ± 12.4 | 63.6 ± 12.0 | 0.808 |
| Tumor | 0.466 | 0.833 | ||||
| Proximal colon | 126 (34.7) | 7 (29.2) | 11 (22.9) | 7 (29.2) | ||
| Distal colon | 101 (27.8) | 5 (20.8) | 10 (20.8) | 5 (20.8) | ||
| Rectum | 136 (37.5) | 12 (50.0) | 27 (56.2) | 12 (50.0) | ||
| Degree of differentiation | 0.572 | 1 | ||||
| Low | 36 (9.9) | 3 (12.5) | 6 (12.5) | 3 (12.5) | ||
| Moderate | 316 (87.1) | 20 (83.3) | 40 (83.3) | 20 (83.3) | ||
| High | 11 (3.0) | 1 (4.2) | 2 (4.2) | 1 (4.2) | ||
| Tumor stage | 0.425 | 1 | ||||
| T1 | 18 (5.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| T2 | 53 (14.6) | 6 (25.0) | 13 (27.1) | 6 (25.0) | ||
| T3 | 200 (55.1) | 13 (54.2) | 25 (52.1) | 13 (54.2) | ||
| T4a | 73 (20.1) | 3 (12.5) | 7 (14.6) | 3 (12.5) | ||
| T4b | 19 (5.2) | 2 (8.3) | 3 (6.2) | 2 (8.3) | ||
After PSM, fewer patients with HIV received chemotherapy than those without [29.2% vs 62.5%, P = 0.008]; however, there were no significant differences in CRC family history, main complications, smoking, drinking, abdominal surgery history, body mass index, or adverse reactions to chemotherapy (Table 2). All patients with HIV infection had latent disease, and no opportunistic infections were recorded. Fifteen patients were diagnosed with HIV infection prior to admission, and others were found to have HIV infection during preoperative screening. Fourteen patients underwent HAART before admission. Most patients were infected with HIV through sexual transmission. The median time difference between HIV and CRC diagnosis was 32 mo (range: 1–192 mo), the median CD4+ cell count before surgery was 459 cells/mm3 (range: 158–1090 cells/mm3), and the CD4+/CD8+ median was 0.71 (range: 0.22–2.10). Follow-up of patients with AIDS showed that only one patient was not medication adherent.
| HIV-positive (n = 24) | HIV-negative (n = 48) | P value | |
| CRC family history | 1 (4.2) | 5 (10.4) | 0.656 |
| Main comorbidity | 8 (33.3) | 18 (37.5) | 0.729 |
| Hypertension | 4 (16.7) | 11 (22.9) | 0.538 |
| Diabetes mellitus | 2 (8.3) | 8 (16.7) | 0.479 |
| CHD | 4 (16.7) | 5 (10.4) | 0.469 |
| COPD | 0 | 2 (4.2) | 0.549 |
| Drinking | 6 (25) | 16 (33.3) | 0.469 |
| Smoking | 4 (16.7) | 11 (22.9) | 0.538 |
| Abdominal surgery history | 5 (20.8) | 8 (16.7) | 0.749 |
| BMI (kg/m2) | 22.06 (19.71, 23.95) | 23.02 (21.03, 25.17) | 0.074 |
| Chemotherapy | 7 (29.2) | 30 (62.5) | 0.008 |
| Adverse reactions of chemotherapy | 2 (28.6) | 15 (50) | 0.416 |
| Fever1 | 0 | 0 | NA |
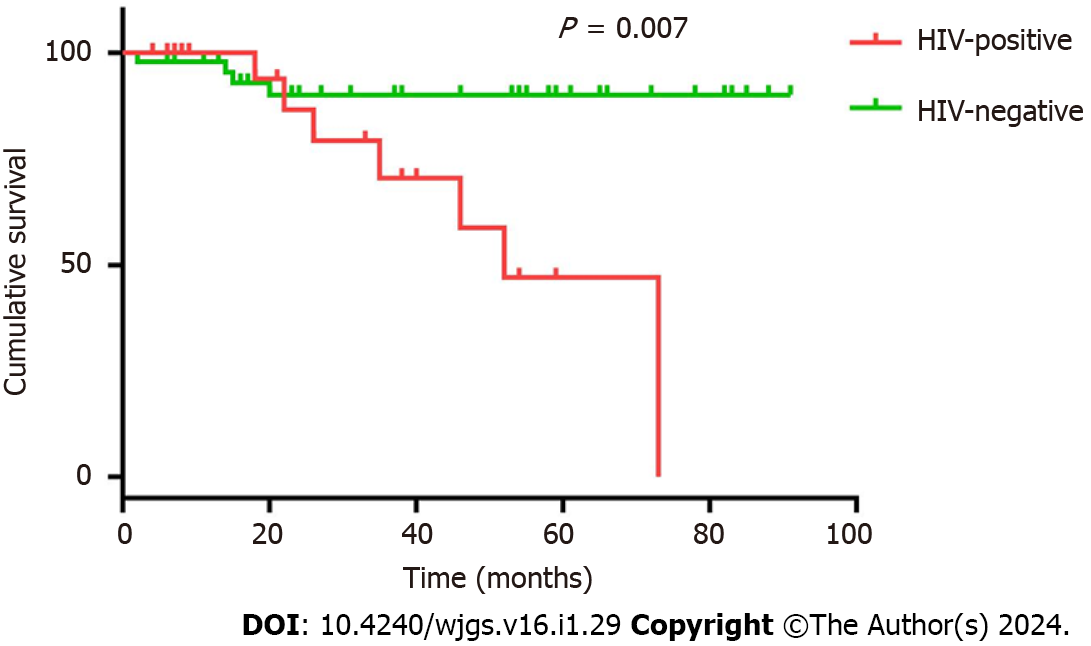
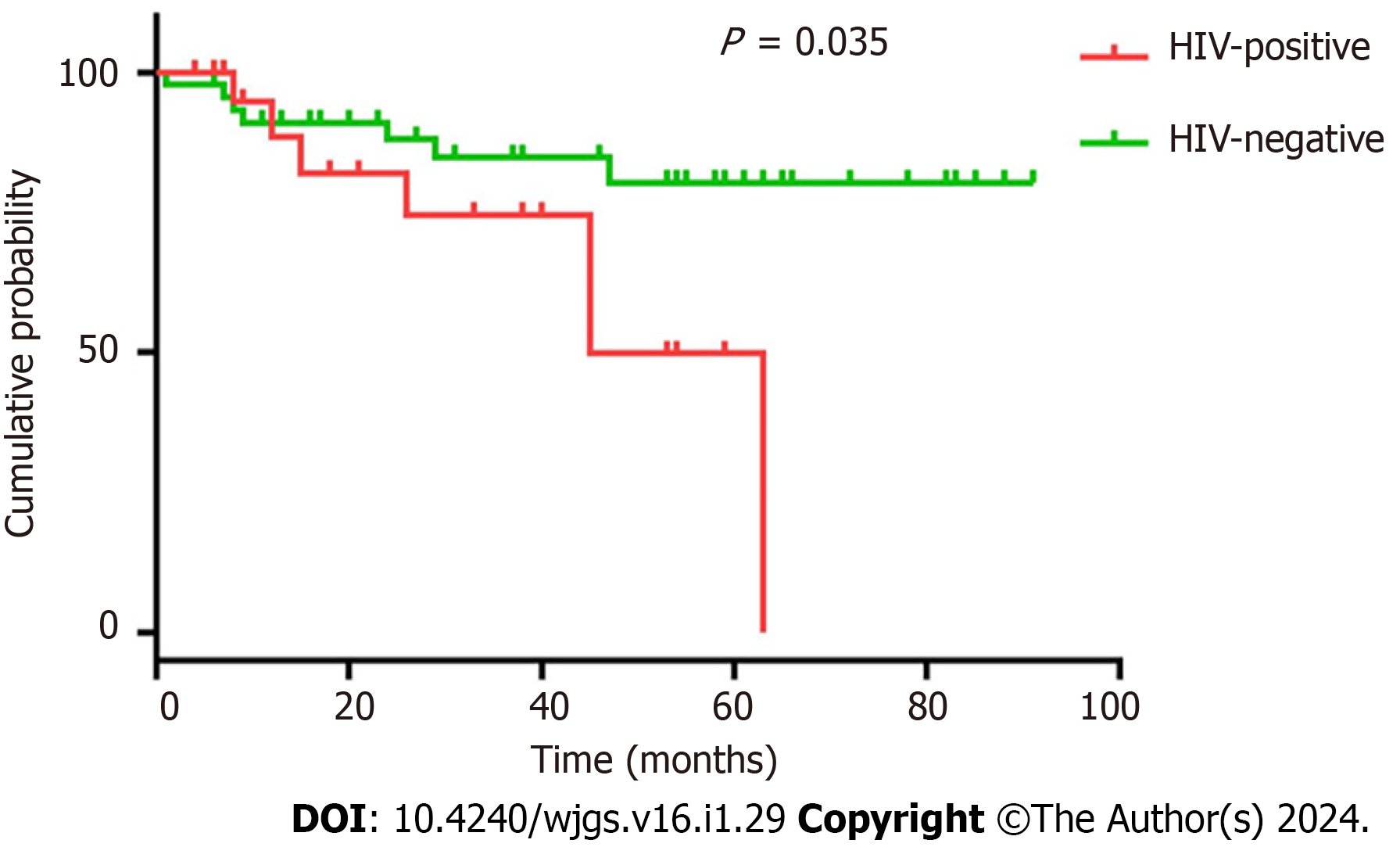
Table 3 presents the major peripheral venous blood indicators of the 72 patients. Compared to patients without HIV infection, patients with HIV infection had fewer preoperative leukocytes [5.36 (3.85, 6.70) vs 5.92 (4.95, 7.50), P = 0.49], postoperative leukocytes [6.91 (5.36, 8.84) vs 8.98 (6.97, 10.89), P = 0.013], and preoperative lymphocytes [1.19 (0.77, 1.48) vs 1.48 (1.18, 1.90), P = 0.028] and lower carcinoembryonic antigen (CEA) levels [2.27 (1.38, 3.10) vs 5.44 (2.90, 20.00), P = 0.012]. No significant differences were observed in preoperative or postoperative hemoglobin, postoperative lymphocytes, preoperative albumin, postoperative albumin and carbohydrate antigen 19-9 levels, or American Society of Anesthesiologists score. Surgical safety between the HIV-positive and -negative groups is shown in Table 4. Patients with HIV infection experienced greater intraoperative blood loss than those without [100 (50, 100) vs 50 (35, 100), P = 0.46]; however, no significant difference was observed between the two groups in terms of operation time, time to first flatus, time to first defecation, time to first liquid intake, time to ambulation, postoperative hospital stay, postoperative complications, admission to intensive care unit, time to discontinuing antibiotics, hospitalization expenses, performance status 1 mo after operation, long-term postoperative gastrointestinal discomfort, decrease in hemoglobin or albumin levels, intraoperative blood transfusion, enterostomy, or American Society of Anesthesiologists score. Two patients in each group had distant metastasis prior to the operation, one patient in the HIV-positive group had liver and lung metastasis, and three patients had only liver metastasis. No readmissions or deaths were recorded within 1 mo in either group. The median follow-up time for both HIV-positive patients and matched controls was 31 mo (2–91). Ten patients (41.7%) died (9 from cancer and 1 from other causes) in the HIV-positive group, while seven (14.6%) died in the control group (6 from cancer and 1 from other causes). CRC patients with HIV infection had a reduced overall survival (26 mo vs 37 mo, respectively) and progression-free survival (23.5 mo vs 37 mo, respectively) compared with the matched controls. The differences in overall survival (P = 0.007) (Figure 1) and progression-free survival (P = 0.035) (Figure 2) between the two groups were significant. However, it should be noted that two missing patients were recorded in each group at the postoperative follow-up.
| HIV-positive (n = 24) | HIV-negative (n = 48) | P value | |
| Preoperative leukocytes (109) | 5.36 (3.85, 6.70) | 5.92 (4.95, 7.50) | 0.049 |
| Postoperative leukocytes (109) | 6.91 (5.36, 8.84) | 8.98 (6.97, 10.89 | 0.013 |
| Preoperative hemoglobin (g/L) | 123.50 (101.25, 139.75) | 128.00 (120.00, 140.00) | 0.229 |
| Postoperative hemoglobin (g/L) | 121.50 (96.00, 127.00) | 119.00 (107.25, 125.25) | 0.976 |
| Preoperative lymphocytes (109) | 1.19 (0.77, 1.48) | 1.48 (1.18, 1.90) | 0.028 |
| Postoperative lymphocyte | 0.75 (0.49, 0.98) | 0.79 (0.61, 1.07) | 0.685 |
| Preoperative albumin (g/L) | 43.00 (37.00, 45.00) | 41.00 (38.00, 44.00) | 0.807 |
| Postoperative albumin (g/L) | 32.00 (28.00, 36.00) | 29.00 (25.00, 34.75) | 0.087 |
| CEA (μg/L) | 2.27 (1.38, 3.10) | 5.44 (2.90, 20.00) | 0.012 |
| CA19-9 (U/mL) | 13.96 (5.40, 20.85) | 15.00 (6.99, 34.68) | 0.983 |
| HIV-positive (n = 24) | HIV-negative (n = 48) | P value | |
| Intraoperative blood loss (mL) | 100 (50, 100) | 50 (34, 100) | 0.046 |
| Operation time (min) | 187.50 (151.25, 227.25) | 195.00 (144.75, 240.00) | 0.77 |
| Time to first flatus (d) | 3 (2, 4) | 3 (2.25, 6) | 0.668 |
| Time to first defecation (d) | 3 (1.25, 4.75) | 3 (2, 6) | 0.257 |
| Time to first liquid intake (d) | 2.5 (2, 3) | 3 (2.25, 4) | 0.064 |
| Time to ambulation (d) | 3 (3, 6.25) | 3.5 (3, 4) | 0.98 |
| Postoperative hospital stay (d) | 9 (7, 13.25) | 8 (7, 11) | 0.655 |
| Postoperative complications | 3 (12.5) | 5 (10.4) | 1.000 |
| Admission to ICU | 1 (4.2) | 9 (18.8) | 0.149 |
| Time to stopping antibiotics (days) | 2 (1, 2.75) | 2 (1, 3.75) | 0.679 |
| Hospitalization expenses (thousand yuan) | 78.7 (65.3, 88.6) | 81.4 (67.5, 134.1) | 0.173 |
| PS at 1 mo after operation | 1 (1, 1) | 1 (1, 1) | 0.48 |
| Long-term postoperative gastrointestinal discomfort | 8 (33.3) | 12 (25) | 0.457 |
| Decrease in hemoglobin (g/L) | 11 (0.5, 18.75) | 12 (4, 22) | 0.385 |
| Decrease in albumin (g/L) | 9.28 (4.75, 13.28) | 12 (7, 15) | 0.071 |
| Intraoperative blood transfusion | 1 (4.2) | 1 (2.1) | 1.000 |
| Enterostomy | 7 (29.2) | 10 (20.8) | 0.433 |
| Readmission within 1 mo | 0 | 0 | NA |
| Death within 1 mo | 0 | 0 | NA |
| ASA score | 3 (2, 3) | 2 (2, 3) | 0.713 |
Patients with HIV infection had more lymph node metastases than patients without [1 (0, 3.5) vs 0 (0, 0), P = 0.001], higher node stage [1 (0, 1.75) vs 0 (0,1), P = 0.005], as well as higher tumor node metastasis (TNM) stage [3 (2, 3) vs 2 (2, 2.75), P = 0.004], whereas the harvested lymph nodes, size of the largest lymph node, metastasis, tumor size, microsatellite instability, RAS gene mutations, BRAF gene mutations, MLH1, MSH2, MSH6, and Ki-67 showed no significant differences. No cases with positive margins were recorded in either group (Table 5). Table 6 shows the number of metastatic lymph nodes, node stage, and TNM stage in patients at different tumor stages.
| HIV-positive (n = 24) | HIV-negative (n = 48) | P value | |
| Number of metastatic lymph nodes | 1 (0, 3.5) | 0 (0, 0) | 0.001 |
| Node stage | 1 (0, 1.75) | 0 (0, 1) | 0.005 |
| Harvested lymph nodes | 13 (10, 15.75) | 14.5 (11.25, 18) | 0.223 |
| Size of largest lymph node | 0.85 (0.525, 1.45) | 0.8 (0.5, 1) | 0.318 |
| Metastasis | 2 (8.3) | 2 (4.2) | 0.597 |
| TNM stage | 3 (2, 3) | 2 (2, 2.75) | 0.004 |
| Tumor size (cm) | 3.75 (2.5, 5) | 4 (3, 5) | 0.497 |
| Margin | 0 | 0 | NA |
| MSI | 1 (11.1) | 1 (6.3) | 1 |
| RAS gene mutation | 3 (37.5) | 13 (76.5) | 0.087 |
| BRAF gene mutation | 0 | 1 (5.8) | 1 |
| MLH1 | 14 (82.4) | 35 (92.1) | 0.359 |
| MSH2 | 16 (94.1) | 37 (97.4) | 0.527 |
| MSH6 | 16 (94.1) | 37 (97.4) | 0.527 |
| Ki-67 (%) | 70 (60, 80) | 60 (50, 80) | 0.159 |
| T2 (n = 19) | T3 (n = 38) | T4a (n = 10) | T4b (n = 5) | |
| Number of metastatic lymph nodes | ||||
| HIV-positive | 1 (0, 1.25) | 1 (0, 3.5) | 0 (0, -) | 6 (4, -) |
| HIV-negative | 0 (0, 0) | 0 (0, 0) | 0 (0, 3) | 1 (0, -) |
| Node stage (0/I/II) | ||||
| HIV-positive | 2/4/0 | 5/5/3 | 2/0/1 | 0/0/2 |
| HIV-negative | 10/3/0/0 | 18/4/3 | 5/1/1 | 1/2/0 |
| TNM stage (I/II/III/IV) | ||||
| HIV-positive | 2/0/4/0 | 0/5/8/0 | 0/2/1/0 | 0/0/0/2 |
| HIV-negative | 11/0/2/0 | 0/20/4/1 | 0/5/2/0 | 0/0/2/1 |
To our knowledge, no studies have yet reported any differences in postoperative pathological features between patients with a combination of HIV infection and CRC and patients with CRC alone at the same tumor stage and tumor site. In this study, after matching factors that may affect lymph node metastasis in CRC using PSM, by comparing the oncological characteristics, surgical safety, and prognosis of the two groups of patients, we discovered that CRC patients with HIV infection had significantly more lymph node metastases than patients without (Table 5). This disparity may be related to the immunosuppression observed in patients with HIV infection. In addition, patients with HIV infection had higher node stage and TNM stage than patients without. Regarding surgical outcomes, although patients with HIV infection had more intraoperative blood loss than patients without, the difference in the decrease in hemoglobin levels between the two groups was not significant. The significant increase in intraoperative blood loss in patients with HIV infection may be etiologically related to AIDS-defining illnesses, other comorbidities, lifestyle, and etiologies related to underlying HIV infection[12,13], while intraoperative blood loss was the expected value for the attending surgeon. Therefore, we concluded that the surgical safety of radical CRC surgery in patients with HIV infection was not worse than that of patients without. However, the overall survival and progression-free survival were shorter in patients with HIV infection.
The CEA levels of CRC patients with HIV infection were lower than those of patients without. Normal and cancerous tissues produce approximately the same amount of CEA[14,15], with healthy adults excreting approximately 50–70 mg of CEA daily in their feces[14]. Most of the CEA produced by the human body is excreted through the intestine. CEA has been indicated to function in innate immunity[16,17] and to prevent microorganisms from invading the intestinal epithelial cells[17]. However, owing to immune deficiency, the intestinal mucosa of people with HIV infection has decreased resistance to intestinal flora, resulting in a greater release of CEA into the intestine to resist microorganisms. Conversely, blood CEA levels decrease[16,17].
The incidence of CRC in China has increased from 17.1/100000 in 2013 to 26.4/100000 in 2020[8,18]; thus, we estimated that the total incidence rate of CRC in China in the last 10 years was 220/100000. Regarding HIV infection, approximately 64000 patients with AIDS survived, and 21000 died in our region as of October 2022. Therefore, we estimated that approximately 140 patients with AIDS and CRC would have been diagnosed in our region over the past 10 years. Admittedly, this estimation method is inaccurate, as we did not consider the influence of AIDS on the incidence of CRC and the different incidences of CRC in different regions of China. However, as we were unable to access data on HIV-positive patients with CRC in our region, we used this rough method for estimation.
According to our hospital data, 65 cases of HIV-positive patients with colorectal adenocarcinoma were recorded during the study period; considering that some patients were not treated, we believe that our hospital admitted more than half of the patients with HIV infection and CRC in our province, which is a relatively high proportion. Although the sample size of the HIV-positive group was only 24, which was limited by the stringent inclusion criteria and low incidence of AIDS, we believe that the sample size of our study is relatively large compared to those previously published in the literature. The prior study by Wasserberg et al[19] included only 11 HIV-positive patients with CRC, some of whom did not undergo surgery. Another study[20] comparing the clinical presentation and prognosis of patients with and without HIV infection included 27 patients with HIV infection and CRC, of whom four HIV-positive patients underwent surgery. Thus, our series of 24 postoperative patients represents the largest study of patients with HIV infection and CRC reported in the literature to date.
Whether HIV infection increases the risk of CRC remains controversial. Most studies suggest that HIV infection decreases immunity even though HARRT increases life expectancy in patients with HIV infection, leading to an increased risk of malignancy[6,7,21,22]. Some studies have reported no difference in CRC prevalence between patients with and without HIV infection[23]. Conversely, some have suggested that patients with HIV infection have a lower risk of CRC[24]. Reinhold et al[21] previously discovered that patients with HIV infection were less likely to undergo CRC screening tests than uninfected patients. This may account for the lower risk of CRC reported by some studies in patients with HIV infection.
In addition, similar to the report of Suneja et al[25,26], we observed that patients with HIV infection were less likely to undergo chemotherapy than patients without. Differences in access to cancer treatment may partially explain the shorter survival of patients with HIV infection and cancer. Suneja et al[25,26] suggested that many treatment providers may believe that patients with HIV infection are in poorer organismal condition, meaning that they will be less tolerant of treatment, and less likely to adhere to treatment regimens than patients without, thus reducing their chances of receiving systemic therapy. In addition, the lack of specific treatment guidelines for patients with HIV infection and cancer is an important reason for the low proportion of patients with HIV infection receiving systemic therapy[26]. From the patient’s perspective, those with HIV infection may be more reluctant to receive systemic therapy for oncology because of concerns about the side-effects of chemotherapy, an inadequate understanding of the need for cancer treatment, or the burden of the dual management of cancer and HIV infection[25]. However, there may be additional medical reasons why patients with HIV infection and CRC have a worse long-term prognosis than those without. Further high-quality studies are needed to explore these reasons.
Available data suggest that CRC patients with HIV infection are more severely ill and younger than those without[9]. In one study, Berretta et al[20] compared the clinical presentation and outcomes of 27 CRC patients with HIV infection and 54 age- and sex-matched CRC controls and concluded that patients with HIV infection had poorer performance status and unfavorable Dukes stages. Further, Bini et al[9] published the results of a screening colonoscopy study in which the prevalence of colon cancer was assessed in 136 asymptomatic CRC patients with HIV infection who were ≥ 50 years old and 272 asymptomatic uninfected controls with CRC matched by age, sex, and CRC family history. The authors discovered that the prevalence of neoplastic lesions was significantly higher in patients with HIV infection than in controls, even after adjusting for potential confounding variables. In the present study, although we eliminated the age difference after PSM, patients with HIV infection still had significantly more metastatic lymph nodes than patients without (Table 5), while overall survival and progression-free survival were significantly shorter in patients with HIV infection than in those without (Figures 1 and 2). This result is consistent with the findings of Berretta et al[20].
André et al[27] and Berretta et al[28] both concluded that the combination of HAART did not increase the toxicity of FOLFOX4. Currently, the advantages of immunotherapy are being gradually explored. Patients with HIV infection have reduced immunity, regardless of the CD4+ T-cell count; thus, because of the fear of increased HIV viral replication and increased toxicity in the presence of T-cell activation[29], they are usually excluded from trials of immune checkpoint inhibitors, and we currently lack data on the efficacy of immunotherapy in this population. The safety and efficacy of immunotherapy for HIV-infected patients with malignancies remain unclear. The phase 1 trial by Uldrick et al[30] revealed that PD-1 monoclonal antibodies are safe for use in patients with HIV infection taking HAART with CD4+ T-cell counts above 100 cells/μL. In addition, the results of Cao et al[31] demonstrated that PD-L1/PD-1 interactions may induce an immune environment favorable for tumor development.
Chemotherapy and immunotherapy have a better safety profile during CRC treatment in patients with well-controlled HIV infection; however, caution should be exercised when treating patients with more severe disease and advanced immunosuppression. Notably, HAART with prophylaxis for opportunistic infections should be administered during treatment, and patients should be closely monitored for CD4+ T-cells and serum viral levels. Nonetheless, the reasons for poorer CRC prognoses in patients with HIV infection are unclear, with more advanced diagnosis, inadequate treatment[32], and decreased immune function being possibilities .
Although this was a retrospective study, we used PSM to reduce the differences in baseline data between the two patient groups and to minimize the impact of baseline differences on the outcomes. This allowed better comparison of the postoperative oncological characteristics, surgical safety, and prognosis of patients with and without HIV infection who had CRC at the same tumor stage and site treated by radical resection. Therefore, we believe that the methodology of our study is scientific and that the conclusions are reliable and meaningful. However, we were unable to obtain specific data on AIDS-related symptoms and preoperative or postoperative HAART treatment. As mentioned earlier, patients with HIV infection are more reluctant to receive postoperative adjuvant therapy for malignancies. In addition, standard treatment protocols may not be possible in patients with poorly controlled HIV infection. Therefore, clinicians should direct their attention towards providing patients with prompt treatment, and research should be directed at the rapid development of appropriate treatments. Moreover, the treatment process should be monitored. We hope that more in-depth studies will be conducted to focus on the efficacy and safety of adjuvant therapy in malignant tumors, to further clarify the interactions of HIV with malignant tumors, and to develop more appropriate treatment plans.
This study had some limitations which should be mentioned. First, although our study had a larger sample size than most AIDS-related clinical studies, the sample size of this study is small compared to that of studies examining the relationship between common diseases and CRC, as it was limited by the low prevalence of AIDS. Second, we did not analyze the relationship between the severity of HIV infection and the prognosis of CRC in the HIV-positive group. Third, because of the small sample size of the two groups of patients with different tumor stages (Table 6), their differences could not be compared. Fourth, we did not specifically analyze the differences in the regimen and cycles of chemotherapy treatments between the two groups of patients. Studies with larger sample sizes are required to further reveal the impact of HIV infection on the oncologic characteristics, prognosis, and safety of surgery in CRC.
Compared to CRC patients without HIV infection, HIV-positive patients with CRC with the same stage and site have a higher number of lymph node metastases and worse postoperative long-term survival; however, surgical risks are not increased. Overall, patients with HIV infection and CRC have a worse prognosis. Therefore, clinicians should focus on treating this population more aggressively and should explore standard treatment options for them. We look forward to further studies on HIV-associated malignancies.
Human immunodeficiency virus (HIV) infection may accelerate the progression of colorectal cancer (CRC). Given the prolonged life expectancy and increased risk of CRC among patients with HIV infection, the prognosis and pathological features of CRC in this population should be examined. This study aimed to compare the differences in oncological features, surgical safety, and prognosis between CRC patients with and without HIV infection.
Differences in oncological features and prognoses between HIV-positive and -negative patients at the same stage and site have rarely been reported.
To compare the oncological characteristics, surgical safety, and prognoses between HIV-positive and -negative patients at the same stage and site.
In this study, after matching the two patient groups for factors that may affect lymph node metastasis in CRC using propensity score matching (PSM), we compared the oncological characteristics, surgical safety, and prognosis of the two groups of patients. Then, Fisher’s exact, Chi-square, and Mann–Whitney U tests were applied to conduct statistical analyses on the demographic characteristics, basic preoperative profile, preoperative HIV treatment, perioperative serological indicators, surgical outcomes, oncological characteristics, and survival of the two groups of patients.
Compared to patients without HIV infection, patients with HIV infection were more reluctant to receive chemotherapy. Clinically, this group of patients had fewer preoperative and postoperative leukocytes, fewer preoperative lymphocytes, lower carcinoembryonic antigen levels, more intraoperative blood loss, more metastatic lymph nodes, higher node stage, higher tumor node metastasis stage, shorter overall survival, and shorter progression-free survival. These findings suggest that the willingness and appropriate treatment of HIV-positive patients with CRC need more attention.
Compared to CRC patients without HIV infection, HIV-positive patients with CRC at the same stage and site have a higher number of lymph node metastases and worse postoperative long-term survival; however, the risk of surgery is not increased.
The reasons that fewer CRC patients with HIV infection receive chemotherapy need to be explored. Appropriate treatments for this patient group should be developed.
The authors are grateful to all their colleagues who helped prepare this article. We thank Yi Xiao for his diligence in data entry. The authors have not applied any AI tools.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ekine-Afolabi B, United Kingdom; Vance D, United States S-Editor: Lin C L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 2. | Nasti G, Martellotta F, Berretta M, Mena M, Fasan M, Di Perri G, Talamini R, Pagano G, Montroni M, Cinelli R, Vaccher E, D'Arminio Monforte A, Tirelli U; GICAT; ICONA. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Berretta M, Cinelli R, Martellotta F, Spina M, Vaccher E, Tirelli U. Therapeutic approaches to AIDS-related malignancies. Oncogene. 2003;22:6646-6659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Martellotta F, Berretta M, Vaccher E, Schioppa O, Zanet E, Tirelli U. AIDS-related Kaposi's sarcoma: state of the art and therapeutic strategies. Curr HIV Res. 2009;7:634-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis. 2010;51:957-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 396] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 7. | Kan M, Wong PH, Press N, Wiseman SM. Colorectal and anal cancer in HIV/AIDS patients: a comprehensive review. Expert Rev Anticancer Ther. 2014;14:395-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 9. | Bini EJ, Green B, Poles MA. Screening colonoscopy for the detection of neoplastic lesions in asymptomatic HIV-infected subjects. Gut. 2009;58:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Reiffel JA. Propensity Score Matching: The 'Devil is in the Details' Where More May Be Hidden than You Know. Am J Med. 2020;133:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083-3107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3915] [Cited by in RCA: 4426] [Article Influence: 276.6] [Reference Citation Analysis (1)] |
| 12. | Chalasani N, Wilcox CM. Gastrointestinal hemorrhage in patients with AIDS. AIDS Patient Care STDS. 1999;13:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Durand M, Sheehy O, Baril JG, LeLorier J, Tremblay CL. Risk of spontaneous intracranial hemorrhage in HIV-infected individuals: a population-based cohort study. J Stroke Cerebrovasc Dis. 2013;22:e34-e41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Matsuoka Y, Matsuo Y, Okamoto N, Kuroki M, Ikehara Y. Highly effective extraction of carcinoembryonic antigen with phosphatidylinositol-specific phospholipase C. Tumour Biol. 1991;12:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Kinugasa T, Kuroki M, Yamanaka T, Matsuo Y, Oikawa S, Nakazato H, Matsuoka Y. Non-proteolytic release of carcinoembryonic antigen from normal human colonic epithelial cells cultured in collagen gel. Int J Cancer. 1994;58:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Virji M. CEA and innate immunity. Trends Microbiol. 2001;9:258-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 913] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 18. | Chen W, Zheng R, Zhang S, Zeng H, Xia C, Zuo T, Yang Z, Zou X, He J. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 348] [Article Influence: 43.5] [Reference Citation Analysis (1)] |
| 19. | Wasserberg N, Nunoo-Mensah JW, Gonzalez-Ruiz C, Beart RW Jr, Kaiser AM. Colorectal cancer in HIV-infected patients: a case control study. Int J Colorectal Dis. 2007;22:1217-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Berretta M, Cappellani A, Di Benedetto F, Lleshi A, Talamini R, Canzonieri V, Zanet E, Bearz A, Nasti G, Lacchin T, Berretta S, Fisichella R, Balestreri L, Torresin A, Izzi I, Ortolani P, Tirelli U. Clinical presentation and outcome of colorectal cancer in HIV-positive patients: a clinical case-control study. Onkologie. 2009;32:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Reinhold JP, Moon M, Tenner CT, Poles MA, Bini EJ. Colorectal cancer screening in HIV-infected patients 50 years of age and older: missed opportunities for prevention. Am J Gastroenterol. 2005;100:1805-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Dal Maso L, Serraino D, Franceschi S. Epidemiology of AIDS-related tumours in developed and developing countries. Eur J Cancer. 2001;37:1188-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Faqih A, Singal AG, Fullington HM, Hewitt B, Burstein E, Gopal P, Wylie A, Abrams J, Murphy CC. Colorectal Neoplasia among Patients with and without Human Immunodeficiency Virus. Cancer Epidemiol Biomarkers Prev. 2020;29:1689-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Coghill AE, Engels EA, Schymura MJ, Mahale P, Shiels MS. Risk of Breast, Prostate, and Colorectal Cancer Diagnoses Among HIV-Infected Individuals in the United States. J Natl Cancer Inst. 2018;110:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Suneja G, Shiels MS, Angulo R, Copeland GE, Gonsalves L, Hakenewerth AM, Macomber KE, Melville SK, Engels EA. Cancer treatment disparities in HIV-infected individuals in the United States. J Clin Oncol. 2014;32:2344-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 26. | Suneja G, Boyer M, Yehia BR, Shiels MS, Engels EA, Bekelman JE, Long JA. Cancer Treatment in Patients With HIV Infection and Non-AIDS-Defining Cancers: A Survey of US Oncologists. J Oncol Pract. 2015;11:e380-e387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | André T, Bensmaine MA, Louvet C, François E, Lucas V, Desseigne F, Beerblock K, Bouché O, Carola E, Merrouche Y, Morvan F, Dupont-André G, de Gramont A. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol. 1999;17:3560-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Berretta M, Lleshi A, Cappellani A, Bearz A, Spina M, Talamini R, Cacopardo B, Nunnari G, Montesarchio V, Izzi I, Lanzafame M, Nasti G, Basile F, Berretta S, Fisichella R, Schiantarelli C C, Garlassi E, Ridolfo A, Guella L, Tirelli U. Oxaliplatin based chemotherapy and concomitant highly active antiretroviral therapy in the treatment of 24 patients with colorectal cancer and HIV infection. Curr HIV Res. 2010;8:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Cecchinato V, Tryniszewska E, Ma ZM, Vaccari M, Boasso A, Tsai WP, Petrovas C, Fuchs D, Heraud JM, Venzon D, Shearer GM, Koup RA, Lowy I, Miller CJ, Franchini G. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439-5447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Uldrick TS, Gonçalves PH, Abdul-Hay M, Claeys AJ, Emu B, Ernstoff MS, Fling SP, Fong L, Kaiser JC, Lacroix AM, Lee SY, Lundgren LM, Lurain K, Parsons CH, Peeramsetti S, Ramaswami R, Sharon E, Sznol M, Wang CJ, Yarchoan R, Cheever MA; Cancer Immunotherapy Trials Network (CITN)-12 Study Team. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced Cancer-A Phase 1 Study. JAMA Oncol. 2019;5:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 31. | Cao Y, Wu Q, Lian S, Deng L. Lymphocytes Infiltration and Expression of PD-1 and PD-L1 in Colorectal Cancer Between HIV-Infected and Non-HIV-Infected Patients: A Propensity Score Matched Cohort Study. Front Oncol. 2022;12:827596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess Mortality among HIV-Infected Individuals with Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26:1027-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |