Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.248
Peer-review started: November 20, 2023
First decision: December 11, 2023
Revised: December 24, 2023
Accepted: January 4, 2024
Article in press: January 4, 2024
Published online: January 27, 2024
Processing time: 65 Days and 18.1 Hours
Intestinal tuberculosis is a chronic disease caused by Mycobacterium tuberculosis that mainly affects the ileum and cecum. Small bowel tuberculosis, characterized by predominant involvement of the small intestine, is an extremely rare condition with highly atypical clinical presentations, making diagnosis even more challenging.
We report three cases of small intestinal tuberculosis, two of the patients pre
Patients with SBTs present with nonspecific symptoms such as abdominal pain, weight loss, and occasional gastrointestinal bleeding. Accurate diagnosis requires a thorough evaluation of clinical symptoms and various tests to avoid misdiagno
Core Tip: Intestinal tuberculosis is a chronic disease caused by Mycobacterium tuberculosis and primarily affects the ileum and cecum. Small bowel tuberculosis (SBT) is rare. We report three cases in which patients with SBTs presented with intestinal stenosis or bleeding. Following a complex diagnostic process involving procedures such as small bowel endoscopy and even surgical intervention, all patients were definitively diagnosed and received standard antituberculosis treatment. SBT manifests primarily with nonspecific symptoms such as abdominal pain, weight loss, and occasional gastrointestinal bleeding. A comprehensive evaluation of clinical symptoms and various examinations including laboratory tests, endoscopy, and pathology, are essential for obtaining an accurate diagnosis.
- Citation: Huang G, Wu KK, Li XN, Kuai JH, Zhang AJ. Intestinal tuberculosis with small bowel stricture and hemorrhage as the predominant manifestation: Three case reports. World J Gastrointest Surg 2024; 16(1): 248-256
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/248.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.248
Tuberculosis (TB) is a chronic granulomatous inflammatory disease with diverse clinical presentations and can affect various systems of the body, such as the lungs, bones, lymphatic system, and intestines[1]. The disease is caused by Mycobacterium tuberculosis, a gram-positive bacterium with high treatment resistance. Although existing drugs can effectively control this disease, tuberculosis still has high contagiousness and fatality rates globally[1-5]. Intestinal tu
This case series aims to help clinicians understand the rare situation of intestinal tuberculosis with primary clinical manifestations of small intestinal bleeding or stenosis.
Case 1: A 49-year-old male patient presented with persistent complaint of melena for the previous 20 d.
Case 2: A 23-year-old female patient presented with recurrent abdominal pain over a span of 5 months.
Case 3: A 63-year-old female patient presented with recurrent abdominal pain that persisted for 2 years.
Case 1: The patient reported three episodes of melena over the last 20 d, amounting to approximately 300 mL. No obvious precipitating factors were identified, with the patient denying significant abdominal pain or bloating, as well as symp
Case 2: The patient suffered from intermittent abdominal pain of unclear origin, primarily around the umbilicus, over the previous 5 months. The associated symptoms included decreased appetite, weight loss, and fatigue, but no fever. The patient had an average of two well-formed bowel movements per day, without mucus or bloody discharge on the stool surface. One month prior, the patient sought medical attention at a local hospital. Laboratory tests indicated a hemog
Case 3: Two years prior, this patient initially presented with recurrent paroxysmal epigastric pain without any clear etiology. These episodes occurred once every 1-2 months and lasted for 4-6 d each. A previous gastrointestinal endoscopy conducted at an external hospital failed to yield any definitive findings. The patient experienced multiple episodes of abdominal pain and sought medical attention at multiple sites. Positron emission (PET)/CT performed three months pre
Case 1: The patient had pulmonary tuberculosis during childhood, which was successfully treated. At the age of 24, the patient developed cervical lymph node tuberculosis and underwent surgical removal of the infected lymph nodes.
Case 2: The patient did not report any significant medical conditions.
Case 3: The patient did not report notable illnesses or conditions.
The personal and family histories of these patients were unremarkable.
Case 1: The patient had a body mass index (BMI) of 19.42 kg/m2 and displayed pallor of the skin and conjunctiva, with no apparent positive chest or abdominal signs.
Case 2: The patient had a BMI of 18.22 kg/m2 and had pale conjunctiva and nail beds. Physical examination of the chest and abdomen revealed no specific positive signs.
Case 3: The patient had a BMI of 19.53 kg/m2 and no apparent positive chest or abdominal signs.
Case 1: Routine blood analysis revealed a hemoglobin level of 106 g/L, with positive fecal occult blood test results. The blood biochemistry and tumor marker levels were found to be within the normal range.
Case 2: The patient's complete blood count showed a hemoglobin level of 101 g/L. Blood biochemistry tests revealed a low albumin level of 20 g/L, an elevated erythrocyte sedimentation rate of 25 mm/h, and a C-reactive protein level of 113 mg/L. Tumor marker, Clostridium difficile, Epstein–Barr virus, and cytomegalovirus tests were negative.
Case 3: The patient's complete blood count, blood biochemistry, and tumor marker levels were within the normal range.
Case 1: Small bowel endoscopy (SBE) revealed multiple ulcers and stenosis in the middle and distal segments of the ileum. (Figure 1A-C). The affected areas exhibited friable mucosal tissue that was prone to bleeding upon touch. Pa
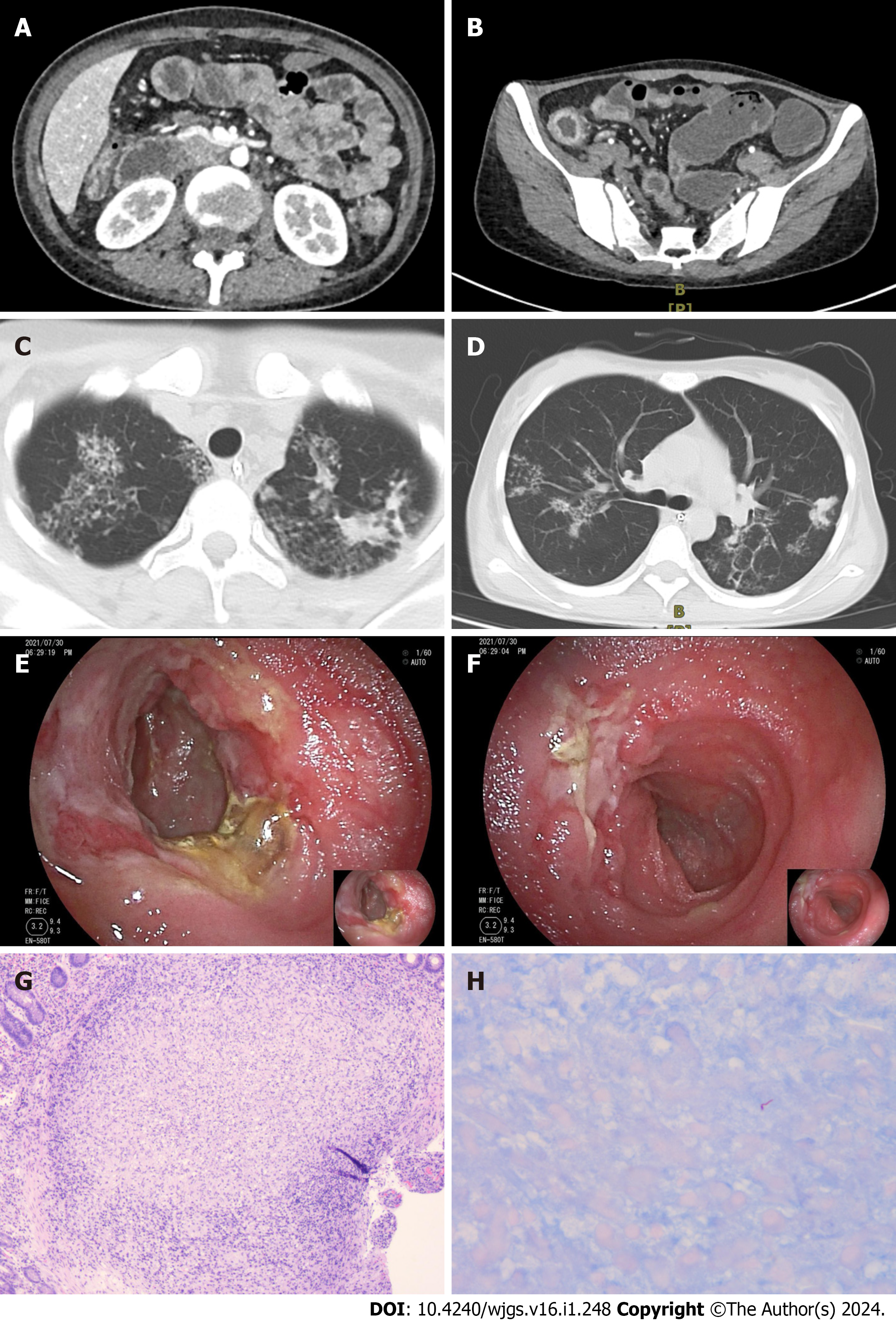
Case 2: Computed tomography enterography (CTE) findings revealed the presence of multiple luminal strictures in both the small intestine and colon, as well as segmental thickening of the intestinal wall, consistent with characteristic features of Crohn's disease (Figure 2A and B). The patient's initial presentation suggested a diagnosis of Crohn's disease. How
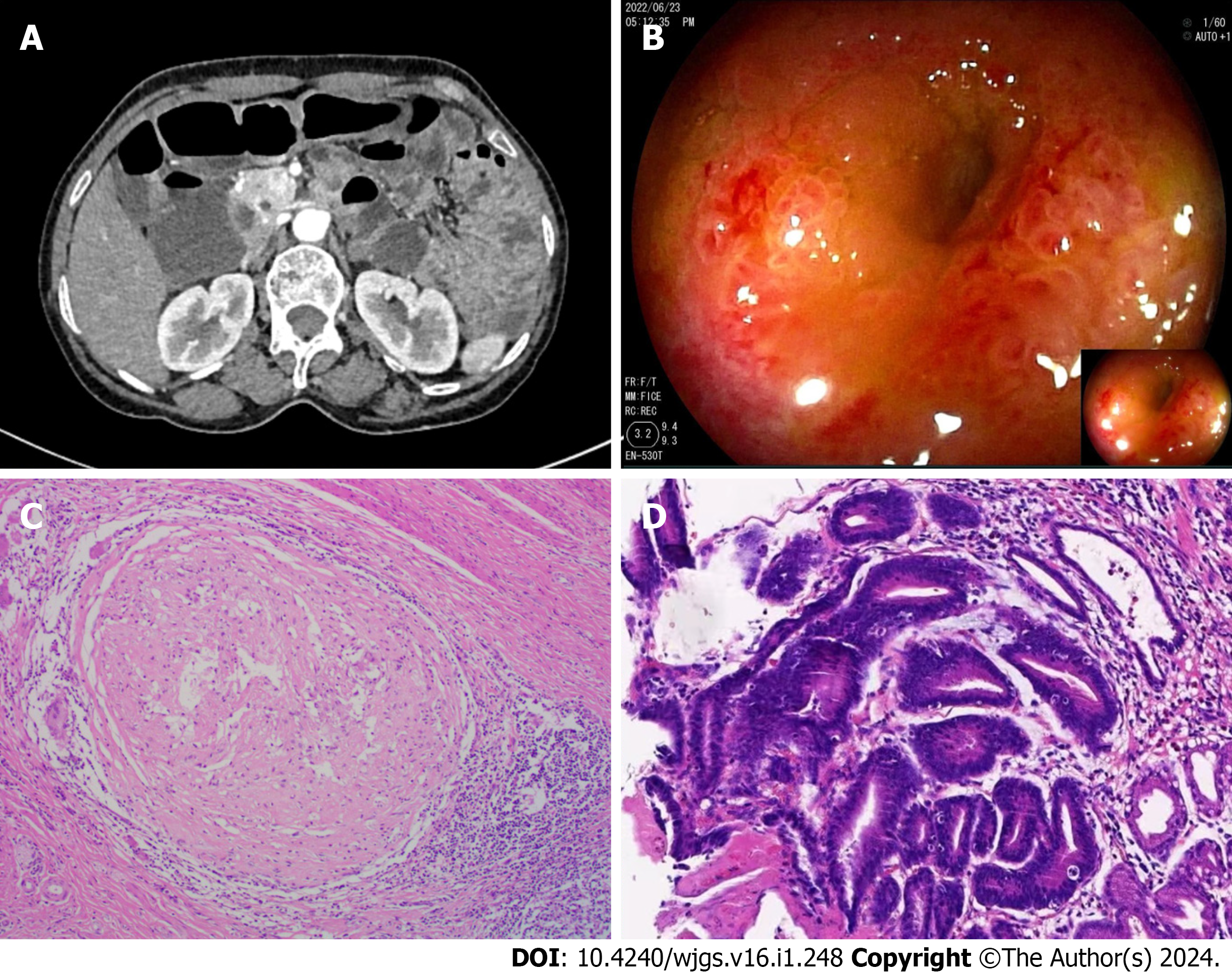
Case 3: CTE revealed localized thickening of the lower left abdominal small intestinal wall (Figure 3A). During SBE, ileal stenosis and gastric mucosal lesions were identified (Figure 3B). Histopathological examination of the gastric mucosal lesions suggested gastric adenocarcinoma, while the histopathological findings of the ileum and jejunum indicated chro
In all of these patients, the final diagnosis was intestinal tuberculosis.
Standard anti-tuberculosis treatment was given.
One week after admission, the patient presented with gastrointestinal bleeding. Blood transfusions were administered, and anti-tuberculosis treatment was initiated, leading to a significant improvement in the symptoms compared to those that the patients had previously.
Standard anti-tuberculosis treatment was given.
These patients recovered well and follow-up was routinely performed. During the telephone follow-up in the 10th month after discharge, the patients did not develop abdominal pain or other obvious discomfort (Table 1).
| Case/gender | Age at diagnosis | Main clinical features | Examinations for tuberculosis | Features of CTE | Endoscopic appearance | Histopathological examination | Management | Outcome |
| 1/male | 49 yr | Melena | PPD (+); TSPOT (+) | Multiple luminal strictures and thickened intestinal walls | Multiple ulcers and stenosis in the small intestine | Multiple granulomas | Standard anti-tuberculosis treatment | No symptoms at the |
| 2/female | 23 yr | Recurrent abdominal pain | T-SPOT (+) | Multiple luminal strictures and thickened intestinal walls | Segmental ulcers were identified throughout the entire ileum and colon | Multiple granulomas; acid-fast staining detects positive bacteria | Standard anti-tuberculosis treatment | No symptoms at the |
| 3/female | 63 yr | Recurrent abdominal pain | PPD (+) | Localized thickening of the small intestine with clustering in the lower left abdomen | Ileal stenosis | Multiple granulomas | Standard anti-tuberculosis treatment | No symptoms at the |
We report three cases of small bowel tuberculosis (SBT) involving the ileocecal region, which is a typical site affected by this disease. The diagnosis and differentiation of intestinal tuberculosis are challenging, and the confirmation of such cases can be further complicated by atypical lesion sites.
A recent series of studies indicated that the most common clinical features of intestinal tuberculosis are abdominal pain, weight loss and fever[8-13]. Abdominal pain is typically chronic and occurs frequently in the right lower quadrant and periumbilical regions, with the symptoms of case 2. However, in case 3, the abdominal pain was mainly localized in the upper abdomen. Weight loss is also a common symptom among patients with intestinal tuberculosis. Among the three patients we described, all had a BMI less than 20 kg/m2, which may be attributed to chronic inflammatory pro
Cases 2 and 3 presented primarily with abdominal pain, while case 1 exhibited small intestine bleeding (SIB) as the main clinical feature. SIB, also known as obscure gastrointestinal bleeding, is commonly caused by malignancies (such as lymphoma), polyposis syndromes, Meckel's diverticulum, inflammatory bowel disease, Dieulafoy's lesions, vascular dilations, or ulcers induced by nonsteroidal anti-inflammatory drugs[15]. There are reports of cases in which colonic tu
Intestinal tuberculosis is a disease known as the "great mimicker" due to its clinical symptoms, which can mimic va
Traditional examinations such as histopathological examination, AFB, and Mycobacterium tuberculosis culture exhibit high specificity but low sensitivity[26]. Various novel molecular-based approaches, including IGRA, GeneXpert, poly
Regarding case 1, the patient had a history of tuberculosis, and both the PPD and T-SPOT results were positive. SBE revealed multiple ulcers and strictures in the small intestine, and histopathological examination revealed granuloma for
Currently, conservative antituberculosis treatment is commonly used for patients with a confirmed diagnosis of SBT. A Cochrane meta-analysis of a randomized controlled trial (328 participants) revealed that patients treated with isoniazid, rifampicin, pyrazinamide, or ethambutol for a shorter duration (6 months) did not have a high rate of recurrence[27]. Additional observational data suggest that in most cases, six months of treatment is sufficient[28,29]. If drug therapy fails to relieve symptoms or if complications such as intestinal obstruction occur, surgical treatment may be considered based on careful evaluation of the patient. In our reported cases, except for case 3 who underwent surgical resection of the intestinal tuberculosis lesion due to gastric malignancy, the main approach was drug therapy, and all the patients achieved satisfactory therapeutic effects.
In summary, the clinical manifestations of SBT are complex and nonspecific, often presenting as abdominal pain, weight loss, and, occasionally, isolated gastrointestinal bleeding. Purely isolated SBTs are relatively rare, with most cases being associated with pulmonary tuberculosis or extrapulmonary tuberculosis. The diagnosis of intestinal tuberculosis relies on a comprehensive assessment of clinical symptom and the laboratory, radiological, endoscopic, bacteriological, and histopathology findings. Only through a thorough analysis of the disease can we distinguish between true and false cases, thus avoiding misdiagnosis and other potential complications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alzerwi NAN, Saudi Arabia S-Editor: Zhang H L-Editor: A P-Editor: Xu ZH
| 1. | Schito M, Migliori GB, Fletcher HA, McNerney R, Centis R, D'Ambrosio L, Bates M, Kibiki G, Kapata N, Corrah T, Bomanji J, Vilaplana C, Johnson D, Mwaba P, Maeurer M, Zumla A. Perspectives on Advances in Tuberculosis Diagnostics, Drugs, and Vaccines. Clin Infect Dis. 2015;61 Suppl 3:S102-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Aregawi AB, Alem AT, Girma A. A Rare Case of Intestinal Tuberculosis with Chronic Partial Small Bowel Obstruction in a 37-Year-Old Ethiopian Man. Int Med Case Rep J. 2022;15:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Merino Gallego E, Gallardo Sánchez F, Gallego Rojo FJ. Intestinal tuberculosis and Crohn's disease: the importance and difficulty of a differential diagnosis. Rev Esp Enferm Dig. 2018;110:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Donoghue HD, Holton J. Intestinal tuberculosis. Curr Opin Infect Dis. 2009;22:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Ma JY, Tong JL, Ran ZH. Intestinal tuberculosis and Crohn's disease: challenging differential diagnosis. J Dig Dis. 2016;17:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Wu YF, Ho CM, Yuan CT, Chen CN. Intestinal tuberculosis previously mistreated as Crohn's disease and complicated with perforation: a case report and literature review. Springerplus. 2015;4:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Park H, Kansara T, Victoria AM, Boma N, Hong J. Intestinal Tuberculosis: A Diagnostic Challenge. Cureus. 2021;13:e13058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kentley J, Ooi JL, Potter J, Tiberi S, O'Shaughnessy T, Langmead L, Chin Aleong J, Thaha MA, Kunst H. Intestinal tuberculosis: a diagnostic challenge. Trop Med Int Health. 2017;22:994-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Shi XC, Zhang LF, Zhang YQ, Liu XQ, Fei GJ. Clinical and Laboratory Diagnosis of Intestinal Tuberculosis. Chin Med J (Engl). 2016;129:1330-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Patel B, Yagnik VD. Clinical and laboratory features of intestinal tuberculosis. Clin Exp Gastroenterol. 2018;11:97-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Gan H, Mely M, Zhao J, Zhu L. An Analysis of the Clinical, Endoscopic, and Pathologic Features of Intestinal Tuberculosis. J Clin Gastroenterol. 2016;50:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Tanoglu A, Erdem H, Friedland JS, Almajid FM, Batirel A, Kulzhanova S, Konkayeva M, Smagulova Z, Pehlivanoglu F, de Saram S, Gulsun S, Amer F, Balkan II, Tekin R, Cascio A, Dauby N, Sirmatel F, Tasbakan M, Erdem A, Wegdan AA, Aydin O, Cesur S, Deniz S, Senbayrak S, Denk A, Duzenli T, Siméon S, Oncul A, Ozseker B, Yakar T, Ormeci N. Clinicopathological profile of gastrointestinal tuberculosis: a multinational ID-IRI study. Eur J Clin Microbiol Infect Dis. 2020;39:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Cheng W, Zhang S, Li Y, Wang J, Li J. Intestinal tuberculosis: clinico-pathological profile and the importance of a high degree of suspicion. Trop Med Int Health. 2019;24:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Maulahela H, Simadibrata M, Nelwan EJ, Rahadiani N, Renesteen E, Suwarti SWT, Anggraini YW. Recent advances in the diagnosis of intestinal tuberculosis. BMC Gastroenterol. 2022;22:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | Awadie H, Zoabi A, Gralnek IM. Obscure-overt gastrointestinal bleeding: a review. Pol Arch Intern Med. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Valainathan SR, Thabut D, Rudler M. Colonic tuberculosis as a cause of massive intestinal bleeding. Clin Res Hepatol Gastroenterol. 2021;45:101365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Nagahashi M, Aoyagi T, Yamada A, Rashid OM, Adams BJ, Takabe K. Intestinal Co-infection of Tuberculosis and CMV can Cause Massive Lower GI Bleeding in a Patient with HIV. J Surg Sci. 2013;1:12-15. [PubMed] |
| 18. | Limsrivilai J, Shreiner AB, Pongpaibul A, Laohapand C, Boonanuwat R, Pausawasdi N, Pongprasobchai S, Manatsathit S, Higgins PD. Meta-Analytic Bayesian Model For Differentiating Intestinal Tuberculosis from Crohn's Disease. Am J Gastroenterol. 2017;112:415-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Jung Y, Hwangbo Y, Yoon SM, Koo HS, Shin HD, Shin JE, Moon HS, Kang SB, Lee JR, Huh KC. Predictive Factors for Differentiating Between Crohn's Disease and Intestinal Tuberculosis in Koreans. Am J Gastroenterol. 2016;111:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 20. | Ye Z, Lin Y, Cao Q, He Y, Xue L. Granulomas as the Most Useful Histopathological Feature in Distinguishing between Crohn's Disease and Intestinal Tuberculosis in Endoscopic Biopsy Specimens. Medicine (Baltimore). 2015;94:e2157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Mehta V, Desai D, Abraham P, Gupta T, Rodrigues C, Joshi A, Deshpande R, Sawant P, Ingle M, Rathi P, Mandot A. Do additional colonoscopic biopsies increase the yield of Mycobacterium tuberculosis culture in suspected ileo-colonic tuberculosis? Indian J Gastroenterol. 2018;37:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Yönal O, Hamzaoğlu HO. What is the most accurate method for the diagnosis of intestinal tuberculosis? Turk J Gastroenterol. 2010;21:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Weng MT, Wei SC, Lin CC, Tsang YM, Shun CT, Wang JY, Shieh MJ, Wang CY, Wong JM. Seminar Report From the 2014 Taiwan Society of Inflammatory Bowel Disease (TSIBD) Spring Forum (May 24th, 2014): Crohn's Disease Versus Intestinal Tuberculosis Infection. Intest Res. 2015;13:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Ko JK, Lee HL, Kim JO, Song SY, Lee KN, Jun DW, Lee OY, Han DS, Yoon BC, Choi HS, Hahm JS, Kim SY. Visceral fat as a useful parameter in the differential diagnosis of Crohn's disease and intestinal tuberculosis. Intest Res. 2014;12:42-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Yadav DP, Madhusudhan KS, Kedia S, Sharma R, Pratap Mouli V, Bopanna S, Dhingra R, Pradhan R, Goyal S, Sreenivas V, Vikram NK, Makharia G, Ahuja V. Development and validation of visceral fat quantification as a surrogate marker for differentiation of Crohn's disease and intestinal tuberculosis. J Gastroenterol Hepatol. 2017;32:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Mehta V, Desai D, Abraham P, Rodrigues C. Making a Positive Diagnosis of Intestinal Tuberculosis with the Aid of New Biologic and Histologic Features: How Far Have We Reached? Inflamm Intest Dis. 2019;3:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Jullien S, Jain S, Ryan H, Ahuja V. Six-month therapy for abdominal tuberculosis. Cochrane Database Syst Rev. 2016;11:CD012163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Mandavdhare HS, Singh H, Dutta U, Sharma V. A real-world experience with 6 months of antitubercular therapy in abdominal tuberculosis. JGH Open. 2019;3:201-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Jha DK, Pathiyil MM, Sharma V. Evidence-based approach to diagnosis and management of abdominal tuberculosis. Indian J Gastroenterol. 2023;42:17-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |