Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.215
Peer-review started: August 31, 2023
First decision: November 9, 2023
Revised: November 24, 2023
Accepted: December 19, 2023
Article in press: December 19, 2023
Published online: January 27, 2024
Processing time: 146 Days and 21.2 Hours
Postoperative complications remain a paramount concern for surgeons and healthcare practitioners.
To present a comprehensive analysis of the Estimation of Physiologic Ability and Surgical Stress (E-PASS) scoring system’s efficacy in predicting postoperative complications following abdominal surgery.
A systematic search of published studies was conducted, yielding 17 studies with pertinent data. Parameters such as preoperative risk score (PRS), surgical stress score (SSS), comprehensive risk score (CRS), postoperative complications, post
Patients experiencing complications after abdominal surgery exhibited significantly higher E-PASS scores compared to those without complications [mean difference and 95% confidence interval (CI) of PRS: 0.10 (0.05-0.15); SSS: 0.04 (0.001-0.08); CRS: 0.19 (0.07-0.31)]. Following the exclusion of low-quality studies, results remained valid with no discernible heterogeneity. Subgroup analysis indicated that variations in sample size and age may contribute to heterogeneity in CRS analysis. Binary variable meta-analysis demonstrated a correlation between high CRS and increased postoperative complication rates [odds ratio (OR) (95%CI): 3.01 (1.83-4.95)], with a significant association observed between high CRS and postoperative mortality [OR (95%CI): 15.49 (3.75-64.01)].
In summary, postoperative complications in abdominal surgery, as assessed by the E-PASS scoring system, are consistently linked to elevated PRS, SSS, and CRS scores. High CRS scores emerge as risk factors for heightened morbidity and mortality. This study establishes the accuracy of the E-PASS scoring system in predicting postoperative morbidity and mortality in abdominal surgery, underscoring its potential for widespread adoption in effective risk assessment.
Core Tip: Excessive surgical stress surpassing the patient’s physiological thresholds could precipitate the occurrence of morbidity and mortality following abdominal surgery, especially for resection of liver, pancreas, spleen, and gastrointestinal tract. As a robust evaluation system, Estimation of Physiologic Ability and Surgical Stress scoring system has been widely recognized and adopted over 20 years. Whether the risk prediction score exhibit precise predictive capability of morbidity and mortality in patients undergoing abdominal surgery and provide a favorable evaluation for surgeons? This systematic review will present you with interesting viewpoints.
- Citation: Pang TS, Cao LP. Estimation of Physiologic Ability and Surgical Stress scoring system for predicting complications following abdominal surgery: A meta-analysis spanning 2004 to 2022. World J Gastrointest Surg 2024; 16(1): 215-227
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/215.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.215
Abdominal surgeries often entail significant visceral trauma, presenting an enduring challenge for clinicians due to the subsequent emergence of postoperative complications. Within the realm of general surgery, the postoperative complication rates vary: Pancreaticoduodenectomy exceeds 40%[1], hepatectomy hovers around 38%[2], gastrectomy ranges from 10% to 15%[3], and colorectal resection is approximately 12%[4]. The incidence of postoperative complications is intricately linked to both patient-specific conditions and the intricacies of clinical treatment processes. Such complications contribute to prolonged hospital stays, escalated hospitalization costs, and, in severe cases, heightened postoperative mortality. Therefore, the effective prediction and assessment of postoperative complications stand as imperative objectives in clinical practice.
Excessive surgical stress surpassing the patient’s physiological thresholds can induce sustained damage to vital organs, precipitating postoperative complications across multiple organ systems. The Estimation of Physiologic Ability and Surgical Stress (E-PASS) scoring system, initially introduced by Haga et al[5], emerged as a predictive tool for post
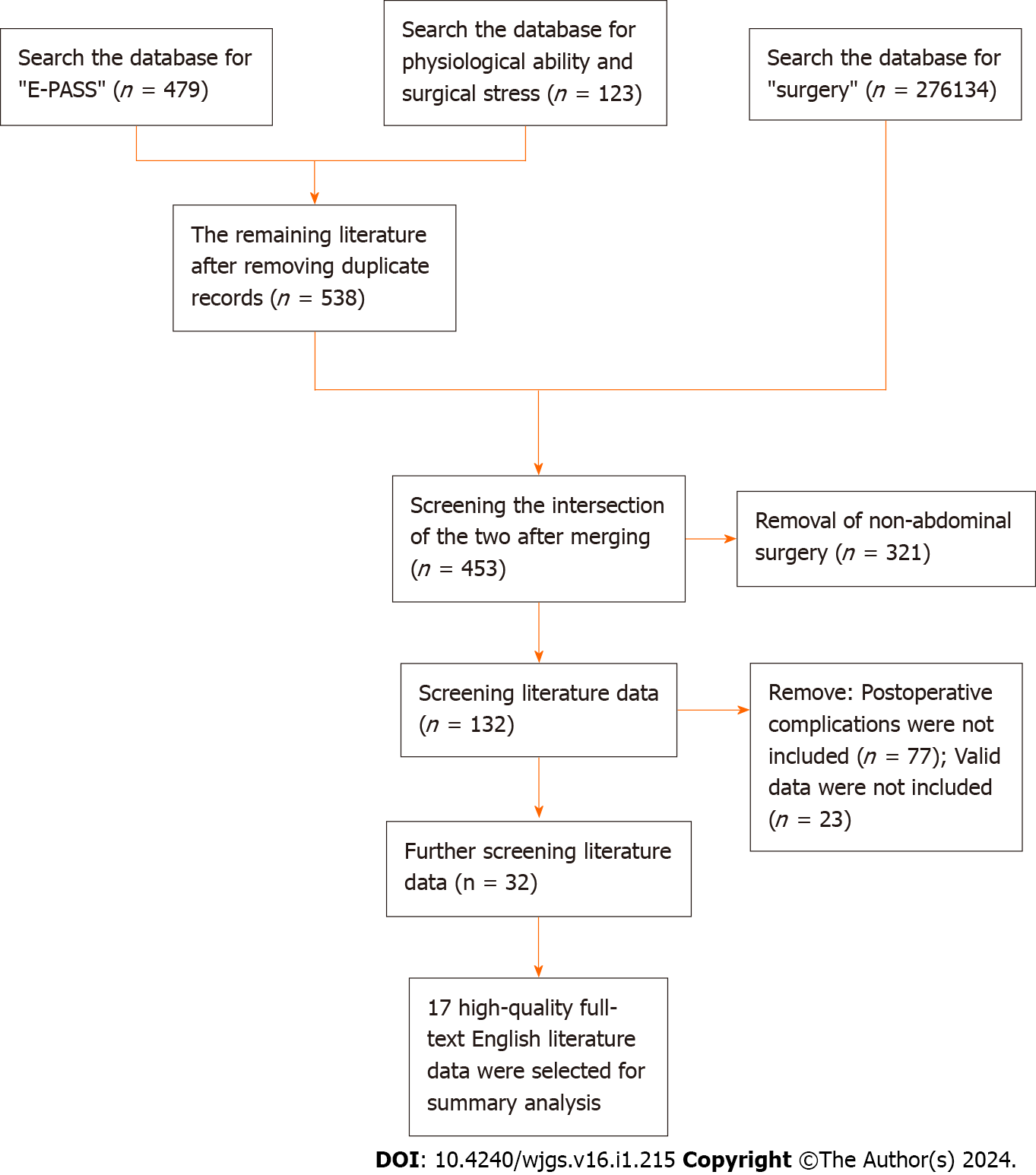
In this study, literature data available up to February 2023 were retrieved from six databases including PubMed, Web of Science, EBSCO, Embase, Ovid and Springerlink. The initial search utilized terms such as “E-PASS” or “physiological ability and surgical pressure” (n = 538 of literature obtained), which were subsequently synthesized and cross-referenced with “surgery” (n = 276134). This multi-step approach resulted in the initial selection of 453 studies. The second phase involved meticulous screening, narrowing down the focus to 132 studies by excluding those unrelated to abdominal surgery (n = 321). In the third stage, 23 studies lacking valid data and 77 studies lacking information on postoperative complications were excluded, yielding 32 effective data points. Finally, stringent exclusion criteria were applied to eliminate non-English and non-full-text literature, culminating in the identification of 17 studies with valuable data for analysis (Figure 1).
The basic data extracted for this study included author information, journal details, publication year, clinical study location (country), number of research institutions, study design (prospective/retrospective), age, sex, surgical site, comprehensive risk score (CRS) truncation value, postoperative complications, postoperative mortality, and E-PASS score. To ensure uniformity, literature data originally presented in median and quartile ranges were transformed into mean and SD values using the methodology devised by Hozo et al[14]. Haga et al[5] initially proposed the E-PASS score that was elaborated from Asian population, similarly, our analysis was also conducted among Asian patients, excluding regional heterogeneity.
The E-PASS scoring system consists of three parts: The preoperative risk score (PRS), the surgical stress score (SSS), and the CRS. The calculation formula is as follows:
PRS = -0.0686 + 0.00345X1 + 0.323X2 + 0.205X3 + 0.153X4 + 0.148X5 + 0.0666X6, X1 represents age; X2 represents the presence (1) or absence (0) of severe heart disease; X3 represents the presence (1) or absence (0) of serious lung disease; X4 represents the presence (1) or absence (0) of diabetes; X5 represents the performance status index (0-4); and X6 stands for [American Society of Anesthesiologists (ASA)] physiological status scale score (0-5).
SSS = -0.342 + 0.0139X1 + 0.0392X2 + 0.352X3, X1 represents blood loss/body weight (g/kg); X2 represents operation time (h); and X3 represents the range of skin incisions: Laparoscopic surgery (0) or open surgery (1).
CRS = -0.328 + 0.936(PRS) + 0.976(SSS).
The six variables of PRS and the three variables of SSS within the E-PASS scoring system serve as unequivocal indicators, employing identical examination methods. Their standardized nature ensures consistency, precluding any potential impact on the assessment of analysis scales across different countries. Notably, all 17 studies retained for the meta-analysis encompassed patients subjected to both laparoscopic and open surgeries. Given that the SSS score incorporates the variable of skin incisions, the consideration of laparoscopy as a factor is integral in ensuring the feasibility and appropriateness of the evaluation across the diverse studies. Postoperative complications were evaluated according to the Clavien-Dindo (CD) classification[15], which was established in 2004[16] and is a simple and feasible classification system for the severity of postoperative complications. CD categorization is a simple grading of post
In this study, Review Manager Software 5.3 was used for meta-analysis. Continuity variables and binary variables were analyzed using forest plots, and heterogeneity was evaluated by the χ2 test P value. The random effects model was adopted when the heterogeneity was large (I2 ≥ 50%), and the fixed effects model was adopted when the heterogeneity was small and the homogeneity was considered (I2 < 50%). For the continuity variable, the mean difference (MD) was selected when the measurement methods or units of the intervention were the same. Heterogeneity tests, P values (validity), and effect scales were analyzed using forest plots, and publication bias was evaluated using funnel plots. The effect size of continuous variables was expressed by the MD and 95% confidence interval (CI), and that of dichotomous variables was expressed by the odds ratio (OR) value and 95%CI. Among the dichotomous variables, risk ratio (RR) for prospective studies and OR for case-control studies were selected. When heterogeneity was high, subgroup analysis was conducted to explore the potential causes of heterogeneity. The literature data are divided into several subgroups according to different attributes, and the subgroup analysis of continuous variables and two categorical variables are analyzed respectively. We affirm that the statistical review of the study was performed by a biomedical statistician and the meta-analysis was based on the PRISMA guidelines.
By exploring the above six databases, 17 studies that included 12744 patients were identified and quantitatively analyzed. The minimum number of samples was 46, and the maximum number was 2495. Of the 17 studies included, 14 studies were from Japan, and the remaining 3 were from China, Switzerland and Turkey; 3 studies were multicenter studies, and the other 14 were single-center studies; 3 studies were prospective, and the remaining 14 were retrospective; 6 studies involved gastrointestinal surgery, and the remaining 11 involved non-gastrointestinal surgery; 6 studies looked specifically at older adults, while the remaining 11 looked at people of all ages; 7 studies defined CRS = 0.5 as the cutoff value, and the remaining 10 defined different CRS cutoff values (Table 1).
| Ref. | Country | Institution | Prescription | Journal | Surgical site | Sample size | Sex (M/F) | Age | Elderly | CRS cutoff |
| Abe et al[19], 2014 | Japan | Single-center | Retrospective | Dig Surg | Gastrointestinal | 73 | 51/22 | 66.0 ± 9.0 | Yes | 0.5 |
| Banz et al[20], 2009 | Switzerland | Single-center | Prospective | World J Surg | Liver | 243 | 131/112 | 61.0 ± 10.5 | No | 0.5 |
| Dai et al[21], 2022 | China | Single-center | Retrospective | Transl Cancer Res | Liver | 236 | 199/37 | 59.73 ± 11.0 | No | 0.126 |
| Hayashi et al[16], 2021 | Japan | Single-center | Retrospective | J Hepatobiliary Pancreat Sci | Pancreas | 343 | 153/190 | 71.0 ± 2.3 | No | 0.5 |
| Kasap et al[22], 2022 | Turkey | Single-center | Retrospective | Int Urol Nephrol | Abdominal | 424 | 248/176 | 49.1 ± 15.3 | No | -0.2996 |
| Kondo et al[23], 2020 | Japan | Single-center | Retrospective | J Anus Rectum Colon | Colorectal | 145 | 72/73 | 87.8 ± 2.0 | Yes | -0.058 |
| Murakami et al[24], 2020 | Japan | Single-center | Retrospective | Dig Surg | Gastrointestinal | 136 | 94/42 | 80.1 ± 4.01 | Yes | 0.2802 |
| Nanashima et al[11], 2011 | Japan | Single-center | Retrospective | J Surg Oncol | Liver | 188 | 152/36 | 65.1 ± 9.5 | Yes | 0.5 |
| Tominaga et al[25], 2016 | Japan | Single-center | Retrospective | Int J Colorectal Dis | Colorectal | 239 | 118/121 | 77.86 ± 5.57 | Yes | 0.2 |
| Yamamoto et al[26], 2020 | Japan | Single-center | Retrospective | Dig Surg | Colorectal | 166 | 91/75 | 80.2 ± 4.5 | Yes | 0.05 |
| Hashimoto et al[7], 2010 | Japan | Single-center | Retrospective | Surg Today | Pancreas | 46 | 19/27 | 63.5 ± 13.4 | No | 0.43 |
| Koushi et al[27], 2011 | Japan | Single-center | Retrospective | Surg Today | Gastrointestinal | 51 | 30/21 | 64.1 ± 14.0 | No | 0.5 |
| Haga et al[28], 2004 | Japan | Multi-center | Prospective | Surgery | Gastrointestinal | 5212 | 2730/2482 | 65.0 ± 16.2 | No | 0.5 |
| Kato et al[29], 2022 | Japan | Multi-center | Retrospective | Int J Surg | Colorectal | 2407 | 1377/1030 | 69.63 ± 11.36 | No | -0.025 |
| Nakanishi et al[30], 2022 | Japan | Multi-center | Retrospective | Surg Today | Gastrointestinal | 2495 | 1790/705 | 67.73 ± 9.996 | No | 0.4179 |
| Norimatsu et al[31], 2022 | Japan | Single-center | Prospective | World J Surg | Hepatobiliary pancreas | 184 | 118/66 | 75.8 ± 1.0 | Yes | 0.049 |
| Oka et al[32], 2005 | Japan | Single-center | Retrospective | World J Surg | Gastrointestinal | 156 | 98/58 | 62.14 ± 9.29 | No | 0.5 |
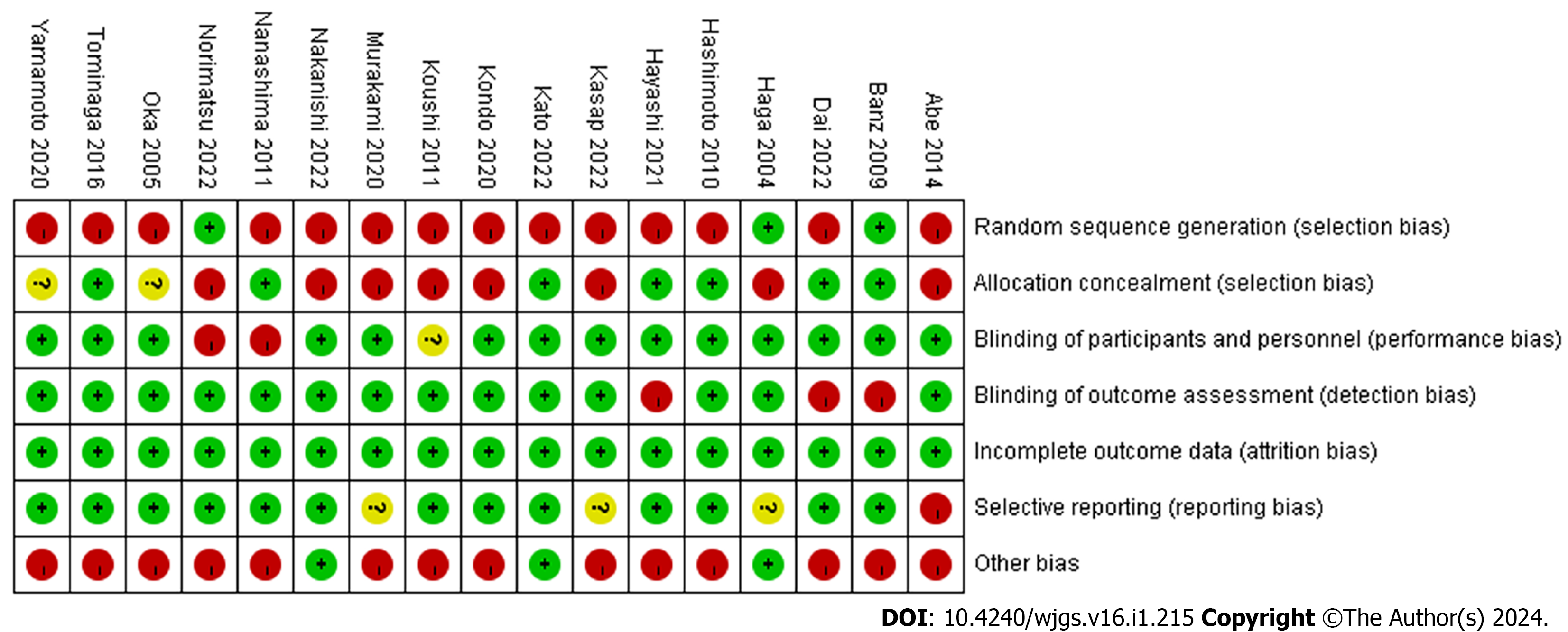
First, an assessment of risk bias was conducted, which was based on the Cochrane Collaboration’s Bias Risk Tool[18] to assess the quality of trials, and it was found that most literature had a little risk bias (Figure 2).
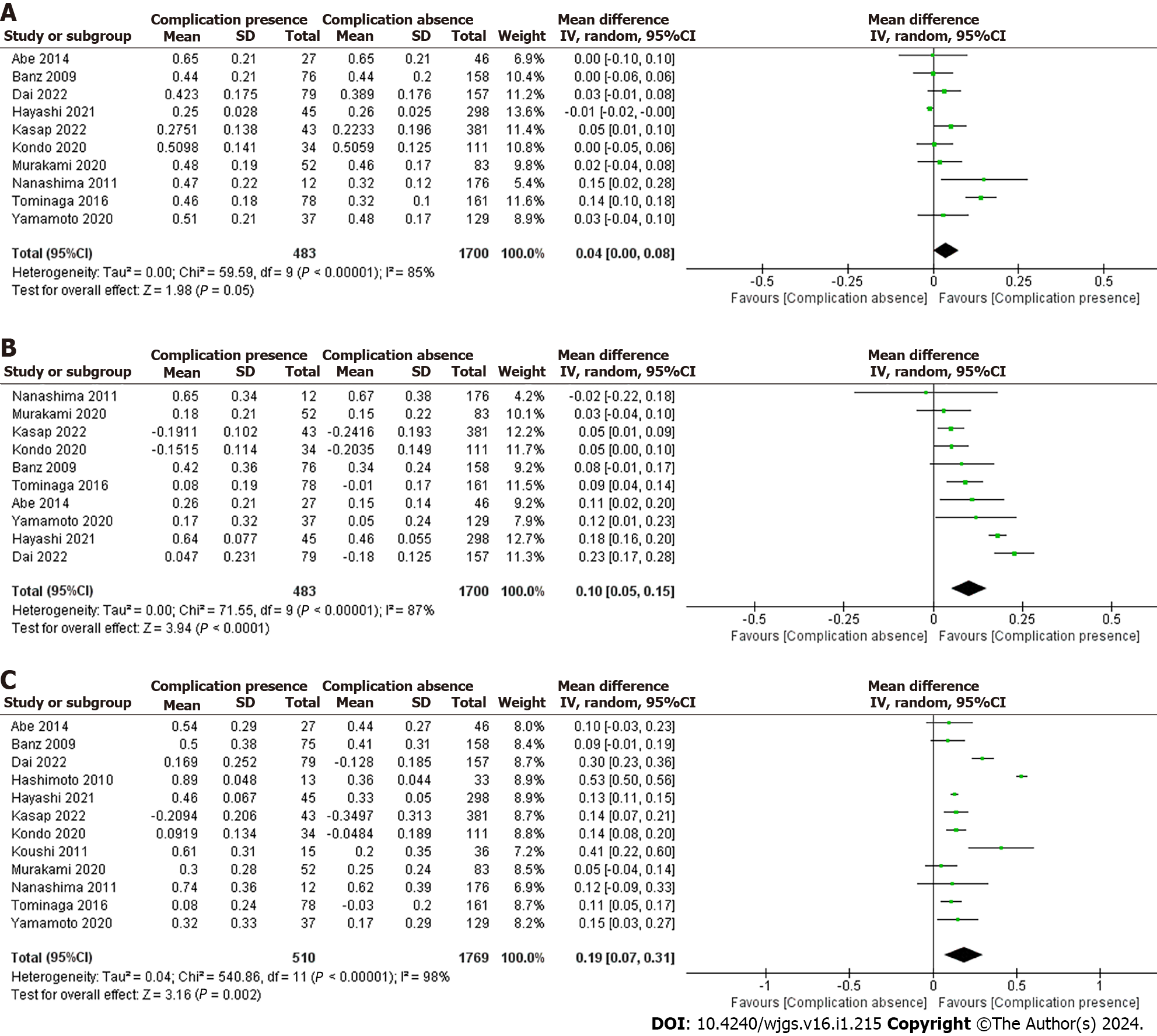
The E-PASS scoring system was analyzed in patients with or without complications after abdominal surgery (Figure 3).
The forest plot was further analyzed to evaluate the heterogeneity effect, and the data were divided into a group with postoperative complications and a group without complications. The PRS, SSS and CRS values are shown from top to bottom in order (Figure 3A). In the PRS analysis, a total of 2183 patients participated in 10 studies[11,16,19-26] that reported the relationship between PRS scores and postoperative complications. The mean value of PRS in the group with complications was 0.45, and that without complications was 0.40. Since the data had heterogeneity (I2 = 85% ≥ 50%, P < 0.0001), we used a random-effects model for the meta-analysis. The result [MD (95%CI): 0.04 (0.001-0.08), P = 0.05] indicated that PRS scores with postoperative complications increased 0.04% more than those without complications, but this difference was not statistically significant.
The results from the sensitivity analysis using a fixed-effect model after removing two low-quality studies[11,25] confirmed the findings that the overall effect of the two groups had no significant difference, and the result was robust [MD (95%CI): -0.01 (-0.01 to 0.001), I2 = 39%, P = 0.20].
We used a random-effects model for the SSS meta-analysis (Figure 3B). A total of 2183 patients participated in 10 studies[11,16,19-26] that reported the relationship between SSS scores and postoperative complications. The mean value of SSS in the group with complications was 0.21, and that without complications was 0.12. The forest plot showed that SSS scores with postoperative complications increased 0.1% more than those without complications, and this difference was statistically significant [MD (95%CI): 0.10 (0.05-0.15), P < 0.0001].
Since the data had significant heterogeneity (I2 = 87%), sensitivity analyses were performed by using a fixed-effect model and removing two low-quality studies[16,21]. The results showed that the SSS score in the group with complications was 0.06% higher than that in the group without complications, which was reliable for the homogeneity of the two groups [MD (95%CI): 0.06 (0.04-0.09), P < 0.0001, I2 = 0].
In the CRS analysis, a total of 2279 patients participated in 12 studies[7,11,16,19-27] that reported the relationship between CRS score and postoperative complications (Figure 3C). The mean value of CRS in the group with complications was 0.37, and that without complications was 0.19. Since the studies had heterogeneity (I2 = 98%), we used a random-effects model for the meta-analysis. The result [MD (95%CI): 0.19 (0.07-0.31), P = 0.002] indicated that CRS scores with postoperative complications were 0.04% higher than those without complications.
Sensitivity analyses were performed by removing two low-quality studies[7,21]. The results showed that the CRS score in the group with complications was 0.13% higher than that in the group without complications, and the result was robust [MD (95%CI): 0.13 (0.11-0.14), P < 0.0001, I2 = 27%].
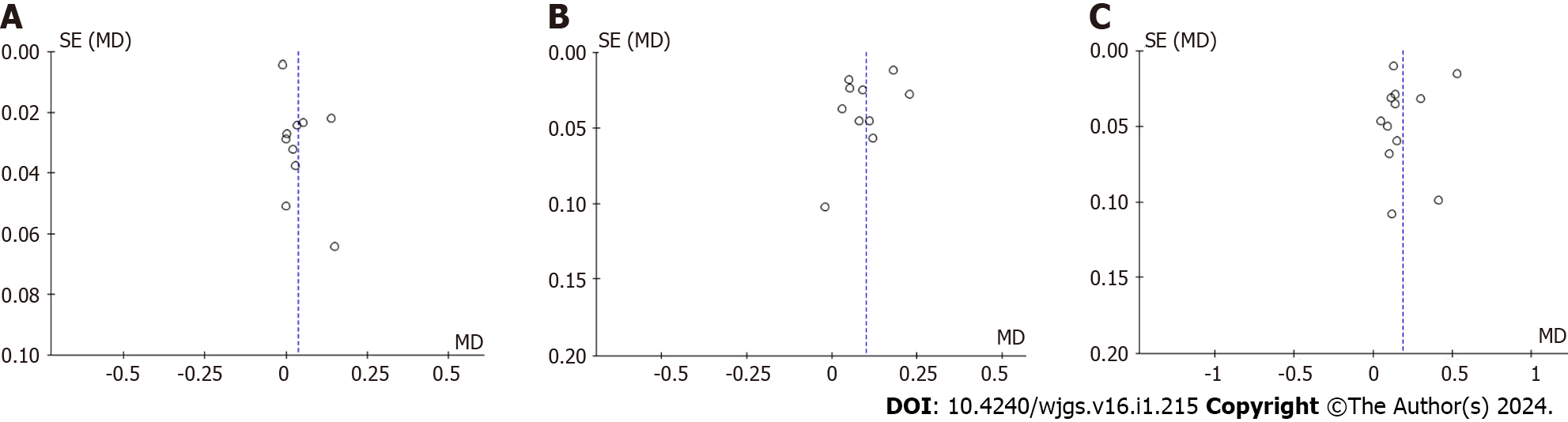
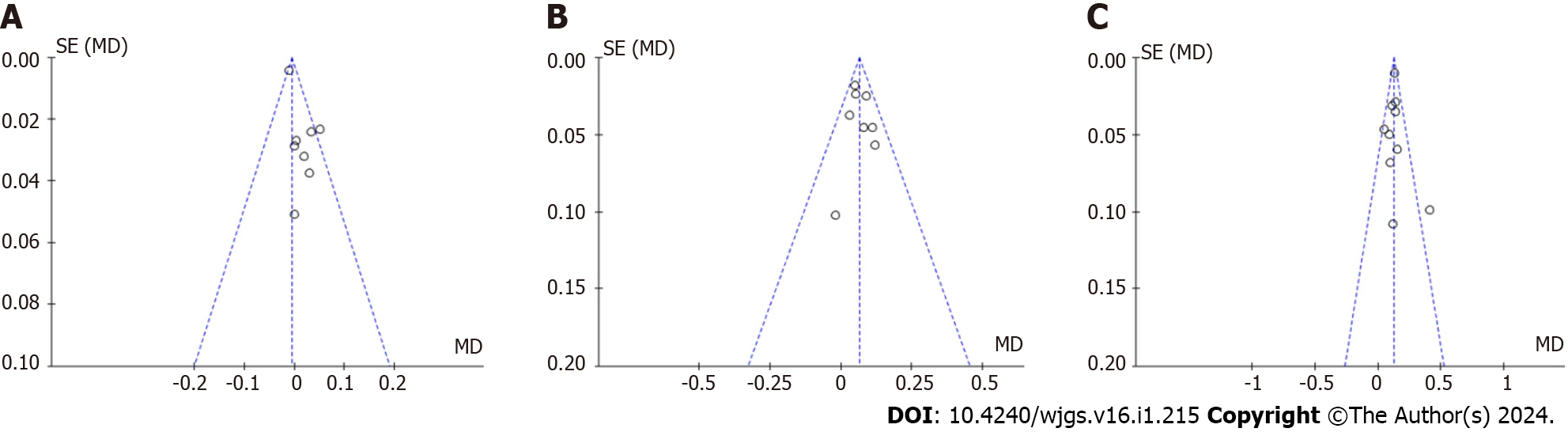
While analyzing the forest maps, the funnel maps were compared correspondingly. The data on both sides of the map are not completely symmetrical, and publication bias might exist (Figure 4). After removing low-quality studies in the PRS, SSS, and CRS analyses, it can be seen that the plots were basically symmetrical on both sides, indicating that there was almost no publication bias in the literature (Figure 5).
To explore the source of CRS heterogeneity, a total of 6 subgroups of 12 studies on CRS score were further analyzed, including sample size (< 100, 100-200, ≥ 200); country (Japan, non-Japan); influence factor (< 2.0, ≥ 2.0); operation site (gastrointestinal tract, hepatobiliary pancreas); age (elderly, nonelderly); and CRS cutoff (< 0.5, ≥ 0.5). Subgroup analysis showed that the sample size grouping (I2 = 99.6%) and the elderly grouping (I2 = 97.7%) had significant heterogeneity, while the other groups had no significant heterogeneity (Supplementary Figures 1-6). Therefore, the differences in sample size and age may be the source of heterogeneity in the CRS analysis (Table 2).
| Subgroup | Classification | Studies | MD (95%CI) | Subgroup I2 | Subgroup effect (P value) | Subgroup overall I2 |
| Sample size | < 100 | 3 | 0.51 (0.48-0.54) | 95% | < 0.0001 | 99.6% |
| 100-200 | 4 | 0.12 (0.08-0.16) | 0 | < 0.0001 | ||
| ≥ 200 | 4 | 0.14 (0.12-0.16) | 85% | < 0.0001 | ||
| Country | Japan | 9 | 0.19 (0.04-0.34) | 98% | 0.01 | 0 |
| Non-Japan | 3 | 0.18 (0.05-0.31) | 88% | 0.005 | ||
| Influence factor | < 2.0 | 3 | 0.19 (0.09-0.28) | 92% | < 0.0001 | 0 |
| ≥ 2.0 | 9 | 0.19 (0.02-0.36) | 98% | 0.03 | ||
| Surgical site | Gastrointestinal tract | 6 | 0.13 (0.07-0.19) | 57% | < 0.0001 | 0 |
| Hepatobiliary pancreas | 5 | 0.24 (0.02-0.46) | 99% | 0.03 | ||
| Age | Elderly | 5 | 0.12 (0.08-0.15) | 0% | < 0.0001 | 97.7% |
| Non-elderly | 7 | 0.25 (0.23-0.26) | 99% | < 0.0001 | ||
| CRS cut-off | < 0.5 | 7 | 0.20 (0.03-0.37) | 98% | 0.02 | 0 |
| ≥ 0.5 | 5 | 0.14 (0.07-0.22) | 55% | < 0.0001 |
In the further meta-analysis of dichotomous variables, we compared the CRS score with postoperative morbidity and mortality studies. A total of 11194 patients with 10 studies were enrolled in the postoperative morbidity analysis[19-21,23,27-32] (Figure 6A); a total of 10557 patients were enrolled in the postoperative mortality analysis, including 6 studies[21,27-30,32] (Figure 6B).
According to the dichotomous variables analysis, patients with a high CRS score had a higher incidence of postoperative complications [OR (95%CI): 3.01 (1.83-4.95), P < 0.0001]. Patients with high CRS scores had a significantly higher incidence of postoperative death, with an OR (95%CI) of 15.49 (3.75- 64.01) (P = 0.0002). There was significant heterogeneity between the two groups (I2 ≥ 50%).
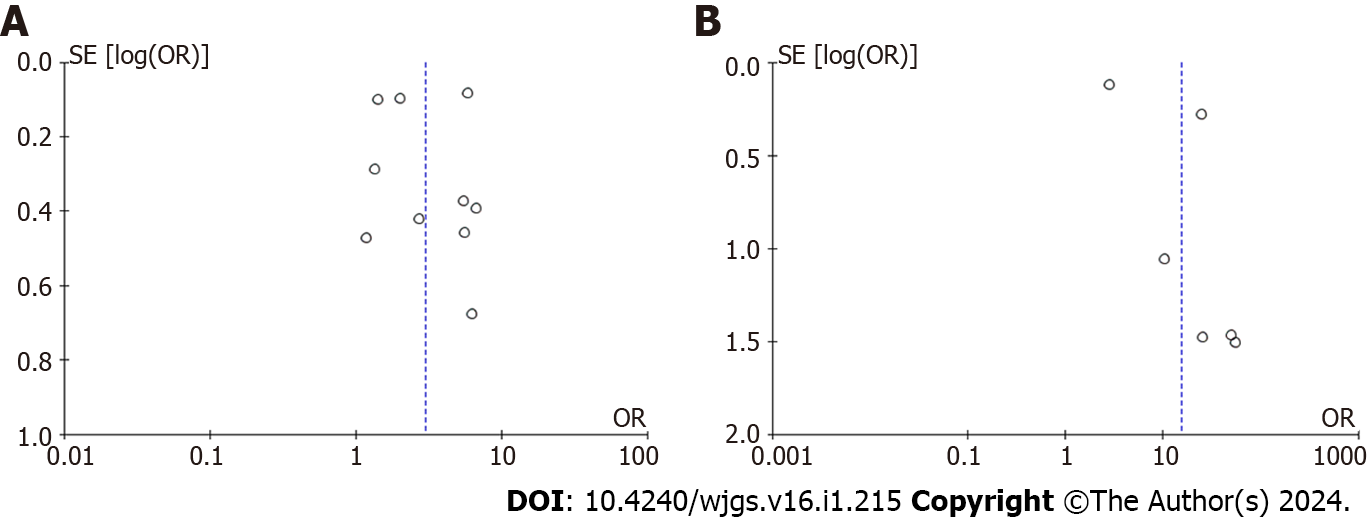
The heterogeneity of CRS and complications did not change when one or more articles were removed, while, when one low-quality study was removed[29], the heterogeneity of CRS and mortality meta-analysis immediately became homogeneous [OR (95%CI): 24.94 (14.72-42.25), P < 0.0001, I2 = 0]. According to the funnel plot analysis of the two groups, the publication bias of postoperative complications was significantly lower than that of postoperative mortality (Figure 7).
The ten studies[19-21,23,27-32] were performed six subgroup analyses as follows: The number of institutions (single-center, multicenter); research time span (retrospective, prospective); sample size (< 200, ≥ 200); influencing factors(< 3.0, ≥ 3.0); operation site (gastrointestinal, nongastrointestinal); and CRS cutoff (< 0.5, ≥ 0.5) (Supplementary Figures 7-12). Subgroup analysis showed that there was no heterogeneity among the groups (subgroup differences I2 = 0), suggesting that the overall heterogeneity was due to certain individual low-quality studies (Table 3).
| Subgroup | Classification | Studies | OR (95%CI) | Subgroup I2 | Subgroup effect (P value) | Subgroup overall I2 |
| Institutions quantity | Single-center | 7 | 3.29 (1.83-5.93) | 72% | < 0.0001 | 0 |
| Multi-center | 3 | 2.57 (1.08-6.10) | 99% | 0.03 | ||
| Time span | Retrospective | 7 | 2.62 (1.75-3.94) | 81% | < 0.0001 | 0 |
| Prospective | 3 | 3.51 (1.26-9.75) | 92% | 0.02 | ||
| Sample size | < 100 | 5 | 3.68 (1.91-7.08) | 60% | < 0.0001 | 0 |
| ≥ 200 | 5 | 2.59 (1.32-5.10) | 97% | 0.006 | ||
| Influence factor | < 3.0 | 5 | 2.71 (1.61-4.56) | 92% | 0.0002 | 0 |
| ≥ 3.0 | 5 | 3.25 (1.39-7.58) | 64% | 0.006 | ||
| Operation site | Gastrointestinal | 6 | 3.30 (1.73-6.29) | 96% | 0.0003 | 0 |
| Non-gastrointestinal | 4 | 2.61 (1.12-6.07) | 80% | 0.03 | ||
| CRS cut-off | < 0.5 | 5 | 2.54 (1.67-3.84) | 82% | < 0.0001 | 0 |
| ≥ 0.5 | 5 | 3.24 (1.45-7.22) | 88% | 0.004 |
Currently, the common predictive surgical scores include the ASA score, Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity (POSSUM) score, E-PASS scoring system, and so on. Developed in the United Kingdom in 1991[33], the POSSUM system integrates 12 physiological factors and 6 surgical factors, generating Physiological score (P score) and Operative score (O score), respectively. However, both POSSUM and P-POSSUM scores that have complex calculation formulas[16] are acknowledged for their tendency to overestimate postoperative mortality in digestive surgery[34,35]. Additionally, the revised modified E-PASS score, while having not been extensively tested[36], shows reduced accuracy in elderly patients undergoing perihilar cholangiocarcinoma surgery[6]. Comparative analyses have consistently shown that E-PASS scores prove more convenient and effective than POSSUM scores and ASA scores, particularly in the realms of hepatobiliary, pancreatic, and gastrointestinal surgeries. Furthermore, E-PASS scores outperform other scoring models in their predictive accuracy for postoperative complications[16,23,25,28]. Widely recognized as a robust evaluation system, the E-PASS scoring system excels in predicting postoperative complications in abdominal surgery. Its capacity to distinguish patients at high and low risk of hospital death following digestive surgery further underscores its clinical utility.
The E-PASS scoring system incorporates PRS, SSS, and CRS. CRS, a composite of PRS and SSS, enhances the comprehensiveness and accuracy of complications prediction. The E-PASS scoring system necessitates the collection of medical history pertaining to heart disease, lung disease, and diabetes. Meanwhile, it involves the assessment of the ASA physiological state rating, along with the retrieval of intraoperative blood loss, preoperative weight, operation time, and surgical incision details. These elements, readily available in medical records, collectively contribute to the effective evaluation of postoperative complication incidence. We believe that each component of the E-PASS can be easily obtained from medical records and directly applied in clinical settings without requiring supplementary calculations.
Numerous prior studies have consistently affirmed the correlation between the E-PASS scoring system and postoperative morbidity and mortality. Nakanishi et al[30] posited the E-PASS score as an independent prognostic factor throughout the stages of gastric cancer. Kato et al[29] validated a significant association between a high CRS and overall survival and recurrence-free survival in colorectal cancer patient’s post-resection, irrespective of disease stage. The CRS score emerges as an indicator of poor prognosis regardless of disease stage. Abe et al[19] advocated the superior predictive value of SSS over PRS, emphasizing the importance of maintaining low surgical pressure rather than solely relying on preoperative patient status for evaluation. Murakami et al[24] suggested that, compared to open surgery, laparoscopic surgery results in a lower CRS score. Specifically, laparoscopic-assisted surgery exhibits a lower SSS score, indicating reduced invasive trauma and a diminished postoperative risk compared to open surgery. Consequently, the recommendation for minimizing postoperative complications in abdominal surgery is to opt for minimally invasive treatment. Kondo et al[23] proposed that almost all elderly patients undergoing laparoscopic surgery exhibited lower mean CRS scores, attributing this to their baseline low CRS values. Overall, approximately 25% of elderly patients experience mortality attributable to noncancer-related comorbidities within the five-year period surgery[37]. Regardless of age, laparoscopic surgery for abdominal visceral diseases is deemed safe and feasible[38,39]. Recognizing that postoperative pain can contribute to complications such as atelectasis, insufficient ventilation, and reduced mobility[40,41], laparoscopic surgery offers patients distinct advantages. These include reduced postoperative pain due to minimized skin incisions, a corresponding decrease in the incidence of lung complications, and enhanced early activity, thereby promoting postoperative recovery.
One notable limitation of this meta-analysis lies in the predominant utilization of retrospective data in the majority of the included studies. The subgroup analysis of binary variables was conducted in Supplementary Figure 8. Potential bias in the risk prediction can be ignored due to that there was no significant heterogeneity between prospective and retrospective studies (subgroup differences I2 = 0). Additionally, certain studies relied on the classification of complications based on the severity criteria defined by the Comprehensive Complication Index (CCI), specifically, a CCI score equal to or exceeding 40 points[42]. Such an approach may introduce outcome bias to some extent. Despite these limitations, the meta-analysis boasts several strengths. Firstly, it consolidates and scrutinizes a substantial volume of literature data, encompassing a diverse array of clinical studies. Secondly, the analysis goes beyond previous studies by intricately examining the heterogeneity and efficacy of the E-PASS system through split analysis, sensitivity analysis, and subgroup analysis of each component. This detailed approach enhances the persuasiveness and granularity of the findings. Third, the meta-analysis furnishes a robust directional guide for predicting the postoperative risk associated with the E-PASS scoring system.
In summary, the amalgamation of findings from 17 studies indicates a positive correlation between postoperative complications in abdominal surgery and elevated scores in PRS, SSS, and CRS within the E-PASS system. Notably, heightened CRS scores emerge as risk factors associated with an increased incidence of postoperative complications and mortality. The simplicity and practicality of the E-PASS scoring system position it as an effective model for accurately predicting postoperative complications. This endorsement underscores the potential for widespread adoption, offering a valuable tool for enhancing risk assessment after abdominal surgery and furnishing a reliable direction for clinical practice.
Postoperative complications have always been a close concern to surgeons. Numerous studies affirm the simplicity and efficacy of the Estimation of Physiologic Ability and Surgical Stress (E-PASS) scoring system, which demonstrates superior predictive capabilities for postoperative complications in hepatobiliary, pancreatic, and gastrointestinal surgeries compared to alternative models.
How can doctors exhibit precise predictive capability for the risk of morbidity and mortality in patients undergoing abdominal surgery?
The main objective is to present a comprehensive analysis of the E-PASS scoring system’s efficacy in predicting postoperative complications following abdominal surgery.
A systematic search of published studies was conducted, yielding 17 studies with pertinent data. Preoperative risk score, surgical stress score, comprehensive risk score (CRS), postoperative complications, postoperative mortality, and other clinical data were collected for meta-analysis. Continuity variables and binary variables were analyzed using forest plots, and heterogeneity was evaluated by the χ2 test P value. Heterogeneity tests, P values (validity), and effect scales were analyzed using forest plots, and publication bias was evaluated using funnel plots. The literature data are divided into several subgroups according to different attributes, and the subgroup analysis of continuous variables and two categorical variables are analyzed respectively.
Patients experiencing complications after abdominal surgery exhibited significantly higher E-PASS scores compared to those without complications. Subgroup analysis indicated that variations in sample size and age may contribute to heterogeneity in CRS analysis. Binary variable meta-analysis demonstrated a correlation between high CRS and increased postoperative complication rates, with a significant association observed between high CRS and postoperative mortality.
The E-PASS scoring system is simple and practical to be used as a good model to predict the postoperative complications with accuracy, being expected to popularize effective risk assessment after abdominal surgery.
We confirmed that the E-PASS scoring system can predict the postoperative morbidity and mortality of abdominal surgery with accuracy, worth to be popularized for effective risk assessment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S, Algeria; Tenreiro N, Portugal S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 439] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 2. | Noba L, Rodgers S, Chandler C, Balfour A, Hariharan D, Yip VS. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Improve Clinical Outcomes in Liver Surgery: a Systematic Review and Meta-Analysis. J Gastrointest Surg. 2020;24:918-932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Guerrini GP, Esposito G, Magistri P, Serra V, Guidetti C, Olivieri T, Catellani B, Assirati G, Ballarin R, Di Sandro S, Di Benedetto F. Robotic versus laparoscopic gastrectomy for gastric cancer: The largest meta-analysis. Int J Surg. 2020;82:210-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 4. | Huang ZX, Zhou Z, Shi HR, Li TY, Ye SP. Postoperative complications after robotic resection of colorectal cancer: An analysis based on 5-year experience at a large-scale center. World J Gastrointest Surg. 2021;13:1660-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 5. | Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Coelen RJ, Olthof PB, van Dieren S, Besselink MG, Busch OR, van Gulik TM. External Validation of the Estimation of Physiologic Ability and Surgical Stress (E-PASS) Risk Model to Predict Operative Risk in Perihilar Cholangiocarcinoma. JAMA Surg. 2016;151:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Hashimoto D, Takamori H, Sakamoto Y, Tanaka H, Hirota M, Baba H. Can the physiologic ability and surgical stress (E-PASS) scoring system predict operative morbidity after distal pancreatectomy? Surg Today. 2010;40:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Hashimoto D, Takamori H, Sakamoto Y, Ikuta Y, Nakahara O, Furuhashi S, Tanaka H, Watanabe M, Beppu T, Hirota M, Baba H. Is an estimation of physiologic ability and surgical stress able to predict operative morbidity after pancreaticoduodenectomy? J Hepatobiliary Pancreat Sci. 2010;17:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Haga Y, Wada Y, Ikenaga M, Takeuchi H, Ikejiri K. Evaluation of modified estimation of physiologic ability and surgical stress in colorectal carcinoma surgery. Dis Colon Rectum. 2011;54:1293-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Haga Y, Ikejiri K, Takeuchi H, Ikenaga M, Wada Y. Value of general surgical risk models for predicting postoperative liver failure and mortality following liver surgery. J Surg Oncol. 2012;106:898-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Nanashima A, Abo T, Nonaka T, Fukuoka H, Hidaka S, Takeshita H, Ichikawa T, Sawai T, Yasutake T, Nakao K, Nagayasu T. Prognosis of patients with hepatocellular carcinoma after hepatic resection: are elderly patients suitable for surgery? J Surg Oncol. 2011;104:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Menezes FH, Ferrarezi B, Souza MA, Cosme SL, Molinari GJ. Results of Open and Endovascular Abdominal Aortic Aneurysm Repair According to the E-PASS Score. Braz J Cardiovasc Surg. 2016;31:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Tang T, Walsh SR, Fanshawe TR, Gillard JH, Sadat U, Varty K, Gaunt ME, Boyle JR. Estimation of physiologic ability and surgical stress (E-PASS) as a predictor of immediate outcome after elective abdominal aortic aneurysm surgery. Am J Surg. 2007;194:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6890] [Article Influence: 344.5] [Reference Citation Analysis (0)] |
| 15. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8608] [Article Influence: 538.0] [Reference Citation Analysis (0)] |
| 16. | Hayashi H, Kawabata Y, Nishi T, Kishi T, Nakamura K, Kaji S, Fujii Y, Tajima Y. Accurate prediction of severe postoperative complications after pancreatic surgery: POSSUM vs E-PASS. J Hepatobiliary Pancreat Sci. 2021;28:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24805] [Article Influence: 1181.2] [Reference Citation Analysis (0)] |
| 18. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24807] [Article Influence: 1771.9] [Reference Citation Analysis (3)] |
| 19. | Abe H, Mafune K, Minamimura K, Hirata T. Validation of the Estimation of Physiologic Ability and Surgical Stress (E-PASS) score for maintenance hemodialysis patients undergoing elective abdominal surgery. Dig Surg. 2014;31:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Banz VM, Studer P, Inderbitzin D, Candinas D. Validation of the estimation of physiologic ability and surgical stress (E-PASS) score in liver surgery. World J Surg. 2009;33:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Dai Y, Chen G, Chen Y, Shi Z, Pan J, Fan X, Lin H. Usefulness of the estimation of physiologic ability and surgical stress (E-PASS) system for prediction of complication and prognosis in hepatocellular carcinoma patients after hepatectomy. Transl Cancer Res. 2022;11:2700-2712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Kasap Y, Senel S, Tastemur S, Olcucuoglu E. Feasibility of E-PASS score to predict postoperative complications in laparoscopic nephrectomy. Int Urol Nephrol. 2022;54:2149-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Kondo H, Hirano Y, Ishii T, Hara K, Obara N, Wang L, Asari M, Kato T, Yamaguchi S. E-PASS Scoring System May Be Useful for Prediction of Postoperative Complications in Super Elderly Colorectal Cancer Surgery Patients. J Anus Rectum Colon. 2020;4:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Murakami Y, Saito H, Shimizu S, Kono Y, Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y, Ashida K, Fujiwara Y. Evaluation of the Estimation of Physiologic Ability and Surgical Stress Score as a Prognostic Indicator for Older Patients with Gastric Cancer. Dig Surg. 2020;37:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Tominaga T, Takeshita H, Takagi K, Kunizaki M, To K, Abo T, Hidaka S, Nanashima A, Nagayasu T, Sawai T. E-PASS score as a useful predictor of postoperative complications and mortality after colorectal surgery in elderly patients. Int J Colorectal Dis. 2016;31:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Yamamoto M, Saito H, Uejima C, Tanio A, Tada Y, Matsunaga T, Sakamoto T, Honjo S, Ashida K, Fujiwara Y. Estimation of Physiological Ability and Surgical Stress Score Is a Useful Prognostic Indicator for Elderly Patients with Colorectal Cancer. Dig Surg. 2020;37:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Koushi K, Korenaga D, Kawanaka H, Okuyama T, Ikeda Y, Takenaka K. Using the E-PASS scoring system to estimate the risk of emergency abdominal surgery in patients with acute gastrointestinal disease. Surg Today. 2011;41:1481-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Haga Y, Wada Y, Takeuchi H, Kimura O, Furuya T, Sameshima H, Ishikawa M. Estimation of physiologic ability and surgical stress (E-PASS) for a surgical audit in elective digestive surgery. Surgery. 2004;135:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Kato Y, Shigeta K, Tajima Y, Kikuchi H, Hirata A, Nakadai J, Sugiura K, Seo Y, Kondo T, Okui J, Matsui S, Seishima R, Okabayashi K, Kitagawa Y. Comprehensive risk score of the E-PASS as a prognostic indicator for patients after elective and emergency curative colorectal cancer surgery: A multicenter retrospective study. Int J Surg. 2022;101:106631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | Nakanishi K, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Kobayashi D, Shimizu D, Tanaka C, Fujiwara M, Murotani K, Kodera Y. E-PASS scoring system serves as a predictor of short- and long-term outcomes in gastric cancer surgery. Surg Today. 2022;52:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Norimatsu Y, Ito K, Takemura N, Inagaki F, Mihara F, Kokudo N. Estimation of Physiologic Ability and Surgical Stress (E-PASS) Predicts Postoperative Major Complications After Hepato-Pancreato Biliary Surgery in the Elderly. World J Surg. 2022;46:2788-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 32. | Oka Y, Nishijima J, Oku K, Azuma T, Inada K, Miyazaki S, Nakano H, Nishida Y, Sakata K, Izukura M. Usefulness of an estimation of physiologic ability and surgical stress (E-PASS) scoring system to predict the incidence of postoperative complications in gastrointestinal surgery. World J Surg. 2005;29:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1136] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 34. | Hong S, Wang S, Xu G, Liu J. Evaluation of the POSSUM, p-POSSUM, o-POSSUM, and APACHE II scoring systems in predicting postoperative mortality and morbidity in gastric cancer patients. Asian J Surg. 2017;40:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Liu N, Cui J, Zhang Z, Zhao Z, Li W, Fu W. [Value of E-PASS and mE-PASS in predicting morbidity and mortality of gastric cancer surgery]. Zhonghua Zhong Liu Za Zhi. 2015;37:753-758. [PubMed] |
| 36. | Haga Y, Ikejiri K, Wada Y, Takahashi T, Ikenaga M, Akiyama N, Koike S, Koseki M, Saitoh T. A multicenter prospective study of surgical audit systems. Ann Surg. 2011;253:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Ariake K, Ueno T, Takahashi M, Goto S, Sato S, Akada M, Naito H. E-PASS comprehensive risk score is a good predictor of postsurgical mortality from comorbid disease in elderly gastric cancer patients. J Surg Oncol. 2014;109:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Zong L, Wu A, Wang W, Deng J, Aikou S, Yamashita H, Maeda M, Abe M, Yu D, Jiang Z, Seto Y, Ji J. Feasibility of laparoscopic gastrectomy for elderly gastric cancer patients: meta-analysis of non-randomized controlled studies. Oncotarget. 2017;8:51878-51887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Wang JF, Zhang SZ, Zhang NY, Wu ZY, Feng JY, Ying LP, Zhang JJ. Laparoscopic gastrectomy versus open gastrectomy for elderly patients with gastric cancer: a systematic review and meta-analysis. World J Surg Oncol. 2016;14:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Egbert AM, Parks LH, Short LM, Burnett ML. Randomized trial of postoperative patient-controlled analgesia vs intramuscular narcotics in frail elderly men. Arch Intern Med. 1990;150:1897-1903. [PubMed] |
| 41. | Emile SH, Barsom SH. Short-term outcomes of single-incision compared to multi-port laparoscopic gastrectomy for gastric cancer: A meta-analysis of randomized controlled trials. Laparosc Endosc Robo Sur 2023. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Efanov M, Alikhanov R, Zamanov E, Melekhina O, Kulezneva Y, Kazakov I, Vankovich A, Koroleva A, Tsvirkun V. Combining E-PASS model and disease specific risk factors to predict severe morbidity after liver and bile duct resection for perihilar cholangiocarcinoma. HPB (Oxford). 2021;23:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |