Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.173
Peer-review started: November 6, 2023
First decision: November 16, 2023
Revised: December 3, 2023
Accepted: December 20, 2023
Article in press: December 20, 2023
Published online: January 27, 2024
Processing time: 79 Days and 21.2 Hours
Recently, research has linked Helicobacter pylori (H. pylori) stomach infection to colonic inflammation, mediated by toxin production, potentially impacting colorectal cancer occurrence.
To investigate the risk factors for post-colon polyp surgery, H. pylori infection, and its correlation with pathologic type.
Eighty patients who underwent colon polypectomy in our hospital between January 2019 and January 2023 were retrospectively chosen. They were then randomly split into modeling (n = 56) and model validation (n = 24) sets using R. The modeling cohort was divided into an H. pylori-infected group (n = 37) and an H. pylori-uninfected group (n = 19). Binary logistic regression analysis was used to analyze the factors influencing the occurrence of H. pylori infection after colon polyp surgery. A roadmap prediction model was established and validated. Finally, the correlation between the different pathological types of colon polyps and the occurrence of H. pylori infection was analyzed after colon polyp surgery.
Univariate results showed that age, body mass index (BMI), literacy, alcohol consumption, polyp pathology type, high-risk adenomas, and heavy diet were all influential factors in the development of H. pylori infection after intestinal polypectomy. Binary multifactorial logistic regression analysis showed that age, BMI, and type of polyp pathology were independent predictors of the occurrence of H. pylori infection after intestinal polypectomy. The area under the receiver operating characteristic curve was 0.969 [95% confidence interval (95%CI): 0.928–1.000] and 0.898 (95%CI: 0.773–1.000) in the modeling and validation sets, respectively. The slope of the calibration curve of the graph was close to 1, and the goodness-of-fit test was P > 0.05 in the two sets. The decision analysis curve showed a high rate of return in both sets. The results of the correlation analysis between different pathological types and the occurrence of H. pylori infection after colon polyp surgery showed that hyperplastic polyps, inflammatory polyps, and the occurrence of H. pylori infection were not significantly correlated. In contrast, adenomatous polyps showed a significant positive correlation with the occurrence of H. pylori infection.
Age, BMI, and polyps of the adenomatous type were independent predictors of H. pylori infection after intestinal polypectomy. Moreover, the further constructed column-line graph prediction model of H. pylori infection after intestinal polypectomy showed good predictive ability.
Core Tip:Helicobacter pylori (H. pylori) infection is reportedly a risk factor for the development of colonic adenomas, especially progressive or multiple adenomas. However, few studies have examined the risk factors for H. pylori infection after therapeutic colon polypectomy and the type of polyp pathology associated with its occurrence. This randomized study evaluated the risk factors for the development of H. pylori infections in patients with colon polyps and the relationship between their pathology and the development of H. pylori infections.
- Citation: Zhang ZS. Predictive factors and model validation of post-colon polyp surgery Helicobacter pylori infection. World J Gastrointest Surg 2024; 16(1): 173-185
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/173.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.173
Colonic polyps are tumor-like lesions that grow on the mucosal surface of the colon, usually in the form of protruding or elevated masses or fleshy lesions[1]. They do not invade surrounding tissues and are clinically classified based on their histologic features and susceptibility to malignant transformation. Mainly, there are colorectal adenomatous polyps, inflammatory polyps, and hyperplastic polyps[2]. Colonic adenomatous polyps are abnormal tissues that may develop into colorectal cancer (CC); colonic adenomatous polyps are the most common in clinical practice and carry a higher risk of developing cancer[3]. According to the 2016 United States Guidelines for Follow-up after Colorectal Polypectomy[4], the presence of adenomas that are more than 1 cm in diameter, three or more in number, or that exhibit villous or high-grade intraepithelial neoplasia, along with the occurrence of any of the aforementioned criteria, suggests a high risk of cancer development in colorectal polyp case. To reduce the risk of CC, further development of colonic adenomatous polyps must be inhibited to the greatest extent possible, through prevention and early treatment[5]. In the last decade, Helicobacter pylori (H. pylori) infection of the stomach has been demonstrated to induce an inflammatory response in the colon through the production of toxins, thereby promoting the development of CC, to some extent[6]. Considering the increasing number of patients with colonic polyps in our country and the large number of H. pylori infections, an in-depth understanding of the current status and risk factors for H. pylori infections in these patients is essential[7]. A previous study[8] revealed that the development of colon tumors is significantly associated with H. pylori infection. Simultaneously, H. pylori infection is also identified as a risk factor for the development of colon adenomas, especially progressive or multiple adenomas. Therefore, this study aimed to analyze the risk factors for the development of H. pylori infection in patients with colonic polyps, and the relationship between their pathological type and the development of H. pylori infection.
Eighty patients who underwent colon polypectomy at our hospital between January 2019 and January 2023 were retrospectively selected as participants. They were randomly divided into a modeling cohort (n = 56) and a model validation cohort (n = 24) at a ratio of 7:3 using the R language.
Inclusion criteria: (1) Participants who met the indications for colonoscopic polypectomy; (2) those who underwent the 14C-urea breath test; (3) had no immune system disease or immune dysfunction; (4) no psychiatric disorders and were able to communicate and interact normally; and (5) had complete clinical data. Participants that met all the above criteria were included in this study.
Exclusion criteria: Participants who met any one of the following criteria were excluded from the study: (1) Participants with a previous history of gastrointestinal disease or colon tumor; (2) those who presented with coagulation disorders after discontinuing oral anticoagulant medication for < 1 wk; and (3) those who were on medication prior to H. pylori screening.
The modeling cohort was divided into an H. pylori-infected group (n = 37) and an H. pylori-uninfected group (n = 19) according to whether the patients developed an H. pylori infection. Patients were monitored for the occurrence of H. pylori infection, which served as the endpoint.
The general information of the patients was collected through electronic medical records. This included general information [sex, age, body mass index (BMI), exercise, education, smoking and drinking habits, history of hypertension and diabetes mellitus, and heavy diet consumptions] and specialty information (number, size, location, and pathological type of the polyps, and whether they were high-risk adenomas).
We analyzed the risk factors for developing H. pylori infection after colon polyp surgery by observing the age, sex, BMI, and exercise of patients in the modeling cohort (H. pylori-infected and H. pylori-uninfected groups). In addition, we assessed whether or not they smoked, consumed alcohol, suffered from high blood pressure, consumed a heavy diet, and had diabetes mellitus. The number, size, location, and the pathological type of polyps, and the presence of high-risk adenomas, were also assessed. All of the information was used to develop and validate a roadmap prediction model. Finally, the correlation between the different pathological types and the occurrence of H. pylori after colon polyp surgery was analyzed.
SPSS 26.0 software and R software were used to analyze the data. The collected count data were expressed as cases (%); χ2 or Fisher exact test was used for unordered data, and the Mann–Whitney U test was used for ordered data. Univariate and multivariate binary logistic regression analyses were used to analyze the factors influencing the development of H. pylori infection after colon polyp surgery and to develop a column-line graph prediction model. The discriminative power of the validation set and calibration graphs were used to assess the accuracy of the column-line graphs. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the discriminative ability of the column diagram. Calibration curves for the model were calculated, and the consistency of the model was verified using the Hosmer–Lemeshow test. Decision curve analysis (DCA) was performed to evaluate the discriminative ability of the model. P < 0.05 was considered statistically significant, and correlations were tested using Spearman’s test.
A total of 93 patients with severe traumatic brain injury were included in the study: 56 in the modeling cohort and 24 in the validation cohort. All patients were aged 30–72 years at the time of diagnosis; 42 (52.50%) were male and 38 (47.50%) were female. Other baseline information regarding the modeling and validation cohorts is shown in Table 1.
| Sports event | Total population (n = 80) | Modeling queues (n = 56) | Validation queue (n = 24) | |
| Gender [n (%)] | Male | 42 (52.50) | 30 (53.57) | 12 (50.00) |
| Female | 38 (47.50) | 26 (46.43) | 12 (50.00) | |
| Age (yr, mean ± SD) | 36.15 ± 11.79 | 36.67 ± 10.00 | 35.92 ± 13.58 | |
| BMI [n (%)] | 22.42 ± 3.44 | 22.20 ± 2.32 | 22.64 ± 4.55 | |
| Movement [n (%)] | < 1 h/wk | 38 (47.50) | 27 (48.21) | 11 (45.83) |
| ≥ 1 h/wk | 42 (52.50) | 29 (51.79) | 13 (54.17) | |
| Literacy [n (%)] | Primary and below | 30 (37.50) | 20 (35.71) | 10 (41.67) |
| Junior high school and secondary school | 29 (36.25) | 21 (37.50) | 8 (33.33) | |
| Junior college or above | 21 (26.25) | 15 (26.79) | 6 (25.00) | |
| Smoking [n (%)] | Be | 33 (41.25) | 24 (42.86) | 9 (37.50) |
| Clogged | 45 (56.25) | 32 (57.14) | 13 (54.17) | |
| Alcohol consumption [n (%)] | Be | 44 (55.00) | 31 (55.36) | 13 (54.17) |
| Clogged | 34 (42.50) | 25 (44.64) | 9 (37.50) | |
| History of hypertension [n (%)] | Be | 32 (40.00) | 22 (39.29) | 10 (41.67) |
| Clogged | 48 (60.00) | 34 (60.71) | 14 (58.33) | |
| History of diabetes [n (%)] | Be | 14 (17.50) | 10 (17.86) | 4 (16.67) |
| Clogged | 66 (82.50) | 46 (82.14) | 20 (83.33) | |
| Number of polyps [n (%)] | An odd one | 38 (47.50) | 27 (48.21) | 11 (45.83) |
| Multi- (faceted, ethnic etc.) | 42 (52.50) | 29 (51.79) | 13 (54.17) | |
| Polyp size [n (%)] | < 1 cm | 33 (41.25) | 23 (41.07) | 10 (41.67) |
| ≥ 1 cm | 47 (58.75) | 33 (58.93) | 14 (58.33) | |
| Polyp site [n (%)] | Proximal | 27 (33.75) | 19 (33.93) | 8 (33.33) |
| Far end | 25 (31.25) | 18 (32.14) | 7 (29.17) | |
| Whole colon | 28 (35.00) | 19 (33.93) | 9 (37.50) | |
| Type of polyp pathology [n (%)] | Adenomatous polyp | 35 (43.75) | 24 (42.86) | 11 (45.83) |
| Non-adenomatous polyp | 45 (56.25) | 32 (57.14) | 13 (54.17) | |
| High-risk adenomas [n (%)] | Be | 23 (28.75) | 16 (28.57) | 7 (29.17) |
| Clogged | 57 (71.25) | 40 (71.43) | 17 (70.83) | |
| Heavy diet [n (%)] | Be | 46 (57.50) | 32 (57.14) | 1 4 (58.33) |
| Clogged | 34 (42.50) | 24 (42.86) | 10 (41.67) | |
There were no statistically significant differences in sex composition, exercise and smoking status, history of hypertension and diabetes mellitus, number of polyps, polyp size, or polyp site in the model cohort (P > 0.05). The differences in age, BMI, literacy level, alcohol consumption, polyp pathological type, high-risk adenomas, and heavy diet consumption in the H. pylori-infected group were statistically significant when compared with the H. pylori-uninfected group (P < 0.05; Table 2).
| Sports event | Hp infection group (n = 37) | Hp uninfected group (n = 19) | χ2 value | P value | |
| Gender [n (%)] | Male | 20 (54.05) | 10 (52.63) | 0.010 | 0.920 |
| Female | 17 (45.95) | 9 (47.37) | |||
| Age (yr, mean ± SD) | 46.58 ± 3.50 | 4.788 | 0.000 | ||
| BMI [n (%)] | 20.37 ± 1.65 | 5.114 | 0.000 | ||
| Movement [n (%)] | < 1 h/wk | 18 (48.65) | 9 (47.37) | 0.008 | 0.928 |
| ≥ 1 h/wk | 19 (51.35) | 10 (52.63) | |||
| Literacy [n (%)] | Primary and below | 16 (43.24) | 4 (21.05) | 2.348 | 0.019 |
| Junior high school and secondary school | 15 (40.54) | 6 (31.58) | |||
| Junior college or above | 6 (16.22) | 9 (47.37) | |||
| Smoking [n (%)] | Be | 16 (43.24) | 8 (42.11) | 0.007 | 0.935 |
| Clogged | 21 (56.76) | 11 (57.89) | |||
| Alcohol consumption [n (%)] | Be | 24 (64.86) | 7 (36.84) | 3.989 | 0.046 |
| Clogged | 13 (35.14) | 12 (63.16) | |||
| History of hypertension [n (%)] | Be | 15 (40.54) | 7 (36.84) | 0.072 | 0.788 |
| Clogged | 22 (59.46) | 12 (63.16) | |||
| History of diabetes [n (%)] | Be | 9 (24.32) | 1 (5.26) | 1.946 | 0.163 |
| Clogged | 28 (75.68) | 18 (94.74) | |||
| Number of polyps [n (%)] | An odd one | 17 (45.95) | 10 (52.63) | 0.225 | 0.636 |
| Multi- (faceted, ethnic etc.) | 20 (54.05) | 9 (47.37) | |||
| Polyp size [n (%)] | < 1 cm | 15 (40.54) | 8 (42.11) | 0.013 | 0.910 |
| ≥ 1 cm | 22 (59.46) | 11 (57.89) | |||
| Polyp site [n (%)] | Proximal | 10 (27.03) | 9 (47.37) | 2.326 | 0.313 |
| Far end | 13 (35.14) | 5 (26.32) | |||
| Whole colon | 14 (37.84) | 5 (26.32) | |||
| Type of polyp pathology [n (%)] | Adenomatous polyp | 20 (56.76) | 4 (21.05) | 5.583 | 0.018 |
| Non-adenomatous polyp | 17 (27.03) | 15 (52.63) | |||
| High-risk adenomas [n (%)] | Be | 14 (37.84) | 2 (10.53) | 4.588 | 0.032 |
| Clogged | 23 (62.16) | 17 (89.47) | |||
| Heavy diet [n (%)] | Be | 25 (67.57) | 7 (36.84) | 4.839 | 0.028 |
| Clogged | 12 (32.43) | 12 (63.16) | |||
In the model cohort, H. pylori infection was the dependent variable and assigned 1, and its absence was assigned 0. Variables with P < 0.05 in the clinical data were included in the univariate analysis. The univariate results showed that age, BMI, literacy level, alcohol consumption, type of polyp pathology, high-risk adenomas, and a heavy diet consumption were all influential factors in the occurrence of H. pylori infections after intestinal polypectomy (Table 3).
| Considerations | B | SE | Wals | P value | OR | 95%CI |
| Age | -0.169 | 0.049 | 12.137 | 0.000 | 0.844 | 0.768-0.929 |
| BMI | 0.738 | 0.216 | 11.708 | 0.001 | 2.093 | 1.371-3.194 |
| Educational attainment | -0.912 | 0.393 | 5.383 | 0.020 | 0.402 | 0.186~0.868 |
| Drinking wine | 1.152 | 0.587 | 3.850 | 0.050 | 3.165 | 1.001-10.004 |
| Types of polyp pathology | 1.484 | 0.652 | 5.178 | 0.023 | 0.227 | 0.063-0.814 |
| High-risk adenoma | 1.644 | 0.821 | 4.010 | 0.045 | 5.174 | 1.035-25.852 |
| Heavy diet | 1.399 | 0.596 | 5.506 | 0.019 | 4.052 | 1.259-13.038 |
Variables with P < 0.05 in the univariate analysis were included in the binary multivariate logistic regression analysis, which showed that age, BMI, and pathologic type of polyp were independent predictors of the development of H. pylori infection after intestinal polypectomy, with the model equation: Logistic = −3.798 – 0.342 × age + 1.222 × BMI − 3.760 × type of polyp pathology (Table 4).
| considerations | B | SE | Wals | P value | OR | 95%CI |
| Age | -0.342 | 0.145 | 5.574 | 0.018 | 0.710 | 0.535-0.944 |
| BMI | 1.222 | 0.446 | 7.524 | 0.006 | 3.395 | 1.418-8.130 |
| Types of polyp pathology | -3.760 | 1.772 | 4.505 | 0.034 | 0.023 | 0.001-0.750 |
| Constant | -3.798 | 7.216 | 0.277 | 0.599 | 0.022 | - |
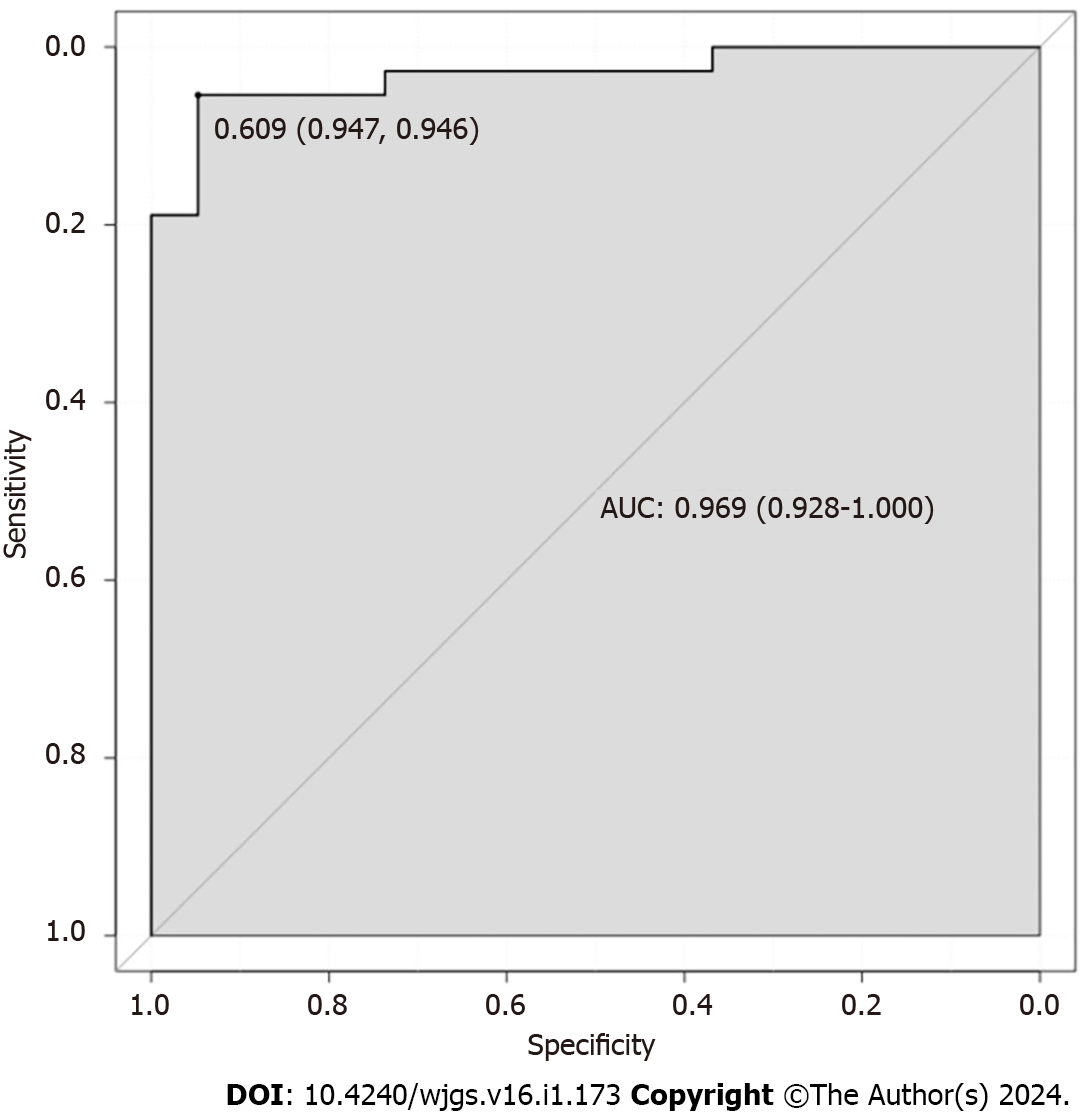
The resulting three independent risk factors (age, BMI, and polyp pathology type) were used to construct a prediction model using R software, and subsequent column-line graph model, as shown in Figure 1. The C-statistic of the model was calculated using the R language software as 0.809, with a 95% confidence interval (95%CI) of 0.761–0.890 and a standard error of 0.030 (P < 0.001). The C-statistic was calculated using the R language software as 0.818, with a standard error of 0.030 (P < 0.001), and the 10000 Bootstrap calculated a C statistic of 0.818. The slope of the generated column-line graph calibration curve was close to 1 (Figure 2), with a goodness-of-fit test of P > 0.05 and a high degree of consistency between the predicted and actual events. The area under the ROC curve of the column-line diagram prediction model was 0.969 (95%CI: 0.928–1.000) (Figure 3). The decision analysis curve is shown in Figure 4, where the X-axis indicates the threshold probability, the Y-axis indicates the net return, and the black solid line indicates the net return using the column-line diagram prediction model, which shows a higher return and further confirms the effectiveness of the column-line diagram prediction model.
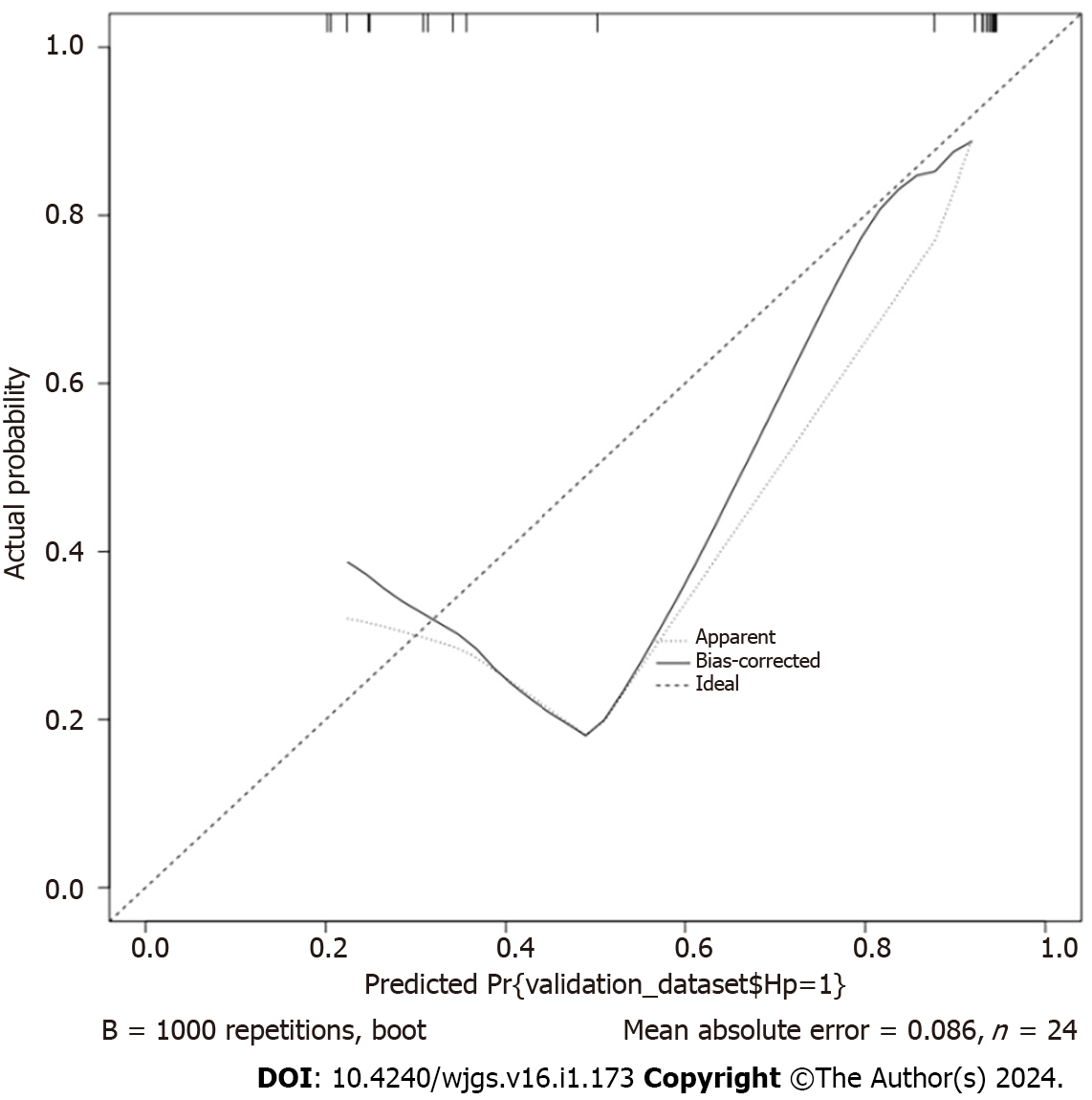
Based on the validation cohort (n = 24), which was divided into H. pylori-infected (n = 16) and H. pylori-uninfected (n = 8) groups, the column-line diagram of the risk of H. pylori infection was externally validated using an ROC curve, and the lower product of the ROC curve was 0.898 (95%CI: 0.773–1.000) (Figure 5). The slope of the generated calibration curve for the column-line diagram was close to 1 (Figure 6), and the result of the Hosmer-Lemeshow test was, χ2 =10.609, P = 0.157 > 0.05. The decision curve showed a higher net benefit of the model (Figure 7), suggesting that the calibration of the column-line diagram model in the validation group was better.
Three pathologic types were identified in the model cohort of patients with colon polyps. In the H. pylori-infected group, these included seven cases of inflammatory polyps, 10 cases of hyperplastic polyps, and 20 cases of adenomatous polyps. In the H. pylori-uninfected group, these included five cases of inflammatory polyps, 10 cases of hyperplastic polyps, and four cases of adenomatous polyps. Correlation analysis of the occurrence of different pathological types and H. pylori infection after colon polyp surgery was performed. The results of the correlation analysis showed no significant correlation between hyperplastic polyps, inflammatory polyps, and the occurrence of H. pylori infection. In contrast, adenomatous polyps showed a significant positive correlation with the occurrence of H. pylori infection (Table 5).
| Inflammatory polyp | Hyperplastic polyp | Adenomatous polyp | ||
| Helicobacter pylori infection | Correlation coefficient | -0.085 | -0.253 | 0.316 |
| Sig. (bilateral) | 0.532 | 0.060 | 0.018 | |
H. pylori is a bacterium that parasitizes areas such as the stomach or duodenum, and can survive for long periods of time under conditions of little oxygen. It not only has the ability to secrete toxic substances, which contribute to gastroin
By comparing the clinical data of patients with gastrointestinal polyps, we found that there were statistically significant differences between the H. pylori-infected and H. pylori-uninfected groups in terms of age, BMI, literacy, alcohol consumption, polyp pathology type, presence of high-risk adenomas, and heavy diets consumption. There were no significant differences in the other indicators. The results of the binary logistic one-way regression analysis assigned a value of 1 to the occurrence of H. pylori infection and a value of 2 to the non-occurrence of H. pylori infection as the dependent variables. Moreover, the factors with significant differences in the aforementioned clinical data as the covariates, showed that age, BMI, literacy, alcohol consumption, polyp pathology type, high-risk adenomas, and heavy diets consumption were the factors influencing the occurrence of H. pylori infection after intestinal polypectomy. Subsequently, we performed a binary logistic regression analysis, of the factors with significant differences in the univariate analysis as covariates and found that age, BMI, and polyp pathology type were independent predictors of the occurrence of H. pylori infection after intestinal polypectomy. Among them, younger age is associated with a greater likelihood of developing H. pylori infection after intestinal polypectomy. This may be because younger patients, with continuous changes in their social environment, are presented with increasing work and life pressures, which tend to result in lower resistance of their bodies and thus are more susceptible to H. pylori infection[12]. Additionally, adolescents tend to favor convenient diets, such as high-fat, high-sugar, and high-salt foods, which subsequently increase the risk of H. pylori infection[13]. In contrast, older people have a more regular lifestyle, pay more attention to healthy eating and living habits, have frequent medical checkups, and follow their doctors' advice. This reduces their likelihood of becoming infected with H. pylori[14,15].
In contrast to the age trend, regarding BMI and polyp pathology type, we found that higher BMI is associated with a greater likelihood of H. pylori infection after intestinal polypectomy. Thus patients with adenomatous polyps on polyp pathology had a greater likelihood of H. pylori infection. It has been reported in the literature[16,17] that this can be because there is an association between BMI and H. pylori infection, and that the two factors can interact with each other. Due to the long-term intake of excessively high calories, the immune environment of their organs is changed, which leads to the expansion of adipose tissues and the activation of macrophages through the secretion of chemokines, subsequently causing a localized inflammatory response. Consequently, the immune microenvironment of obese patients creates favorable conditions for the survival of H. pylori; thus, obese people are more likely to be infected with H. pylori. This is similar to the findings of Xie et al[18] Additionally, changes in the intragastric microenvironment due to H. Pylori may lead to intestinal microecological disorders, further affecting the intestinal microecology of the patients. This may lead to intestinal tumor-like lesions and adenomatous polyps[19]. Thus, adenomatous polyps in patients are often accompanied by H. pylori infection. The results of a study by Zhang et al[20] showed that the proportion of adenomatous polyps occurring in H. pylori-infected populations was significantly higher than that in H. pylori-uninfected populations. This is similar to the results of the present study and further supports the findings of the present study.
Additionally, to further clarify the predictive value of age, BMI, and polyp pathology type in the occurrence of H. pylori infection after intestinal polypectomy, we utilized the R software to establish a column-line graph model. The C statistic of this model was calculated using the R language software as 0.809, which indicated that the model had a stronger discriminatory ability and was able to distinguish patients with high likelihood to develop H. pylori infection. The slope of the calibration curve of the column-line graph it generated was close to 1, and the test of goodness of fit was P > 0.05, which showed that the model had a strong calibration ability. The consistency between the predicted events and the actual events was high, and the area under the ROC curve was 0.969 (95%CI: 0.928-1.000). This indicated that the model was more efficacious in predicting the risk of H. pylori infection. Furthermore, the AUC value was closer to 1, indicating that the model is more capable of discriminating risk. The decision analysis curve showed a higher yield, further confirming the validity of the column–line graph prediction model. Further external validation ROC curve product under the curve was 0.898 (95%CI: 0.773–1.000), which indicated that the model also performed well in the external validation cohort and had good generalization ability. The slope of the generated column-line graph calibration curve was close to 1, with a Hosmer–Lemeshow test result of P > 0.05. Moreover, the decision curve showed a higher net gain of the model, suggesting that the column-line graph model had a better calibration ability in the validation cohort. The column-line diagram model of H. pylori infection risk obtained in this study showed good predictive and calibration abilities for both in-sample and out-of-sample validations. According to the visualized form of the column-line diagram, age ≤ 50 years, lower education level, and higher BMI are associated with higher risk of H. pylori infection after intestinal polypectomy. Moreover, patients with adenomatous polyps often have H. pylori infection. This showed effective clinical discrimination of the high-risk group of H. pylori infection after intestinal polypectomy, based on the information of patients in the aforementioned key factors. Therefore, the present study illustrated simple predictors that are favorable for the early prevention of H. pylori infection.
In conclusion, age, BMI, and polyp pathology of the adenomatous type were independent predictors of H. pylori infection after intestinal polypectomy. In addition, the columnar graph prediction model of H. pylori infection after intestinal polypectomy showed good predictive ability, which provided assistance in the clinical identification of high-risk groups of H. pylori infection after intestinal polypectomy. This is beneficial for the timely prevention of H. pylori infection. However, because this study was a retrospective analysis, the sample size was limited, and more clinical indicators should be added for further comprehensive assessment and establishment of a more comprehensive prediction model.
Colon polyps are tumor-like lesions that grow on the surface of the colonic mucosa, usually in the form of a protruding or bulging mass, or meaty lesion. They are abnormal tissue that can develop into colorectal cancer. Considering that the number of patients with colon polyps in our country has been rising and that a large number of Helicobacter pylori (H. pylori) infections also exist, an in-depth understanding of the current status of H. pylori infections in patients with colonic polyps in our country and the risk factors for these infections is necessary.
The development of colon tumors is significantly associated with H. pylori infection, of which colonic adenomatous polyps may develop into colon cancer. It is also a risk factor for the development of colonic adenomas, especially progressive or multiple adenomas. However, few clinical studies have investigated the correlation between the pathological types of colonic polyps and H. pylori infection.
To investigate the risk factors for the development of H. pylori infection after colon polyp surgery, and to establish the relationship between the type of pathology and its occurrence.
Eighty patients who underwent colon polypectomy in our hospital from January 2019 to January 2023 were retrospectively selected as participants, and randomly divided into a modeling cohort (n = 56) and a model validation cohort (n = 24) at a ratio of 7:3 using R. Simultaneously, based on whether the patients were infected with H. pylori, the modeling cohort was divided into an H. pylori-infected group (n = 37) and an H. pylori-uninfected group (n = 19). The risk factors for H. pylori after colon polyp surgery were analyzed by comparing the age, sex, body mass index (BMI), and exercise status of patients in the modeling cohort (H. pylori-infected and H. pylori-uninfected groups). In addition, whether or not they smoked, consumed alcohol, suffered from hypertension and diabetes mellitus, and had heavy diets, and the number, size, location, and the pathological type of the polyps, and whether or not they were high-risk adenomas, were also analyzed. A binary logistic regression analysis was used to analyze the factors influencing the occurrence of H. pylori infection after colon polyp surgery. A roadmap prediction model was therefore established and validated; receiver operating characteristic was used to evaluate the predictive efficacy of the model; calibration curves were used to assess the consistency between predicted and actual events. DCA curves were also used to evaluate the validity of the model; and finally, the correlation between the different pathological types of colon polyps and the occurrence of H. pylori infection was analyzed after colon polyp surgery.
Age, BMI, and polyp pathology type were independent predictors of H. pylori infection after intestinal polypectomy. Additionally, the H. pylori infection risk column-line diagram model obtained in this study demonstrated good predictive and calibration abilities for both in-sample and out-of-sample validations. The visualized form of the column-line diagram showed that for age ≤ 50 years, the lower the education level, the higher the risk of H. pylori infection after intestinal polypectomy, the higher the BMI, the higher the risk of H. pylori infection, and that patients with adenomatous polyps often have H. pylori infection. This is conducive to the effective clinical discrimination of patients at high risk of H. pylori infection, after intestinal polypectomy, based on the information of the above mentioned key factors. Moreover, the predictors obtained in this study are favorable for the early prevention of H. pylori infection.
Age, BMI, and polyp pathology of the adenomatous type were all independent predictors of H. pylori infection after intestinal polypectomy, and the column-line graph prediction model of H. pylori infection after intestinal polypectomy showed good predictive ability. This provides assistance in the clinical identification of high-risk groups for H. pylori infection after intestinal polypectomy and is conducive to timely prevention.
This study was a retrospective analysis with a limited sample size, and additional clinical indicators need to be added for further comprehensive assessment and predictive modeling.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bhansali A, India; Rastogi A, India S-Editor: Lin C L-Editor: A P-Editor: Yu HG
| 1. | Yan Z, Sun YM, Gao F, Lang HB, Zhang J. Analysis of risk factors for delayed bleeding after endoscopic mucosal resection of colon polyps. Zhongguo Yiyao Daobao. 2023;20:132-135. [DOI] [Full Text] |
| 2. | Han L, Jiang SL. Observation on the efficacy of endoscopic mucosal resection in patients with colonic polyps. Xiandai Yixueyujiankang Yanjiu (Dianziban). 2023;7:56-59. [DOI] [Full Text] |
| 3. | Sninsky JA, Shore BM, Lupu GV, Crockett SD. Risk Factors for Colorectal Polyps and Cancer. Gastrointest Endosc Clin N Am. 2022;32:195-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Shuai XW, Xie PY. Guidelines for colonoscopic follow-up protocols after resection of colon cancer--Updated consensus of the U.S. Multicenter Task Force on Colorectal Cancer and the American Cancer Society (2006). Zhongguo Neijing Zazhi. 2007;889-891. |
| 5. | Chao G, Zhu Y, Fang L. Retrospective study of risk factors for colorectal adenomas and non-adenomatous polyps. Transl Cancer Res. 2020;9:1670-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Chen CC, Liou JM, Lee YC, Hong TC, El-Omar EM, Wu MS. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes. 2021;13:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (3)] |
| 7. | Hou Y M, Li H, Wang L, Xiang X H, Yang S G. Association of colon polyps with Helicobacter pylori infection and gastric polyps. Zhongguo Neijing Zazhi. 2023;29:73-80. [DOI] [Full Text] |
| 8. | Yang QJ, Zheng J, Yang J, Luo R, Leng J, Jin Q, Ma HL. Study on the effect of Helicobacter pylori infection on nonalcoholic fatty liver disease and its associated colorectal polyps. Jiating Yixue. 2021;4:3855-3862. [DOI] [Full Text] |
| 9. | Yang Z P, Tian Y J, Wang Y Z, Zhao Y D. Relationship between oral Helicobacter pylori infection and Helicobacter pylori gastritis. Beihua Daxue Xuebao(Zirankexueban). 2022;23:352-356. [DOI] [Full Text] |
| 10. | de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, Neves PHM, de Melo FF. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25:5578-5589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 198] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (15)] |
| 11. | Qiu E, Li Z, Han S. Methods for detection of Helicobacter pylori from stool sample: current options and developments. Braz J Microbiol. 2021;52:2057-2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Liu L, Zhu ZH. Influencing factors of Helicobacter pylori infection in gastroenterology patients and its relationship with gastrointestinal diseases. Xiandai Yangsheng (Xiabanyueban). 2020;20:52-55. [DOI] [Full Text] |
| 13. | Cao YF, Li XL, Wang YF, Wu WZ, Xu C, Wang SS, Huang HX. Study on the influencing factors of Helicobacter pylori infection based on age stratification. Zhejiang Yixue. 2023;45:140-144. [DOI] [Full Text] |
| 14. | LI XY, GAO CG. Analysis of the results of 102 cases of 13C-urea breath test for detecting Hp infection in patients and related influencing factors in Xuchang Central Hospital. Yixue Yanjiu. 2021;30:1010-1012. [DOI] [Full Text] |
| 15. | Li X, Wang J. Survey on factors affecting compliance with standardized treatment of Helicobacter pylori infection patients in digestive system and intervention countermeasures. Guizhou Yixue. 2023;47:549-550. [DOI] [Full Text] |
| 16. | Yusuf Tohti, Li K. Research progress on the correlation between Helicobacter pylori infection and obesity. Zhonghua Feipangyudaixiebing Zazhi. 2020;6:196-199. [DOI] [Full Text] |
| 17. | AlAli MN, Bamehriz F, Arishi H, Aldeghaither MK, Alabdullatif F, Alnaeem KA, Alzamil AF, AlHashim IR, Alhaizan S, Aljuhani T, Aldohayan A. Trends in bariatric surgery and incidentalomas at a single institution in Saudi Arabia: a retrospective study and literature review. Ann Saudi Med. 2020;40:389-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Xie Q, He Y, Zhou D, Jiang Y, Deng Y, Li R. Recent research progress on the correlation between metabolic syndrome and Helicobacter pylori infection. Peer J. 2023;11:e15755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Ouyang Y, Zhang W, Huang Y, Wang Y, Shao Q, Wu X, Lu N, Xie C. Effect of Helicobacter pylori eradication on hyperplastic gastric polyps: A systematic review and meta-analysis. Helicobacter. 2021;26:e12838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Zhang P, Wang P, Hong R, Huang ML Q. Clinical characteristics of Hp infection in patients with colorectal polyps and its relationship with G-17, sIL-2R and COX-2. Zhonghua Yiyuan Ganranxue Zazhi. 2023;33:81-85. [DOI] [Full Text] |