Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.113
Peer-review started: October 12, 2023
First decision: December 15, 2023
Revised: December 20, 2023
Accepted: January 10, 2024
Article in press: January 10, 2024
Published online: January 27, 2024
Processing time: 104 Days and 16.6 Hours
With the increasing incidence of proximal gastric cancer, laparoscopic proximal gastrectomy has been applied. However, reflux esophagitis often occurs after traditional esophagogastric anastomosis. In order to solve this problem, several methods of digestive tract reconstruction have emerged, but the most satisfying method remains to be discussed. Therefore, we modified traditional Kamikawa anastomosis to investigate the appropriate digestive tract reconstruction in laparoscopic proximal gastrectomy.
To discuss the clinical efficacy of modified Kamikawa anastomosis in laparoscopic proximal gastrectomy.
A retrospective case series was adopted. Clinicopathological data were collected from 26 patients who underwent laparoscopic proximal gastrectomy and modified Kamikawa anastomosis at our hospital from January 2020 to September 2022. The operation conditions, postoperative recovery, postoperative complications, and follow-up data were collected and analyzed.
All the patients were successfully operated on without conversion to laparotomy. The duration of operation and digestive tract reconstruction were 203.500 (150-224) min and 87.500 (73-111) min, respectively. The intraoperative amount of bleeding was 20.500 mL ± 0.696 mL. The time of postoperative first flatus, the first postoperative fluid intake, and the postoperative length of stay were 2 (1-3) d, 4 (3-5) d, and 9 (8-10) d, respectively. All the patients were followed up for 12-23 months. The body mass index at 6 and 12 months after surgery were 22.577 kg/m2 ± 3.098 kg/m2 and 22.594 kg/m2 ± 3.207 kg/m2, respectively. The nutrition risk screening 2002 score, the patient-generated subjective global assessment score, and the gastroesophageal reflux disease scale score were good at 6 and 12 months after surgery. Reflux esophagitis and anastomotic stenosis were not observed in any of the patients during their 12-month postoperative gastroscopy or upper gastrointestinal tract visits. All the patients exhibited no tumor recurrence or metastasis.
The modified Kamikawa anastomosis is safe and feasible for laparoscopic proximal gastrectomy and has good antireflux effects and nutritional status.
Core Tip: The study retrospectively analyzed clinicopathological data of patients who underwent laparoscopic proximal gastrectomy and modified Kamikawa anastomosis. According to our research, modified Kamikawa anastomosis in laparoscopic proximal gastrectomy is safe, feasible and shows good antireflux effect.
- Citation: Wu CY, Lin JA, Ye K. Clinical efficacy of modified Kamikawa anastomosis in patients with laparoscopic proximal gastrectomy. World J Gastrointest Surg 2024; 16(1): 113-123
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/113.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.113
The incidence of proximal gastric cancer (PGC) has recently increased and has thereby attracted increased amounts of attention from surgeons[1]. A national survey in Korea showed that the incidence of PGC in Korea increased from 11.2% to 16.0% in 2014[2]. The traditional treatment for PGC is total gastrectomy. However, with the development of medicine and improvements in the detection rate of early gastric cancer (GC), maximal preservation of gastric function has become a new requirement for radical excision of tumors. Therefore, proximal gastrectomy for preserving gastric function has emerged. Proximal gastrectomy for early GC does not affect patients’ long-term survival and can effectively improve their postoperative nutritional status. Nevertheless, reflux esophagitis occurring after traditional esophagogastric anastomosis has an incidence as high as 21.8%-71.6% and significantly affects patients’ postoperative quality of life (QOL)[3]. Several gastrointestinal reconstruction methods after proximal gastrectomy have emerged, including tubular esophagogastric anastomosis, esophagogastric lateral anastomosis, double-channel anastomosis, and interposed jejunum[4]. Although these techniques effectively decrease the incidence of postoperative reflux, they are accompanied by nearly hidden dangers involving operation difficulties or anastomotic complications[5]. Therefore, ensuring surgical safety and patient postoperative QOL has become the focus of domestic and foreign research.
In 1998, Kamikawa et al[6] reported of a new esophagogastric double-flap technique, also known as Kamikawa anasto
The retrospective case series method was adopted in this study. Clinicopathological data were collected from 26 patients who underwent laparoscopic proximal gastrectomy and modified Kamikawa anastomosis at our hospital from January 2020 to September 2022. The patients and their families signed informed consent forms. The clinicopathological characteristics of the patients are shown in Table 1.
| Item | Value |
| Age (mean ± SD, yr) | 68.846 ± 1.352 |
| Sex | |
| Male | 22 |
| Female | 4 |
| Tumor location | |
| Esophagogastric junction | 3 |
| Upper stomach | 23 |
| Maximum tumor diameter (mean ± SD, cm) | 2.069 ± 0.164 |
| Histological grade | |
| Poor | 6 |
| Moderately | 10 |
| Well | 10 |
| Tumor stage | |
| T stage | |
| T1 | 15 |
| T2 | 11 |
| BMI (mean ± SD, kg/m2) | 22.623 ± 3.103 |
| NRS2002 score [M (range)] | 2 (1-2) |
| PG-SGA score [M (range)] | 1(1-3) |
The inclusion criteria were as follows: (1) PGC confirmed through pathological examination via gastroscopic biopsy before the operation; (2) a tumor diameter < 4 cm; (3) a clinical stage of cT1-2N0M0 according to preoperative enhanced computed tomography (CT) and endoscopic ultrasonography; (4) no distant metastasis before the operation; and (5) lacked a history of abdominal operation.
The exclusion criteria were as follows: (1) A dentate line involved in the tumor; (2) had received neoadjuvant therapies before the operation; (3) had severe cardiopulmonary dysfunction and poor nutritional status and could not tolerate the operation; (4) had complications related to other malignant tumors; and (5) lacked complete clinicopathological data.
In this study, all the operations were performed by the same group of surgeons. The patients were placed in the supine split-leg position, with the head side positioned slightly taller than the rest of the body. After the administration of anesthesia, skin preparation and draping were performed. The surgeons stood on the left side of the patient, and the surgical assistants stood on the right side of the patient. The camera holder was positioned between the legs of the patient. In addition, the five-hole method was adopted. A 12 mm trocar was inserted below the umbilicus as an observation hole to establish pneumoperitoneum, with the pressure maintained at 12-15 mmHg (1 mmHg = 0.133 kPa). A 12 mm trocar and a 5 mm trocar were inserted 2 cm below the costal margin on the left anterior axillary line and 2 cm above the umbilicus on the left midclavicular line as operation holes, respectively. A 5 mm trocar was inserted at the corresponding parts on the right as operation holes. The location and size of the tumor, the degree of infiltration of the tumor, and its relationship with peripheral organ tissues were probed by using a laparoscope.
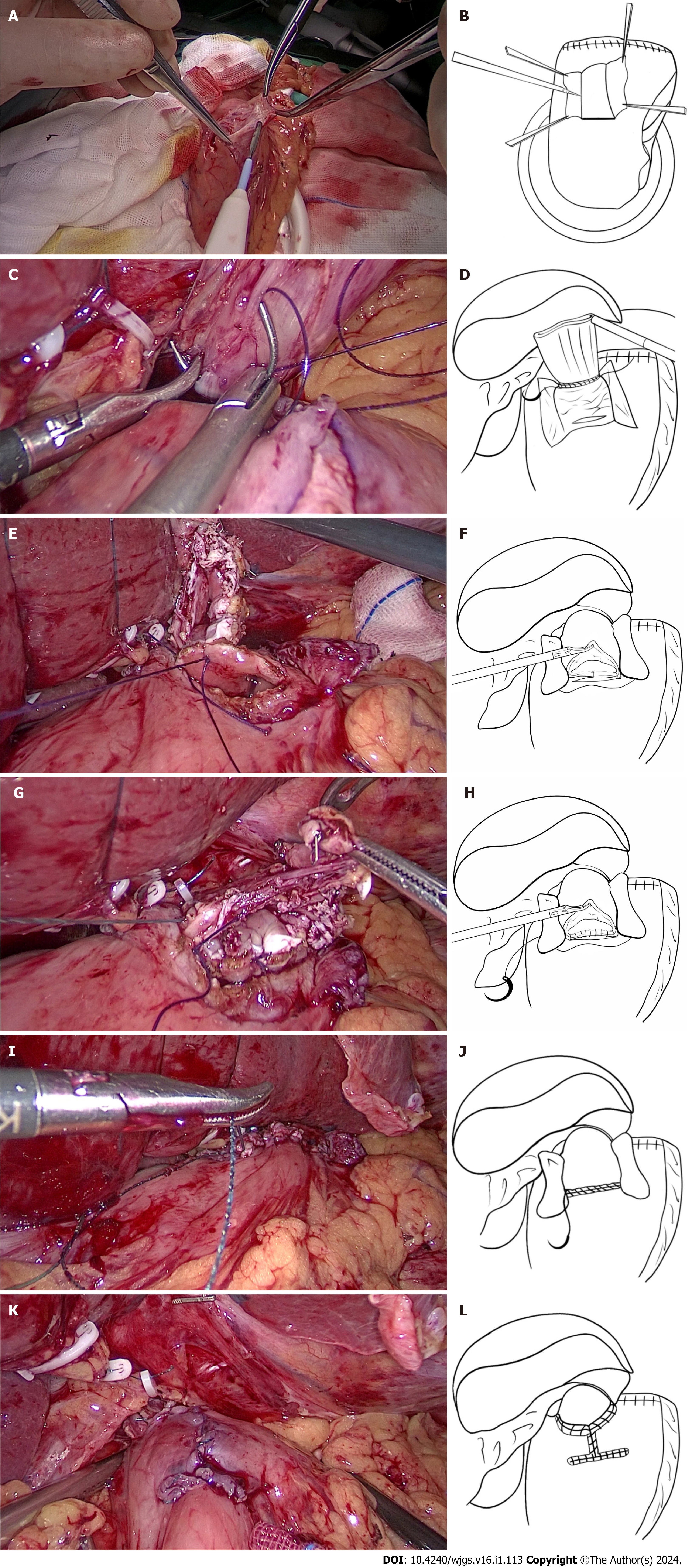
Laparoscopic proximal gastrectomy and modified Kamikawa anastomosis were performed. First, the falciform ligament was cut. The left deltoid ligament and part of the omentum were separated. The left external lobe of the liver was dissociated, displaced, and placed over the right lobe of the liver by cutting the falciform ligament. The liver was fixed and suspended with a purse suture to enable external puncture. The proximal stomach was dissociated, and the D1+ lymph nodes were dissected in accordance with the Japanese GC treatment guidelines (5th edition)[10] and the Japanese classification of gastric carcinoma (3rd edition)[11]. The tumor was located via intraoperative endoscopy. The esophageal hiatus was opened, and the esophagus was fully dissociated, exposed, and severed. The posterior wall at 5 cm from the esophageal stump was marked with gentian violet. The stomach was removed through a small subxiphoid incision. Afterwards, the proximal stomach was severed with a linear cutting stapler 3 cm from the distal end of the tumor, and intermittent sutures were applied to the gastric stump for reinforcement. The intraoperative incisal edge was sent for rapid frozen-section pathological examination. Subsequently, gentian violet was used to mark the anterior wall of the residual stomach (approximately 1.5 cm from the upper incisal edge) near the lesser curvature of the stomach with a 2.5-3.0 cm × 3.5 cm I-shaped mark. The width of the seromuscular flap matched the diameter of the esophagus. Additionally, the superior border of the seromuscular flap was parallel to the upper incisal edge of the residual stomach. Anatomical separation between the submucosa and muscularis layers was performed by using an electrotome along the mark to prepare the seromuscular flap. Care was taken to protect the integrity of the seromuscular flap and gastric mucosa. At this point, a surgical assistant applied upward vertical traction to the muscle flap to close it. The surgeon subsequently used an electrotome to completely dissect the submucosal layer from the mucosa to create the seromuscular flap (Figure 1A and B). The submucosa and mucosa were cut off at the lower margin of the seromuscular flap anasto
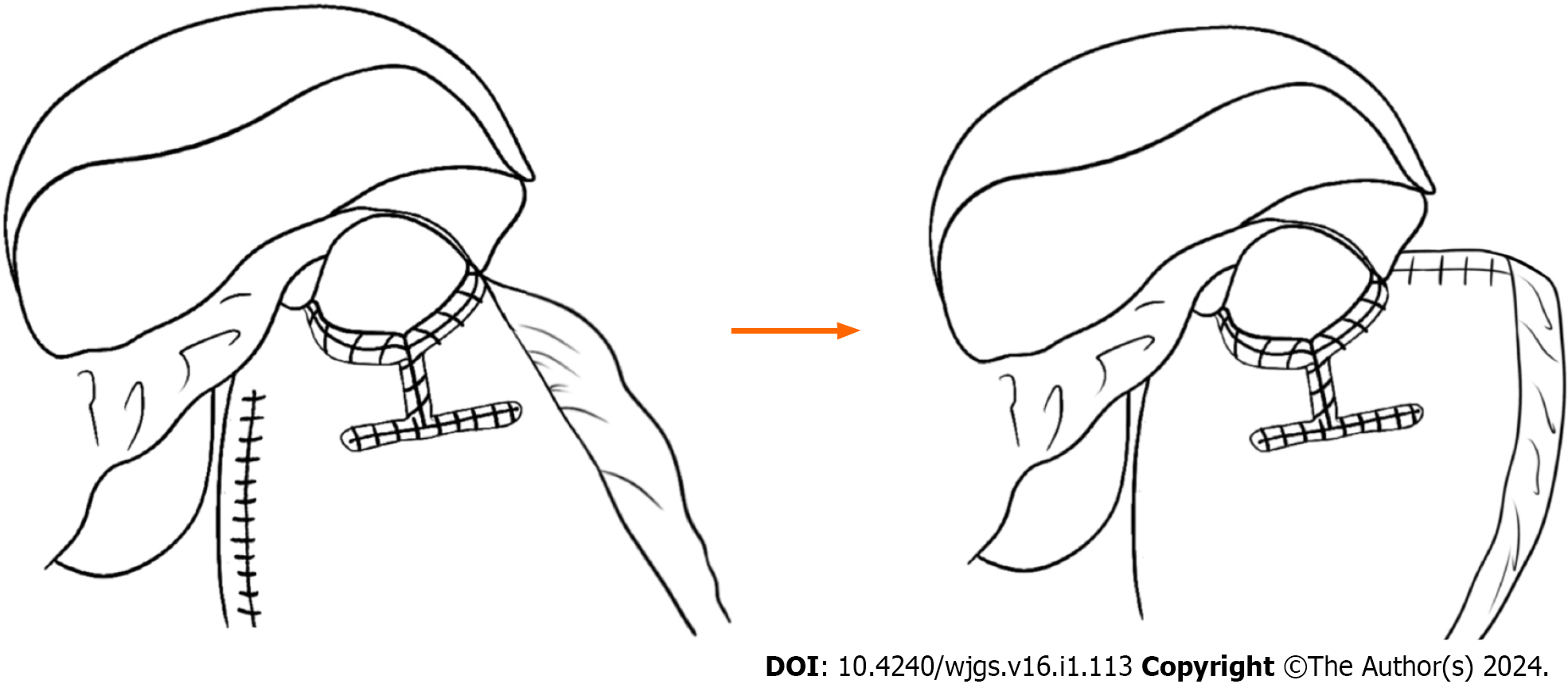
The following points are the main modifications of the original Kamikawa anastomosis that was performed by the author’s center: (1) The left extrahepatic lobe was separated and suspended, thus reducing the blockage, and ensuring a good view and easy operation. Additionally, the operation requires no extra instrumentation, is simple and practicable, and does not cause any trauma to the liver; (2) as the width of the esophagus is commonly 2.5-3.0 cm, the width of the I-shaped seromuscular flap is changed to 2.5-3.0 cm to match the diameter of the esophagus and consequently lower the incidence of anastomotic stenosis; (3) the posterior wall of the esophageal stump opening and the superior border of the anastomotic stoma were first fixed by placing two interrupted sutures on the right and in the middle. When the posterior wall of the esophageal stump opening and the superior border of the anastomotic stoma are fixed, subsequent sutures are easier and less likely to shift and cause postoperative anastomotic stenosis; (4) continuous sutures (including the suture of the posterior wall of the esophageal stump, the anastomotic stoma, and the seromuscular flap) were used to improve the feasibility of the operation, thus preventing tedious interrupted suturing and reducing the duration of the operation; (5) after proximal gastric resection, the blood supply to the incisional margin is poor. A seromuscular muscle flap positioned close to the incisal margin may lead to postoperative ischemia and elicit the antireflux effect. During our operation, the superior border of the I-shaped seromuscular flap was parallel to the upper incisal edge of the gastric remnant to ensure that the seromuscular flap was close to the lesser curvature side of the stomach with a better blood supply, thus improving the blood supply in the seromuscular flap and allowing it to produce the anti-reflux effect; and (6) an angle is formed after traditional Kamikawa anastomosis, whereas the pseudofornix reconstructed by our modified method on the left side of the esophagus is larger and has a better antireflux effect. A detailed diagram illustrating these modifications is shown in Figure 2. Attention should be given to the tension of the seromuscular flap. When the tension is too high during the folded suture of the seromuscular flap, the flap may be directly and obliquely sutured to the esophageal wall to reduce tension and prevent postoperative anastomotic stenosis.
Observation indicators: The following indicators were used in this study: (1) Intraoperative conditions: Duration of operation, digestive tract reconstruction, and intraoperative amount of bleeding; (2) postoperative conditions: time of postoperative first flatus, first fluid intake, length of stay (LOS), and postoperative complications (such as ileus, lymphatic fistula, abdominal hemorrhage, anastomotic stoma hemorrhage, anastomotic stenosis, anastomotic fistula, pulmonary infection, and incisional wound infection); and (3) follow-up: Postdischarge nutritional status, symptoms of esophageal reflux, reflux esophagitis, anastomotic stoma conditions, and tumor recurrence and metastasis.
Evaluation criteria: Postoperative complications were evaluated by using the Clavien-Dindo grading standard[12]. Body mass index (BMI), nutrition risk screening (NRS) 2002 score, and patient-generated subjective global assessment (PG-SGA) score were used to evaluate their nutritional status[13]. The gastroesophageal reflux disease (GERD) scale was used to grade the symptoms of esophageal reflux[14]. Reflux esophagitis was diagnosed by using gastroscopy. The Los Angeles grading standard was adopted to evaluate the severity of the disease[15]. The conditions of the anastomotic stoma were examined by using upper gastrointestinal angiography.
The patients were followed up by using outpatient visits, telephone calls, and WeChat to determine their nutritional status, symptoms of esophageal reflux, reflux esophagitis, conditions of anastomotic stoma, and tumor recurrence and metastasis. The follow-up was conducted every three months after surgery and ended in September 2023.
SPSS 26.0 software was used for statistical analysis. Descriptive statistics were adopted. Measurement data with a skewed distribution are represented as M (range), and the rank-sum test was used for comparisons between groups. Normally distributed data are represented as mean ± SD, and a t test was used for comparisons between groups. P < 0.05 was considered to indicate statistical significance.
A total of 26 patients (22 males and 4 females) were included, and the average age was 68.846 years ± 1.352 years. The other characteristics are included in Table 1.
All 26 patients were successfully operated on without conversion to laparotomy. The duration of the operation was 203.500 (150-224) min. The duration of digestive tract reconstruction was 87.500 (73-111) min. The intraoperative amount of bleeding was 20.500 ± 0.696 mL (Table 2).
| Item | Value |
| Duration of operation [M (range), min] | 203.500 (150-224) |
| Duration of digestive tract reconstruction [M (range), min] | 87.500 (73-111) |
| Intraoperative amount of bleeding (mean ± SD, mL) | 20.500 ± 0.696 |
| Time of postoperative first exhaust [M (range), d] | 2 (1-3) |
| Time of the first postoperative fluid intake [M (range), d] | 4 (3-5) |
| Postoperative LOS [M (range), d] | 9 (8-10) |
| Postoperative complications | - |
| Pulmonary infection | 1 |
| Others | 0 |
The duration of postoperative first flatus was 2 (1-3) d. The duration of the first postoperative fluid intake was 4 (3-5) d. The postoperative LOS was 9 (8-10) d. Among the 26 patients, one patient had a postoperative pulmonary infection, and the Clavien-Dindo grade was 2. The patient’s condition improved after conservative treatments, such as anti-infection treatment, oxygen inhalation, and atomization. The remaining 25 patients had no postoperative complications (Table 2).
All 26 patients were followed up for 12-23 months. The median follow-up period was 13 months. The BMIs of the 26 patients at 6 and 12 months after surgery were 22.577 kg/m2 ± 3.098 kg/m2 and 22.594 kg/m2 ± 3.207 kg/m2, respectively. The NRS2002 scores at 6 and 12 months after surgery were 2 (1-2) points and 2 (1-2) points, respectively; additionally, the PG-SGA scores at 6 and 12 months after surgery were 1 (1-3) point and 1 (1-3) point, respectively. Moreover, the GERD scale scores at 6 and 12 months were 3 (2-4) points and 3 (2-4) points, respectively. There were no significant differences in BMI, NRS2002 score, and PG-SGA score before or after surgery (P > 0.05). Reflux esophagitis was not found in any of the patients during their 6-month postoperative gastroscopy. No anastomotic stenosis was observed in any patient during 12-month postoperative upper gastrointestinal angiography. Furthermore, no patients experienced tumor recurrence or metastasis (Table 3).
| Follow-up period [M (range), m] | 16 (12-23) |
| BMI 6 months after the surgery (mean ± SD, kg/m2) | 22.577 ± 3.098 |
| BMI 12 months after the surgery (mean ± SD, kg/m2) | 22.594 ± 3.207 |
| NRS2002 score 6 months after the surgery [M (range)] | 2 (1-2) |
| NRS2002 score 12 months after the surgery [M (range)] | 2 (1-2) |
| PG-SGA score 6 months after the surgery [M (range)] | 1 (1-3) |
| PG-SGA score 12 months after the surgery [M (range)] | 1 (1-3) |
| GERD scale score 6 months after the surgery [M (range)] | 3 (2-4) |
| GERD scale score 12 months after the surgery [M (range)] | 3 (2-4) |
| Reflux esophagitis | 0 |
| Anastomotic stenosis | 0 |
| Recurrence or metastasis | 0 |
According to the Japanese 5th edition of the Guidelines for the Treatment of GC[10], proximal gastrectomy can be performed for early PGC when more than half of the stomach volume can be preserved after R0 resection. However, due to the specificity of the anatomical site, the original physiological structure and function at the esophagogastric junction are lost after proximal gastrectomy. Therefore, the proper procedure for digestive tract reconstruction is still controversial. In 1988, the Japanese scholars Aikou et al[16] first reported that two-channel anastomosis was indicated for digestive tract reconstruction in most proximal gastrectomy patients, especially for those who had extremely small gastric remnants and impaired glucose tolerance, as well as those who were not qualified for esophagogastric anastomosis. However, the risk of postoperative anastomotic fistula is high because the operation is complicated and involves a large number of anastomotic stomata. Additionally, the extensive use of linear cutting staplers also increases the cost. In 1993, Kameyama et al[17] first proposed the use of the interposition jejunum for digestive tract reconstruction in proximal gastrectomy. An antireflux barrier is constructed by taking advantage of the tolerance and natural peristalsis of the digestive juice from the jejunum itself. However, this procedure is also complicated and may cause postoperative emptying disorders[18]. The length of the interposition jejunum segment is also difficult to control; specifically, if it is too long, then it will affect postoperative gastroscopy, and if it is too short, then it will affect the postoperative antireflux effect. In 1998, Shiraishi et al[19] proposed tubular gastrosophageal anastomosis, which allows food to pass quickly to prevent food retention and has a good antireflux effect. The tubular stomach is long and can be lifted to the mediastinum. This approach is particularly suitable for patients with a relatively high incisal edge of the esophagus. However, the risk of postoperative hemorrhage and gastric fistula increases because of the long incisal edge after gastric wall cutting. Yamashita et al[20] designed a reconstruction method (also known as side overlap anastomosis) for accessing the digestive tract via esophagogastric wall anastomosis after laparoscopic proximal gastrectomy. This approach can effectively reduce the reflux of food and digestive juice after surgery. Side overlap anastomosis was completed by using a linear cutting stapler. A wide anastomotic stoma can effectively reduce postoperative anastomotic stenosis. This procedure is easy to perform, and the duration of the operation is short. However, its application is relatively limited because of the need to preserve the long abdominal segment of the esophagus and large gastric remnant. In 2016, Muraoka et al[21] first used Kamikawa anastomosis for proximal gastrectomy. No patient had reflux esophagitis after the operation. In the same year, Kuroda et al[7] reported on Kamikawa anastomosis under laparoscopy and verified its safe and feasible clinical efficacy for proximal gastrectomy. As reported by Shoji et al[9], the incidence of postoperative anastomotic stoma-associated complications decreases after the application of Kamikawa anastomosis in proximal gastrectomy. Additionally, the incidence of reflux esophagitis is effectively decreased via this approach. According to a multicenter Japanese retrospective study, the incidence of reflux esophagitis 1 year after surgery was only 6% among 464 patients who underwent proximal gastrectomy and Kamikawa anastomosis via gastroscopy[8]. The anastomotic stoma from Kamikawa anastomosis is manually sutured; therefore, the cost is low. However, this approach involves a complicated process and has high surgical requirements, particularly for laparoscopic suturing; moreover, it takes more time to perform than other digestive tract reconstruction methods. If the seromuscular flap is improperly made, such as when the width of the seromuscular flap is too short or the suture of the anastomotic stoma is too tight, then anastomotic stenosis will occur. Therefore, the abovementioned factors limit the popularization of this operation.
With these modifications, the technique has become more feasible than the traditional operation. Compared with the traditional approach, the improved Kamikawa anastomosis in this study can reduce the duration of operation to approximately 50%[22]. In the improved Kamikawa anastomosis, the lower esophagus and anastomotic stoma are embedded within an appropriate seromuscular flap that is made at the anterior wall of the gastric remnant. When increased intragastric pressure is applied to the lower esophagus, double flaps can generate counterpressure and close the esophagus above the anastomotic stoma, thus effectively preventing reflux. Moreover, the risk of operative anastomotic fistula is reduced because the sole anastomotic stoma is covered by the seromuscular flap. The matching of the width of the I-shaped seromuscular flap with the width of the esophagus can avoid the anastomotic stenosis caused by the mismatch of the width after reconstruction. Moreover, the modified seromuscular flap is close to the lesser curvature side of the stomach and provides a better blood supply to avoid postoperative anastomotic stenosis or anastomotic fistula caused by ischemia. No anastomotic stenosis was observed in any patient as examined by using upper gastrointestinal angiography. Therefore, improved Kamikawa anastomosis can prevent anastomotic stenosis. The operation was successful in 26 patients, the postoperative recovery was good, and no complications (such as anastomotic fistula or anastomotic stenosis) occurred, thus indicating that the modified Kamikawa anastomosis was safe and feasible. During the follow-up, upper gastrointestinal angiography and gastroscopy demonstrated no reflux or esophagitis in any patient, thus indicating that the improved Kamikawa anastomosis has a good antireflux effect. The postoperative nutritional indices and scores of all the patients were satisfactory, thus indicating that good nutritional status and QOL can be obtained after proximal gastrectomy.
According to the preliminary analysis of 26 patients at the author’s center, laparoscopic proximal gastrectomy and modified Kamikawa anastomosis are safe and feasible approaches with good postoperative recovery. The expected antireflux effect can be achieved, and good nutritional status can be maintained. This was a retrospective study with a small sample size. The long-term efficacy of these regimens must be further observed and verified by multicenter clinical studies with large sample sizes.
The incidence of reflux esophagitis after proximal gastrectomy is high, and the proper reconstruction of digestive tract is still controversial.
To explore the appropriate digestive tract reconstruction method in laparoscopic proximal gastrectomy to reduce the occurrence of postoperative reflux while ensuring the safety and feasibility of the operation.
To explore the clinical efficacy of modified Kamikawa anastomosis in laparoscopic proximal gastrectomy.
We retrospectively collected clinicopathological data of patients who underwent laparoscopic proximal gastrectomy and modified Kamikawa anastomosis. The intraoperative conditions, postoperative conditions and follow-up were analyzed.
The operation of 26 patients was successful and the postoperative recovery was good. No reflux esophagitis and anastomotic stenosis were found in all patients during postoperative follow-up, and their nutritional status was satisfactory.
The modified Kamikawa anastomosis in laparoscopic proximal gastrectomy shows satisfactory antireflux effect and is safe and feasible during operation. We can use this procedure in laparoscopic proximal gastrectomy.
A multicenter prospective study should be performed to verify our modified method.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Weiss H, Austria S-Editor: Chen YL L-Editor: A P-Editor: Zheng XM
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer. 2016;16:131-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 3. | Shibuya S, Fukudo S, Shineha R, Miyazaki S, Miyata G, Sugawara K, Mori T, Tanabe S, Tonotsuka N, Satomi S. High incidence of reflux esophagitis observed by routine endoscopic examination after gastric pull-up esophagectomy. World J Surg. 2003;27:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Haruta S, Shinohara H, Hosogi H, Ohkura Y, Kobayashi N, Mizuno A, Okamura R, Ueno M, Sakai Y, Udagawa H. Proximal gastrectomy with exclusion of no. 3b lesser curvature lymph node dissection could be indicated for patients with advanced upper-third gastric cancer. Gastric Cancer. 2017;20:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg. 2008;196:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Kamikawa Y, Kobayashi T, Kamikawa S. New esophagogastric anastomosis method for preventing reflux after cardia gastrectomy. Gastrointestinal Surgery. 2001;24:1053-1060. |
| 7. | Kuroda S, Nishizaki M, Kikuchi S, Noma K, Tanabe S, Kagawa S, Shirakawa Y, Fujiwara T. Double-Flap Technique as an Antireflux Procedure in Esophagogastrostomy after Proximal Gastrectomy. J Am Coll Surg. 2016;223:e7-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 8. | Kuroda S, Choda Y, Otsuka S, Ueyama S, Tanaka N, Muraoka A, Hato S, Kimura T, Tanakaya K, Kikuchi S, Tanabe S, Noma K, Nishizaki M, Kagawa S, Shirakawa Y, Kamikawa Y, Fujiwara T. Multicenter retrospective study to evaluate the efficacy and safety of the double-flap technique as antireflux esophagogastrostomy after proximal gastrectomy (rD-FLAP Study). Ann Gastroenterol Surg. 2019;3:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 9. | Shoji Y, Nunobe S, Ida S, Kumagai K, Ohashi M, Sano T, Hiki N. Surgical outcomes and risk assessment for anastomotic complications after laparoscopic proximal gastrectomy with double-flap technique for upper-third gastric cancer. Gastric Cancer. 2019;22:1036-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 10. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1336] [Article Influence: 334.0] [Reference Citation Analysis (2)] |
| 11. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 12. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24813] [Article Influence: 1181.6] [Reference Citation Analysis (0)] |
| 13. | Kondrup J, Rasmussen HH, Hamberg O, Stanga Z; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1755] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 14. | Neto RML, Herbella FAM, Schlottmann F, Patti MG. Does DeMeester score still define GERD? Dis Esophagus. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1653] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 16. | Aikou T, Natsugoe S, Shimazu H, Nishi M. Antrum preserving double tract method for reconstruction following proximal gastrectomy. Jpn J Surg. 1988;18:114-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 17. | Kameyama J, Ishida H, Yasaku Y, Suzuki A, Kuzu H, Tsukamoto M. Proximal gastrectomy reconstructed by interposition of a jejunal pouch. Surgical technique. Eur J Surg. 1993;159:491-493. [PubMed] |
| 18. | Tokunaga M, Hiki N, Ohyama S, Nunobe S, Miki A, Fukunaga T, Seto Y, Sano T, Yamaguchi T. Effects of reconstruction methods on a patient's quality of life after a proximal gastrectomy: subjective symptoms evaluation using questionnaire survey. Langenbecks Arch Surg. 2009;394:637-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Shiraishi N, Hirose R, Morimoto A, Kawano K, Adachi Y, Kitano S. Gastric tube reconstruction prevented esophageal reflux after proximal gastrectomy. Gastric Cancer. 1998;1:78-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (1)] |
| 20. | Yamashita Y, Yamamoto A, Tamamori Y, Yoshii M, Nishiguchi Y. Side overlap esophagogastrostomy to prevent reflux after proximal gastrectomy. Gastric Cancer. 2017;20:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 21. | Muraoka A, Kobayashi M, Kokudo Y. Laparoscopy-Assisted Proximal Gastrectomy with the Hinged Double Flap Method. World J Surg. 2016;40:2419-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 22. | Mine S, Nunobe S, Watanabe M. A Novel Technique of Anti-reflux Esophagogastrostomy Following Left Thoracoabdominal Esophagectomy for Carcinoma of the Esophagogastric Junction. World J Surg. 2015;39:2359-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (1)] |