Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.103
Peer-review started: November 3, 2023
First decision: November 16, 2023
Revised: November 29, 2023
Accepted: January 2, 2024
Article in press: January 2, 2024
Published online: January 27, 2024
Processing time: 83 Days and 2.9 Hours
Endoscopic retrograde cholangiopancreatography (ERCP) is an accurate diagnostic method for choledocholithiasis and treatment option for stone removal. Additionally, ursodeoxycholic acid (UDCA) can dissolve cholesterol stones and prevent their development and reappearance by lowering the cholesterol concentration in bile. Despite these treatment options, there are still patients who experience stone recurrence.
To analyze the risk factors for choledocholithiasis recurrence after ERCP retrograde cholangiopancreatography and the effect of UDCA intervention.
The clinical data of 100 patients with choledochal stones who were hospitalized at the Yixing People’s Hospital and underwent ERCP for successful stone extraction between June 2020 and December 2022 were retrospectively collected. According to the post-ERCP treatment plan, 100 patients were classified into UDCA (n = 47) and control (n = 53) groups. We aimed to assess the clinical efficacy and rate of relapse in the two patient populations. We then collected information (basic demographic data, clinical characteristics, and serum biochemical indicators) and determined the factors contributing to relapse using logistic regression analysis. Our secondary goal was to determine the effects of UDCA on liver function after ERCP.
Compared to the control group, the UDCA group demonstrated a higher clinical effectiveness rate of 92.45% vs 78.72% (P < 0.05). No significant differences were observed in liver function indices, including total bilirubin, direct bilirubin, gamma-glutamyl transpeptidase, alanine aminotransferase, alkaline phosphatase, and aspartate aminotransferase, between the two groups before treatment. After treatment, all liver function indices were significantly reduced. Comparing the control vs UDCA groups, the UDCA group exhibited significantly lower levels of all indices (55.39 ± 6.53 vs 77.31 ± 8.52, 32.10 ± 4.62 vs 45.39 ± 5.69, 142.32 ± 14.21 vs 189.63 ± 16.87, 112.52 ± 14.25 vs 149.36 ± 15.36, 122.61 ± 16.00 vs 171.33 ± 22.09, 96.98 ± 10.44 vs 121.35 ± 11.57, respectively, all P < 0.05). The stone recurrence rate was lower in the UDCA group (13.21%) in contrast with the control group (44.68%). Periampullary diverticula (OR: 6.00, 95%CI: 1.69-21.30), maximum stone diameter (OR: 1.69, 95%CI: 1.01-2.85), stone quantity >3 (OR: 4.23, 95%CI: 1.17-15.26), and positive bile culture (OR: 7.61, 95%CI: 2.07-27.91) were independent factors that influenced the relapse of common bile duct stones after ERCP (P < 0.05). Furthermore, postoperative UDCA was identified as a preventive factor (OR: 0.07; 95%CI: 0.08-0.09).
The intervention effect of UDCA after ERCP for common bile duct stones is adequate, providing new research directions and references for the prevention and treatment of stone recurrence.
Core Tip: Choledocholithiasis is a common biliary disorder that can be treated by endoscopic retrograde cholangiopancreatography. However, postoperative recurrence of bile duct stones is a common complication. Ursodeoxycholic acid (UDCA) is used to treat biliary disorders mainly by lowering cholesterol saturation, facilitating bile flow, and reducing inflammation. Through these actions, UDCA improves symptoms, prevents and treats gallstone formation, and promotes biliary health in patients with biliary tract disorders.
- Citation: Yuan WH, Zhang Z, Pan Q, Mao BN, Yuan T. Risk factors for recurrence of common bile duct stones after surgical treatment and effect of ursodeoxycholic acid intervention. World J Gastrointest Surg 2024; 16(1): 103-112
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/103.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.103
In recent years, the occurrence of choledocholithiasis has increased, causing serious discomfort and health risks for patients. Choledocholithiasis is mainly treated through endoscopic lithotomy or traditional open surgery[1,2]. Endoscopic retrograde cholangiopancreatography (ERCP) for stone extraction is favored by patients due to the lower risk of trauma[3,4]. However, after stone removal via ERCP, the postoperative stone recurrence rate is between 4% to 25%[5], which presents difficulties for both clinicians and patients.
Numerous studies have been conducted on stone recurrence; however, the causes of and risk factors for recurrence have not been well characterized. Ursodeoxycholic acid (UDCA) has been proposed as a treatment for stone recurrence. UDCA is a drug that dissolves cholesterol stones and prevents stone formation and recurrence by lowering the cholesterol concentration in bile[6]. Chen et al[7] showed that postoperative application of UDCA after percutaneous hepatic perforation balloon dilatation for choledocholithiasis is a feasible and effective treatment modality. UDCA has been shown to reduce cholesterol levels in the bile and promote the dissolution and elimination of stones. Therefore, we analyzed the effect of UDCA after ERCP in patients with common bile duct stones and screened high-risk individuals for factors that may contribute to relapse in order to alleviate the suffering caused by stone recurrence and improve the prognosis.
The clinical records of a cohort comprising 100 patients with choledochal stones who were hospitalized at Yixing People’s Hospital and underwent ERCP for successful stone extraction between June 2020 and December 2022 were retrospectively collected. According to the post-ERCP treatment plan, 100 patients were classified into UDCA (n = 47) and control (n = 53) groups. Inclusion criteria: (1) Those who fulfilled the diagnostic criteria of choledocholithiasis and whose diagnosis was confirmed by computerized tomography (CT) or abdominal ultrasonography; (2) those who had not taken other medications for the treatment of choledocholithiasis in the last month; and (3) those whose age was ≥ 18 years old. Exclusion criteria: (1) People with combined acute cholangitis, biliary pancreatitis, severe cardiopulmonary insufficiency, or other contraindications to surgery without ERCP; (2) people with a previous history of pancreaticoduodenal or gastrointestinal anastomosis and other surgical procedures to change the normal structure of the bile ducts; (3) people with biliary ductal abnormalities, pancreatic tumors, and duodenal papilloma tumors detected in the ERCP; (4) individuals within the study population who exhibit drug allergies or whose clinical data are incomplete; and (5) pregnant and lactating women.
ERCP: The patients underwent standard fasting protocols that included restriction of solid food and water intake before the operation and a clean enema 2 h in advance on the day of the operation. The day before the operation, the doctor explained in detail to the patients and their families their conditions, the benefits and possible risks of the operation, and relevant precautions during the perioperative period.
First, the patient was given 10% lidocaine for local anesthesia of the oral pharynx. The endoscope was inserted through the mouth and gradually passed through the esophagus and stomach, eventually reaching the duodenum. During this process, physicians observed and evaluated the morphology and lesions in the bile and pancreatic ducts. Subsequently, a fine guidewire was carefully inserted using an endoscope and guided into the bile and pancreatic ducts. Subsequently, a certain dose of muscle relaxant was injected to relax the sphincter. Once the sphincter relaxed, a special contrast agent was injected into the bile and pancreatic ducts. Distribution and flow of the contrast agent in the ducts were recorded using a PHILIPS BV Pulsera C-arm (Royal Philips, Dutch). This helped determine the structure of the ducts and detect any abnormalities. Based on the imaging results, doctors could perform various therapeutic procedures, such as stone removal, duct dilation, and placement of biliary stents. Finally, after confirming the absence of stones through another imaging procedure, the doctor slowly withdrew the endoscope from the intestine and concluded the procedure. Throughout the process, the doctor closely monitored the patient's vital signs and promptly managed potential complications.
Control group: The control group was administered the routine postoperative treatment, including oral anti-inflammatory choleretic tablets, 1.5 g per dose, three times a day. Aspirin tablets (0.5 g) were orally administered three times daily for 4 wk.
UDCA group: The UDCA group received the same treatment as the control group as well as underwent combination therapy, including the use of UDCA capsules. The UDCA capsules were administered orally at a dose of 25 mg once daily for 4 wk.
Clinical effects: Evaluation criteria: the treatment was considered markedly effective if the imaging examination of the bile duct showed no evident dilation, there were no obvious residual stones, and clinical symptoms such as jaundice, high fever, chills, and upper abdominal colic were noticeably relieved. The treatment was considered effective if the imaging examination showed no dilation of the bile duct, there was a small amount of residual stones, and some improvement in clinical symptoms such as jaundice, high fever, chills, and upper abdominal colic. The treatment was considered ineffective if the imaging examination showed residual stones and clinical symptoms, such as jaundice, high fever, chills, and upper abdominal colic, did not improve after treatment.
Rate of relapse: Postoperative follow-up was conducted on all patients at intervals of 3-6 mo until June 2023. If the following conditions occurred, stone relapse was considered: (1) During the follow-up period, if patients began to experience symptoms of acute biliary disease, such as fever, jaundice, and right upper quadrant pain, and the recurrence of common bile duct stones was confirmed through abdominal ultrasound, CT, Magnetic resonance cholangiopancreatography (MRCP), or other imaging examinations; and (2) in patients without typical clinical symptoms but suspected of having small stones, endoscopic ultrasonography was used to determine stone relapse.
Analysis of risk factors: We collected information including age, gender, presence of jaundice, presence of hypertension, diabetes, biliary conditions (history of biliary surgery, common bile duct diameter, periampullary diverticulum), stone characteristics (maximum stone diameter, number of stones), laboratory tests [preoperative white blood cell count (WBC), procalcitonin (PCT), total bilirubin (TBiL), direct bilirubin (DBiL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), etc.], and bile culture results as predictive variables.
Recovery of liver function: A 5-milliliter aliquot of venous blood was collected from the patient in the morning while fasting, both before and after treatment. The blood collection tube was centrifuged (speed of 3000 r/min, radius of 10 cm) for 10 min, and the upper layer of serum was taken after centrifugation, and serum TBiL, DBiL, IBiL, AST, ALT, GGT were detected by PUZS-300X automatic analyzer (Nanjing Plan Medical Equipment Co., Ltd., Nanjing, Jiangsu Province, China). The analyzer was used to detect serum TBiL, DBiL, IBiL, AST, ALT, and GGT levels.
Data were analyzed using SPSS 23.0. (IBM Corporation, Armonk, New York, NY, United States). The age, common bile duct diameter, maximum stone diameter, and laboratory test results were described using mean ± SD or median (P25, P75). Subsequently, we used a t-test or non-parametric Mann-Whitney U test to compare the differences between the two groups for continuous variables. Sex, the presence or absence of jaundice and hypertension, and diabetes were expressed as constituent ratios. The differences in rates between the two groups were compared using the chi-square test or Fisher's exact test. Logistic regression analysis was used to analyze risk factors. Statistical significance was defined as a two-sided P value of < 0.05.
The 100 patients included were 39-73 years old with a median (P25, P75) of 53.00 (48.00, 60.00) years old, and included 44 males (44.0%) and 56 females (56.0%). The descriptive characteristics of the participants are presented in Table 1.
| Variable | Total (n = 100) | Control (n = 47) | UDCA (n = 53) | |
| Age (yr), median (P25, P75) | 53.00 (48, 60) | 51 (47, 60) | 54 (48.5, 60) | |
| Gender, n (%) | Male | 44 (44) | 21 (44.68) | 23 (23.40) |
| Female | 56 (56) | 26 (55.32) | 30 (56.60) | |
| Jaundice, n (%) | Yes | 27 (27) | 12 (25.53) | 15 (28.30) |
| No | 73 (73) | 35 (74.47) | 38 (71.70) | |
| Hypertension, n (%) | Yes | 25 (25) | 12 (25.53) | 14 (26.42) |
| No | 75 (75) | 35 (74.47) | 39 (73.58) | |
| Diabetes, n (%) | Yes | 32 (32) | 14 (29.79) | 18 (33.96) |
| No | 68 (68) | 33 (70.21) | 35 (66.04) | |
| History of biliary tract surgery, n (%) | Yes | 14 (14) | 6 (12.77) | 8 (15.09) |
| No | 86 (86) | 41 (87.23) | 45 (84.91) | |
| Parapillary diverticulum, n (%) | Yes | 36 (36) | 23 (48.94) | 13 (24.53) |
| No | 64 (64) | 24 (51.06) | 40 (75.47) | |
| No. of stones, n (%) | ≤ 3 | 49 (49) | 25 (53.19) | 24 (45.28) |
| > 3 | 51 (51) | 22 (46.81) | 29 (54.72) | |
| Bile culture positive, n (%) | 37 (37) | 20 (42.55) | 17 (32.08) | |
| Common bile duct diameter (mm), median (P25, P75) | 14.00 (13.00, 16.00) | 15.0 (13.0, 16.0) | 14.0 (12.0, 16.0) | |
| Maximum stone diameter (mm), median (P25, P75) | 13.00 (12.00, 15.00) | 13.0 (12.0, 15.0) | 13.0 (11.5, 14.5) | |
| ERCP time (min), median (P25, P75) | 64.00 (61.00, 68.00) | 63 (61, 67) | 64 (61, 71) | |
| WBC (109/L, mean ± SD) | 6.60 ± 0.96 | 6.24 ± 1.24 | 6.60 ± 0.97 | |
| PCT (ng/mL), median (P25, P75) | 0.18 (0.12, 0.27) | 0.17 (0.12, 0.25) | 0.18 (0.13, 0.28) |
In comparison to the control group, the UDCA group demonstrated a higher clinical effectiveness rate of 92.45%, with statistically significant differences (P < 0.05; Table 2).
| Group | Efficacious | Effective | Ineffective | χ2 value | P value |
| Control, n (%) | 16 (34.04) | 21 (44.68) | 10 (21.28) | 3.990 | 0.048 |
| UDCA, n (%) | 22 (41.51) | 27 (50.94) | 4 (7.55) |
No significant differences were observed in the liver function indices and TBiL, DBiL, GGT, ALT, ALP, and AST levels between the two patient populations before treatment (P > 0.05). After treatment, all liver function indices significantly reduced. Moreover, the UDCA group exhibited significantly lower levels of these indices than the control group (P < 0.05) (Table 3).
| Group | n | TBiL (μmon/L, mean ± SD) | DBiL (μmon/L, mean ± SD) | GGT (U/L, mean ± SD) | |||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||
| Control | 47 | 96.65 ± 11.24 | 77.31 ± 8.52 | 63.74 ± 7.45 | 45.39 ± 5.69 | 328.65 ± 20.45 | 189.63 ± 16.87 |
| UDCA | 53 | 98.63 ± 11.42 | 55.39 ± 6.53 | 63.36 ± 7.96 | 32.10 ± 4.62 | 330.25 ± 20.64 | 142.32 ± 14.21 |
| t value | 0.872 | -14.532 | -0.251 | -12.896 | 0.388 | -15.217 | |
| P value | 0.385 | < 0.001 | 0.803 | < 0.001 | 0.699 | < 0.001 | |
| ALT (U/L, mean ± SD) | ALP (U/L, mean ± SD) | AST (U/L, mean ± SD) | |||||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||
| Control | 47 | 274.63 ± 25.41 | 149.36 ± 15.36 | 256.66 ± 24.12 | 171.33 ± 22.09 | 201.32 ± 16.35 | 121.35 ± 11.57 |
| UDCA | 53 | 268.54 ± 21.23 | 112.52 ± 14.25 | 262.36 ± 25.78 | 122.61 ± 16.00 | 201.11 ± 15.47 | 96.98 ± 10.44 |
| t value | -1.306 | -12.440 | 1.139 | -12.733 | -0.066 | -11.072 | |
| P value | 0.195 | < 0.001 | 0.257 | < 0.001 | 0.947 | < 0.001 | |
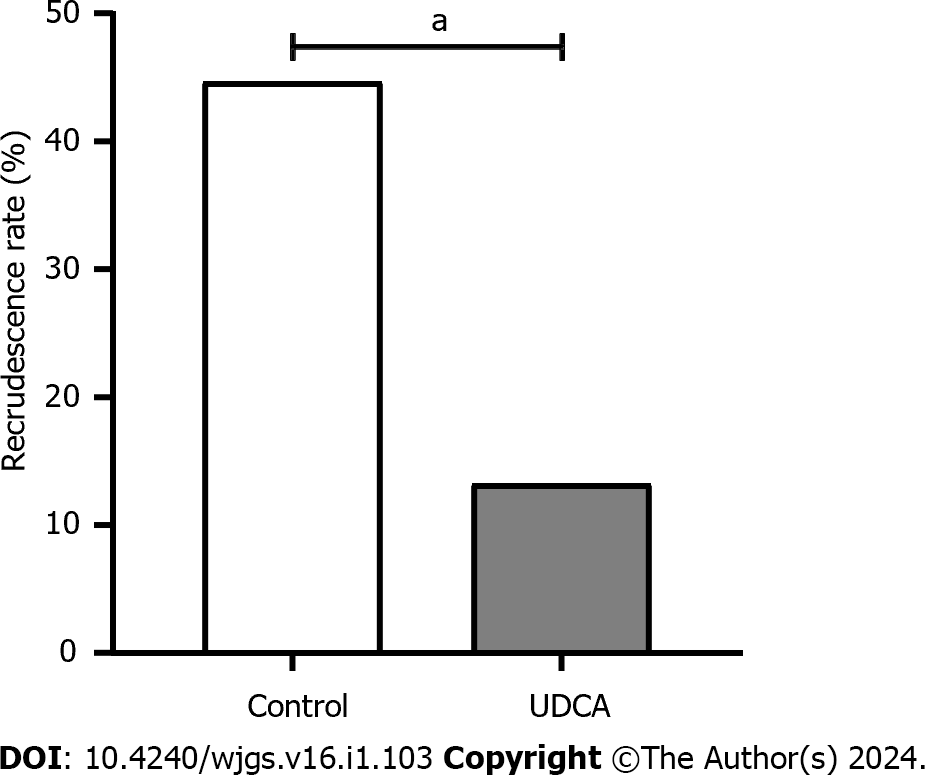
There were 7 cases (13.21%) of recurrence in the UDCA group and 21 cases (44.68%) in the control group, with statistically significant differences in the recurrence rate between the two groups (P < 0.05; Figure 1).
The non-recurrent and recurrent groups included parapapillary diverticulum, number of stones, positive bile culture, postoperative treatment, common bile duct diameter, and maximum stone diameter, with statistically significant differences (P < 0.05; Table 4).
| Variables | Non-recurrent group (n = 72) | Recurrent group (n = 28) | χ2 value | P value | |
| Age (yr, mean ± SD) | 54.64 ± 6.62 | 51.43 ± 8.74 | -1.983 | 0.050 | |
| Gender, n (%) | Male | 31 (43.06) | 13 (46.43) | 0.093 | 0.760 |
| Female | 41 (56.94) | 15 (53.57) | |||
| Jaundice, n (%) | Yes | 19 (26.39) | 8 (28.57) | 0.049 | 0.825 |
| No | 53 (73.61) | 20 (71.43) | |||
| Hypertension, n (%) | Yes | 18 (25.00) | 8 (28.57) | 0.134 | 0.715 |
| No | 54 (75.00) | 20 (71.43) | |||
| Diabetes, n (%) | Yes | 27 (37.50) | 5 (17.86) | 3.575 | 0.059 |
| No | 45 (62.50) | 23 (82.14) | |||
| History of biliary tract surgery, n (%) | Yes | 12 (16.67) | 2 (7.14) | 1.519 | 0.218 |
| No | 60 (83.33) | 26 (92.86) | |||
| Parapillary diverticulum, n (%) | Yes | 18 (25.00) | 18 (64.29) | 13.504 | < 0.001 |
| No | 54 (75.00) | 10 (35.71) | |||
| No. of stones, n (%) | ≤ 3 | 46 (63.89) | 11 (39.29) | 4.979 | 0.026 |
| > 3 | 26 (36.11) | 17 (60.71) | |||
| Bile culture positive, n (%) | 19 (26.39) | 18 (64.29) | 12.421 | < 0.001 | |
| Postoperative treatment, n (%) | Control | 26 (36.11) | 21 (75.00) | 12.240 | < 0.001 |
| UDCA | 46 (63.89) | 7 (25.00) | |||
| Common bile duct diameter(mm), median (P25, P75) | 14.00 (12.00, 15.00) | 16.00 (14.00, 17.00) | -3.265 | 0.001 | |
| Maximum stone diameter(mm), median (P25, P75) | 12.00 (11.25, 13.75) | 15.00 (12.00, 16.00) | -3.599 | < 0.001 | |
| ERCP time (min, mean ± SD) | 63.93 ± 5.12 | 65.82 ± 5.33 | 1.640 | 0.140 | |
| WBC (× 109/L, mean ± SD) | 6.45 ± 1.05 | 6.38 ± 1.27 | -0.290 | 0.772 | |
| PCT (ng/mL), median (P25, P75) | 0.18 (0.12, 0.27) | 0.18 (0.12, 0.28) | -0.088 | 0.930 | |
| TBiL (μmon/L, mean ± SD) | 97.85 ± 10.95 | 97.32 ± 12.43 | -0.211 | 0.833 | |
| DBiL (μmon/L, mean ± SD) | 64.13 ± 7.64 | 62.02 ± 7.76 | -1.234 | 0.220 | |
| GGT (U/L, mean ± SD) | 330.90 ± 19.82 | 325.88 ± 21.99 | -1.102 | 0.273 | |
| ALT (U/L, mean ± SD) | 272.31 ± 22.87 | 269.09 ± 24.88 | -0.617 | 0.538 | |
| ALP (U/L, mean ± SD) | 259.81 ± 25.79 | 259.36 ± 23.50 | -0.079 | 0.937 | |
| AST (U/L, mean ± SD) | 201.12 ± 16.02 | 201.44 ± 15.55 | 0.089 | 0.929 |
The characteristics in Table 5 with P < 0.05 were used as independent variables, and recurrence (no vs yes) after ERCP was the dependent variable. Periampullary diverticula (OR: 6.00, 95%CI: 1.69-21.30), maximum stone diameter (OR: 1.69, 95%CI: 1.01-2.85), stone quantity > 3 (OR: 4.23, 95%CI: 1.17-15.26), and positive bile culture (OR: 7.61, 95%CI: 2.07-27.91) were independent factors that influenced the relapse of common bile duct stones after ERCP (P < 0.05). Furthermore, postoperative UDCA was identified as a preventive factor (OR: 0.07; 95%CI: 0.08-0.09). The results of the logistic regression analysis are presented in Table 6.
| Variables | Assignment |
| Parapillary diverticulum | 0 = no, 1 = yes |
| No. of stones | 0 = ≤ 2, 1 = > 3 |
| Bile culture positive | 0 = no, 1 = yes |
| Postoperative treatment | 0 = UDCA, 1 = control |
| Common bile duct diameter | Original value |
| Maximum stone diameter | Original value |
| Variables | β | SE | Wald χ2 | P value | OR (95%CI) |
| Parapillary diverticulum | 1.792 | 0.646 | 7.692 | 0.006 | 6.003 (1.692-21.303) |
| No. of stones > 3 | 1.443 | 0.654 | 4.863 | 0.027 | 4.233 (1.174-15.263) |
| Bile culture positive | 2.029 | 0.663 | 9.357 | 0.002 | 47.606 (2.073-27.910) |
| Postoperative UDCA | -1.287 | 0.628 | 4.199 | 0.040 | 0.072 (0.080-0.094) |
| Maximum stone diameter | 0.527 | 0.265 | 3.957 | 0.047 | 1.694 (1.008-2.847) |
| Constant | -8.895 | 2.360 | 14.211 | < 0.001 | - |
The incidence of common bile duct stones has gradually increased in recent years, rising from 8% to 20%[8]. Since its introduction, ERCP, a minimally invasive procedure with low risk, fast recovery, high success rate, and lower cost than traditional surgery, has gradually replaced traditional open surgery for the treatment of choledocholithiasis[9]. However, ERCP may damage liver function and slow its recovery of liver function after surgery, leading to cholestasis and stone recurrence. Routine postoperative liver protection and anti-infection treatments can reduce harm caused by surgery; however, their effect on preventing stone recurrence is not obvious. Therefore, the use of drugs to prevent stone recurrence during the postoperative period may have good long-term efficacy.
In this study, the prophylactic use of UDCA after ERCP achieved an ideal therapeutic effect with a clinical efficacy rate of 92.45%, and TBiL, DBiL, GGT, ALT, ALP, and AST levels were reduced, demonstrating an adequate hepatoprotective effect. UDCA is a dihydroxy bile acid extracted from bear-bile powder. It has the ability to enhance liver detoxification, improve liver function, protect liver cells, inhibit liver cell apoptosis, and regulate immune response in the body[10,11]. UDCA also promotes bile secretion, reduces cholesterol synthase activity, inhibits hepatic synthesis of cholesterol, reduces the amount of cholesterol in bile, and depolymerizes free cholesterol crystals in bile to a microcolloid state that is dissolved in bile[12]. UDCA inhibits the binding of lipids to hydrophobic bile acids on the granular membrane of hepatocytes, thus preventing bile acids from attacking the liver, reducing cytochrome release from mitochondria, and decreasing the permeability of mitochondrial cells, which protect hepatocytes and prevent apoptosis[11,13]. Choi et al[14] and Mulliri et al[15] found that prophylactic UDCA in patients undergoing gastric or bariatric surgery prevented gallstone formation and ensured clinical benefits while reducing the burden of late cholecystectomy. Lee et al[1] recom-mended the postoperative use of UDCA to prevent recurrence in children with small or large numbers of gallstones treated with cholecystectomy. Thus, UDCA may be a new treatment strategy for reducing the likelihood of recurrence. This is reflected in the results of this study. However, there is insufficient evidence regarding the improvement in liver function.
The rate of relapse stones was lower in the UDCA group (13.21%) in contrast with the control group (44.68%) after ERCP. This finding supports the observations made in previous studies. In our study, parapapillary diverticulum was significantly associated with the recurrence of choledocholithiasis which is consistent with previous studies[16,17]. It is possible that the papilla located within the diverticulum interferes with the normal functioning of the duodenal papilla. As a result, compression occurs in the lower segment of the common bile duct and dilation occurs in the upper segment, which may lead to increased biliary pressure or biliary spasm and consequent obstruction of bile outflow, increasing the risk of stone recurrence. The presence of large stones causes dilatation of the common bile duct and reduces smooth muscle fiber retraction. Thus, bile excretion is difficult, and bile is prone to cholestasis and bacterial infections[18], inducing stone formation. Deng et al[16] found that the risk of common bile duct stone recurrence after ERCP was 1.599 times higher for patients with stone diameter ≥ 10 mm compared to those with stone diameter < 10 mm. A study involving 1148 patients found that stone diameter > 12 mm was more likely to recur[19]. Although multiple studies have established that common bile duct diameter increases the risk of common bile duct stone recurrence, predictive cut-offs are controversial. In our study, we observed that mean maximum stone diameter was a significant risk factor for stone recurrence after ERCP. Unfortunately, the cutoff value was not analyzed further. In addition, stones > 3 in diameter were a risk factor for choledochal stone recurrence after ERCP. Multiple stones are not easily removed, and recurrent choledocholithiasis can eventually develop due to negligence of the surgeon or the inability to detect small residual stones via CT. However, results from Akay et al[20] differ from ours. Akay et al[20] reported that the recurrence rate after endoscopic treatment of common bile duct stones is associated with a wide common bile duct (≥ 10 mm), but not with the number of stones. Therefore, the findings are inconclusive and require further investigation.
In this study, positive bile bacterial culture was found to be a significant risk factor. The specific mechanism is not yet clear, but we speculate that it may be related to the biological activity of intestinal microecology. Biliary tract bacteria may induce an inflammatory response and promote stone formation through changes in KEGG metabolic pathway activity. Alternatively, biliary tract bacteria synthesize many enzymes that participate in bile metabolism, increasing free bilirubin, fatty acids, and other organic components and inducing stone formation[21]. However, there have been few studies on biliary bacterial microorganisms and stone recurrence, which can be supplemented in the future.
Although the risk factors for stone relapse have been extensively studied, we also evaluated UDCA as a preventive factor. The main cause of stone formation is an imbalance in bile composition, resulting in increased cholesterol and decreased bile acid concentrations. Correcting the imbalance in bile composition is a key measure in preventing stone recurrence. UDCA has high solubility, cytoprotective effects, and membrane stability without biotransformation, and can inhibit the absorption of cholesterol in the intestinal tract and reduce cholesterol levels[12,22]. In addition, UCDA can act as a bile transporter that promotes bile acid secretion and reabsorption by increasing hydrophilic bile acids, thus improving endogenous bile acid excretion[23,24]. In conclusion, UDCA can correct the bile composition, effectively alleviate the indications for postoperative cholestasis, and reduce the stone recurrence rate.
This study has some limitations, such as a potentially small sample size, which limits the generalizability of the results. The study may not have accounted for all the possible confounding factors, which could impact the results. The limited follow-up duration may affect the assessment of long-term recurrence rates and intervention effects. Further research with larger sample sizes and rigorous study designs is needed to confirm and expand upon these findings.
We retrospectively studied patients who underwent ERCP for choledocholithiasis, with a special emphasis on the use of UDCA. The main findings of this study were as follows: (1) Prophylactic use of UDCA after ERCP helps reduce intrahepatic bile stasis, promotes hepatic function recovery, and effectively reduces the rate of stone recurrence; and (2) Parapapillary diverticulum, number of stones > 3, positive bile culture, and maximum stone diameter are independent correlates of increased recurrence rates after ERCP in patients with choledochal stones. Postoperative UDCA level was found to be a preventive factor.
Future research directions could include the following aspects: (1) Further exploration of other potential factors that may influence the recurrence of common bile duct stones (CBDS), such as the patients’ lifestyles, genetic factors, etc.; (2) Investigation of new intervention measures to reduce the recurrence rate after endoscopic treatment of CBDS, such as the use of UDCA for intervention; (3) Study the optimal retreatment strategies for patients with recurrent CBDS to improve treatment outcomes and reduce recurrence rates; (4) Comparison of the effectiveness of different treatment methods, such as endoscopic treatment and surgical treatment, in terms of recurrence rates and complications; and (5) Further research on the pathogenesis of common bile duct stones is needed to better prevent and treat this disease.
Endoscopic retrograde cholangiopancreatography (ERCP) is a commonly used modality for the treatment of choledocholithiasis, with a stone clearance rate of up to 95%; however, the recurrence rate has not decreased. Ursodeoxycholic acid (UDCA) is a postoperative drug used to prevent stone recurrence; however, its effectiveness is yet to be explored. Therefore, this study focused on biliopancreatic surgery to investigate the interventional effect of UDCA after ERCP for choledocholithiasis and analyze the risk factors for recurrence.
Recurrence of choledocholithiasis after ERCP brings pain to patients; therefore, this paper retrospectively analyzes the intervention effect of UDCA after ERCP for choledocholithiasis and the risk factors of recurrence, in order to provide a new research direction and reference for the prevention and treatment of stone recurrence.
To analyze the intervention effect of the prophylactic use of UDCA after ERCP and the influencing factors of postoperative recurrence, and to explain the mechanism of action.
The clinical records of 100 cases after ERCP were retrospectively selected, the therapeutic effects of non-UDCA and UDCA after ERCP and their effects on liver function were evaluated, and the rate of relapse within the two patient populations was compared. The risk factors for relapse were determined.
The clinical efficacy rates were 92.45% in UDCA group and 78.72% in control groups. The factors associated with recurrence after ERCP for choledochal stones included parapapillary diverticulum, number of stones > 3, positive bile culture, postoperative UDCA, and maximum stone diameter.
The administration of UDCA to patients with common bile duct stones following ERCP can enhance liver function recovery and effectively decrease relapse.
Future studies should explore the relevant mechanisms of action of UDCA treatment and construct a risk prediction model to evaluate its clinical benefits.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Knospe L, Germany; Shimamura Y, Japan S-Editor: Yan JP L-Editor: A P-Editor: Chen YX
| 1. | Lee YJ, Park YS, Park JH. Cholecystectomy is Feasible in Children with Small-Sized or Large Numbers of Gallstones and in Those with Persistent Symptoms Despite Medical Treatment. Pediatr Gastroenterol Hepatol Nutr. 2020;23:430-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Akmal AM, Putra BP, Darmaningrat CIAA, Nariswari IGARC, Srigede LD, Budyono C. Management of Cholelithiasis with Concomitant Choledocholithiasis. Acta Med Indones. 2022;54:151-157. [PubMed] |
| 3. | Troncone E, Mossa M, De Vico P, Monteleone G, Del Vecchio Blanco G. Difficult Biliary Stones: A Comprehensive Review of New and Old Lithotripsy Techniques. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Cianci P, Restini E. Management of cholelithiasis with choledocholithiasis: Endoscopic and surgical approaches. World J Gastroenterol. 2021;27:4536-4554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (15)] |
| 5. | Wu Y, Xu CJ, Xu SF. Advances in Risk Factors for Recurrence of Common Bile Duct Stones. Int J Med Sci. 2021;18:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Chang HY, Wang CJ, Liu B, Wang YZ, Wang WJ, Wang W, Li D, Li YL. Ursodeoxycholic acid combined with percutaneous transhepatic balloon dilation for management of gallstones after elimination of common bile duct stones. World J Gastroenterol. 2018;24:4489-4498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Chen X, Yan XR, Zhang LP. Ursodeoxycholic acid after common bile duct stones removal for prevention of recurrence: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97:e13086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Cai JS, Qiang S, Bao-Bing Y. Advances of recurrent risk factors and management of choledocholithiasis. Scand J Gastroenterol. 2017;52:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Manes G, Paspatis G, Aabakken L, Anderloni A, Arvanitakis M, Ah-Soune P, Barthet M, Domagk D, Dumonceau JM, Gigot JF, Hritz I, Karamanolis G, Laghi A, Mariani A, Paraskeva K, Pohl J, Ponchon T, Swahn F, Ter Steege RWF, Tringali A, Vezakis A, Williams EJ, van Hooft JE. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51:472-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 373] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 10. | Wang L, Rui X, He HW, Zhou X, Long Y. Ursodeoxycholic Acid (UDCA) Reduces Hepatocyte Apoptosis by Inhibiting Farnesoid X Receptor (FXR) in Hemorrhagic Shock (HS). Curr Mol Med. 2023;23:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Buryova H, Chalupsky K, Zbodakova O, Kanchev I, Jirouskova M, Gregor M, Sedlacek R. Liver protective effect of ursodeoxycholic acid includes regulation of ADAM17 activity. BMC Gastroenterol. 2013;13:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Jiang R, Zheng X, Lei S, Huang F, Xie G, Kwee S, Yu H, Farrar C, Sun B, Zhao A, Jia W. Ursodeoxycholic acid accelerates bile acid enterohepatic circulation. Br J Pharmacol. 2019;176:2848-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Beuers U, Trampert DC. [Ursodeoxycholic acid: history and clinical implications]. Ned Tijdschr Geneeskd. 2022;166. [PubMed] |
| 14. | Choi JH, Lee SH, Cho IR, Paik WH, Ryu JK, Kim YT. Ursodeoxycholic acid for the prevention of gallstone and subsequent cholecystectomy following gastric surgery: A systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. 2021;28:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Mulliri A, Menahem B, Alves A, Dupont B. Ursodeoxycholic acid for the prevention of gallstones and subsequent cholecystectomy after bariatric surgery: a meta-analysis of randomized controlled trials. J Gastroenterol. 2022;57:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Deng F, Zhou M, Liu PP, Hong JB, Li GH, Zhou XJ, Chen YX. Causes associated with recurrent choledocholithiasis following therapeutic endoscopic retrograde cholangiopancreatography: A large sample sized retrospective study. World J Clin Cases. 2019;7:1028-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Tantau M, Mercea V, Crisan D, Tantau A, Mester G, Vesa S, Sparchez Z. ERCP on a cohort of 2,986 patients with cholelitiasis: a 10-year experience of a single center. J Gastrointestin Liver Dis. 2013;22:141-147. [PubMed] |
| 18. | Mu H, Gao J, Kong Q, Jiang K, Wang C, Wang A, Zeng X, Li Y. Prognostic Factors and Postoperative Recurrence of Calculus Following Small-Incision Sphincterotomy with Papillary Balloon Dilation for the Treatment of Intractable Choledocholithiasis: A 72-Month Follow-Up Study. Dig Dis Sci. 2015;60:2144-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Nzenza TC, Al-Habbal Y, Guerra GR, Manolas S, Yong T, McQuillan T. Recurrent common bile duct stones as a late complication of endoscopic sphincterotomy. BMC Gastroenterol. 2018;18:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Akay T, Sari E. Identification of risk factors involved in recurrence after common bile duct stone removal with ERCP: A retrospective observational study. Medicine (Baltimore). 2022;101:e29037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Wu SD, Uchiyama K, Fan Y. The role and mechanism of fatty acids in gallstones. Hepatobiliary Pancreat Dis Int. 2007;6:399-401. [PubMed] |
| 22. | Roma MG, Toledo FD, Boaglio AC, Basiglio CL, Crocenzi FA, Sánchez Pozzi EJ. Ursodeoxycholic acid in cholestasis: linking action mechanisms to therapeutic applications. Clin Sci (Lond). 2011;121:523-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 23. | Huang L, Li S, Chen J, Zhu Y, Lan K, Zeng L, Jiang X, Zhang L. Efficacy and safety of ursodeoxycholic acid in children with cholestasis: A systematic review and meta-analysis. PLoS One. 2023;18:e0280691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Halilbasic E, Steinacher D, Trauner M. Nor-Ursodeoxycholic Acid as a Novel Therapeutic Approach for Cholestatic and Metabolic Liver Diseases. Dig Dis. 2017;35:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |