Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.2003
Peer-review started: June 30, 2023
First decision: July 18, 2023
Revised: July 24, 2023
Accepted: August 8, 2023
Article in press: August 8, 2023
Published online: September 27, 2023
Processing time: 84 Days and 4.1 Hours
Esophageal gastric anastomosis is a common surgical technique used to treat patients with gastric cancer who undergo total gastrectomy. However, using simple anastomosis techniques alone may not meet the needs of patients in some cases and can lead to complications such as anastomotic stenosis and ulceration. In order to overcome these issues and improve patient prognosis, muscle flap reconstruction technique has emerged. Muscle flap reconstruction is a method of improving gastric-esophageal anastomosis by transplanting muscle tissue. By covering the anastomotic site with muscle tissue, it not only enhances the stability of the anastomosis site but also increases blood supply, promoting healing and recovery of the anastomosis. Therefore, the use of muscle flap reconstruction technique in esophageal gastric anastomosis during total gastrectomy for gastric cancer is increasingly widely applied.
To determine the effectiveness of esophagogastric anastomosis using the muscle flap reconstruction technology in total abdominal gastrectomy for gastric cancer and perform follow-up experiments to understand the factors affecting patients’ prognosis.
The study subjects were 60 patients with gastric cancer who were admitted to our hospital between October 2018 and January 2022. All patients underwent esopha
The operation time was 318 ± 43 min, the formation time of esophageal double muscle flap anastomosis was 110 ± 13 min, the number of lymph node dissections was 26 ± 6, the incision length was 3 ± 0.6 cm, intraoperative bleeding volume was 48 ± 15 mL, first anal exhaust time was 5.3 ± 1.8 d, first meal time was 6.0 ± 1.6 d, length of hospital stay was 11.8 ± 2.5, and treatment cost was 5.8 ± 0.7 thousand yuan. The patient experienced three post
Esophagogastric anastomotic using muscle flap reconstruction exhibits good effects on patients who undergo total abdominal gastrectomy for cancer. Postoperative adjuvant radiotherapy and chemotherapy are the main factors affecting patient prognosis.
Core Tip: This study evaluated the effectiveness of esophagogastric anastomosis using muscle flap reconstruction technology in total abdominal gastrectomy for gastric cancer. The study found that this technique had positive effects on patient outcomes, and postoperative adjuvant radiotherapy and chemotherapy were important factors affecting prognosis. Univariate analysis revealed that histological types, tumor size, tumor-node-metastasis staging, vascular invasion, and postoperative adjuvant radiotherapy and chemotherapy were major factors affecting the prognosis of surviving patients. Cox regression analysis showed that postoperative adjuvant radiotherapy and chemotherapy were the main factors affecting overall patient prognosis. The findings of this study may contribute to improving treatment options and decision-making for patients with gastric cancer undergoing total abdominal gastrectomy, ultimately leading to better patient outcomes.
- Citation: Shi JK, Wang B, Zhang XS, Lv P, Chen YL, Ren SY. Multifactor analysis of the technique in total laparoscopic gastric cancer. World J Gastrointest Surg 2023; 15(9): 2003-2011
- URL: https://www.wjgnet.com/1948-9366/full/v15/i9/2003.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i9.2003
Gastric cancer is one of the most common tumors of the digestive system worldwide. Although gastric cancer may not have significant manifestations in the early stage, as the disease progresses, systemic symptoms such as emaciation, anemia, and gastric perforation are observed[1]. Surgery is the main treatment strategy for gastric cancer. With recent advances in total laparoscopy, total laparoscopic radical resection has gradually become an important treatment strategy for gastric cancer. Conventional laparoscopic surgery may require at least 5-6 incisions, whereas total laparoscopic surgery requires only 3-4 small incisions, decreasing surgical trauma and postoperative pain[2]. Furthermore, because total laparoscopic surgery is less invasive than conventional laparoscopic surgery, patients can generally return to normal living and working conditions more quickly[3]. Moreover, total laparoscopic surgery does not leave obvious surgical scars; therefore, it is advantageous for patients who pay attention to appearance[4]. Esophagogastrostomy is a method used to repair gastrointestinal anastomosis, called the “double muscle valve”. This technique requires folding the fundus of the stomach, followed by sealing it with two layers of tissue, forming a structure similar to a valve. The application of esophagogastrostomy to total laparoscopic radical resection for gastric cancer can effectively decrease the incidence of complications such as anastomotic incontinence and bile reflux and improve the surgical cure rate and postoperative quality of life, which is a recent topic of interest for surgeons. At present, systematic multivariate analyses of the application effects of esophagogastrostomy in total laparoscopic surgery for gastric cancer and their effects on prognosis remain scarce[5]. In the present study, we conducted surgery and postoperative follow-up of patients with gastric cancer and collected relevant clinical data for esophagogastric anastomosis during postoperative resection for gastric cancer to provide a reference for the clinical improvement of surgical effects, treatment levels, and postoperative rehabilitation efficiency.
To obtain a definite diagnosis, the study subjects were 60 patients with gastric cancer who were admitted to our hospital from October 2018 to January 2022. The inclusion criteria were as follows: (1) Patients with gastric cancer; (2) Patients whose preoperative pathology was adenocarcinoma; (3) Preoperative computed tomography, ultrasound, and magnetic resonance imaging confirmed tumor presence without distant organ metastasis; (4) Patients who underwent total laparoscopic esophageal plasty; (5) Patients with no history of abdominal surgery; and (6) Patients with complete clinical data. The exclusion criteria were as follows: (1) Tumor involving the dentate line and lower esophageal segment; (2) Patients who did not undergo surgery; (3) Patients who received preoperative radiotherapy, chemotherapy, or targeted therapy; (4) Patients with severe disease and dysfunction; (5) Patients with other or tumor history; (6) Patients with missing follow-up data; and (7) Patients with mental and psychological illnesses.
For all patients with intravenous inhalation compound anesthesia, supine position, according to the laparoscopic radical gastric cancer conventional 5-hole placement Trocar, laparoscopic conventional exploration, along the lower edge of the liver ligament, lower separation to the right cardia, cut the right diaphragm, suspension liver, complete lymph node dissection, laparoscopic linear cutter from the esophagus, stomach, specimen in specimen bag, close pneumoperitoneum, all around the umbilical mouth (3.5 cm) specimen, confirm the tumor far and near. The pneumoperitoneum was rebuilt to maintain a pressure of 10-12 mmHg and the “H” shape was labeled at the tip of the remnant stomach, with a width of approximately 2.5 cm and a spacing of 3.5 cm up and down. The plasma muscle layer and middle muscle layer were prepared and incised to prepare the cytoplasmic muscle flap of the anterior gastric wall. Next, the mucosal layer was incised under the H-shaped transverse flap to prepare for subsequent esophageal anastomosis. The posterior wall of the esophagus was pulled 4 cm from the broken end of the esophagus and the plasma muscle layer was continuously stitched on the gastric wall using barbed threads. The broken end and remnant stomach were fixed, the closed section of the esophagus was incised, and the broken end and remnant stomach were anatomized. The whole layer of the posterior wall of the broken end and the mucosal layer and submucosa of the remnant stomach was closed. A barbed thread was used to continuously suture the full layer of the anterior wall of the broken end and the H shape of the remnant stomach. The anterior gastric wall was sutured using a Y-shaped intermittent suture to realize wrapping around the anastomosis. During surgery, a gastroscope was used to check the esophagus and residual gastric anastomosis, including whether the ana
Perioperative index: The perioperative index was observed, and the operating room nurse recorded the operation time, shaping time of esophagogastric double muscle flap anastomosis, number of lymph node dissections, incision length, and intraoperative bleeding volume (calculated using the sterile gauze weighing method). On the other hand, the inpatient nurse recorded postoperative first anal exhaust time, first feeding time, hospitalization time, treatment cost, and the probability of complications during postoperative hospitalization. Information on sex, age, Borrmann classification, histological type, tumor size, tumor-node-metastasis (TNM) stage, vascular invasion, postoperative adjuvant chemoradiotherapy, and lymph node metastasis was collected by inquiring or consulting medical records. Among them, the Borrmann classification can be divided into types I-IV, which refer to mushroom umbrella-type nodules (tumor nodules, polyp shape, ulcer, and ulcer surface can be shallow), local ulcer-type nodules (ulcer degree, edge, and tumor limitation), infiltration ulcer-type nodules (ulcer chassis, unclear edge, and deep infiltration), and diffuse infiltration-type nodules (infiltration of cancer tissue in the stomach wall), respectively. The follow-up records of the patients within 1 year postoperatively were analyzed and patients were grouped based on whether they survived or died. The clinicopathological characteristics of the two patient groups were observed. Statistically significant indicators were included in the Cox regression model, and the relevant factors affecting patient prognosis were analyzed.
SPSS27.0 was used for data processing, with (n, %), and cross χ2 test. Measurement data showing normal distribution are expressed as (mean ± SD), using the independent sample t-test. Relevant factors that affected prognosis were analyzed by Cox regression analysis. Values at P < 0.05 were considered statistically significant.
The following perioperative indicators were observed: Operation time (318 ± 43 min); time of esophageal anastomosis double muscle valve forming (110 ± 13 min); number of lymph node dissection (26 ± 6); incision length (3.4 ± 0.6 cm); intraoperative bleeding volume (48 ± 15 mL); anal first vent time (5.3 ± 1.8 d); first feeding time (6.0 ± 1.6 d); hospitalization time (11.8 ± 2.5); and treatment cost (5.8 ± 0.7 ten thousand yuan). The specific bar chart ratio is shown in Figure 1. The patients suffered from three postoperative complications, two pulmonary infection-related and one respiratory discomfort-related complication. The number of complications in Figure 2.
The univariate analysis showed histological type, tumor size, TNM stage, vascular invasion, and postoperative adjuvant chemoradiotherapy as the main factors affecting the prognosis (P < 0.05). Details are presented in Table 1.
| Factor | Classify | Survival (n = 50) | Death (n = 10) | χ2 | P value |
| Sex | Male | 34 (68.00) | 6 (60.00) | 0.240 | 0.624 |
| Female | 16 (32.00) | 4 (40.00) | |||
| Age | > 60 yr | 21 (42.00) | 7 (70.00) | 2.625 | 0.105 |
| Admidia 60 yr | 29 (58.00) | 3 (30.00) | |||
| Bommann classification | I-II mold | 35 (70.00) | 4 (40.00) | 3.297 | 0.069 |
| III-IV mold | 15 (30.00) | 6 (60.00) | |||
| Histological type | Poorly differentiated | 30 (60.00) | 1 (10.00) | 10.141 | 0.006 |
| Moderately differentiated | 11 (22.00) | 3 (30.00) | |||
| Well-differentiated | 9 (18.00) | 6 (60.00) | |||
| Tumor size | ≤ 5 cm | 38 (76.00) | 2 (20.00) | 11.760 | < 0.001 |
| > 5 cm | 12 (24.00) | 8 (80.00) | |||
| TNM by stages | I-II designated time | 35 (70.00) | 1 (10.00) | 12.500 | < 0.001 |
| III-IV designated time | 15 (30.00) | 9 (90.00) | |||
| Vascular invasion | Have | 13 (26.00) | 6 (60.00) | 4.452 | 0.035 |
| Not have | 37 (74.00) | 4 (40.00) | |||
| Postoperative adjuvant chemoradiation therapy | Not have | 16 (32.00) | 8 (80.00) | 8.000 | 0.005 |
| Have | 34 (68) | 2 (20) | |||
| Lymphatic metastasis | Have | 10 (20) | 3 (30) | 0.491 | 0.483 |
| Not have | 40 (80) | 7 (70) |
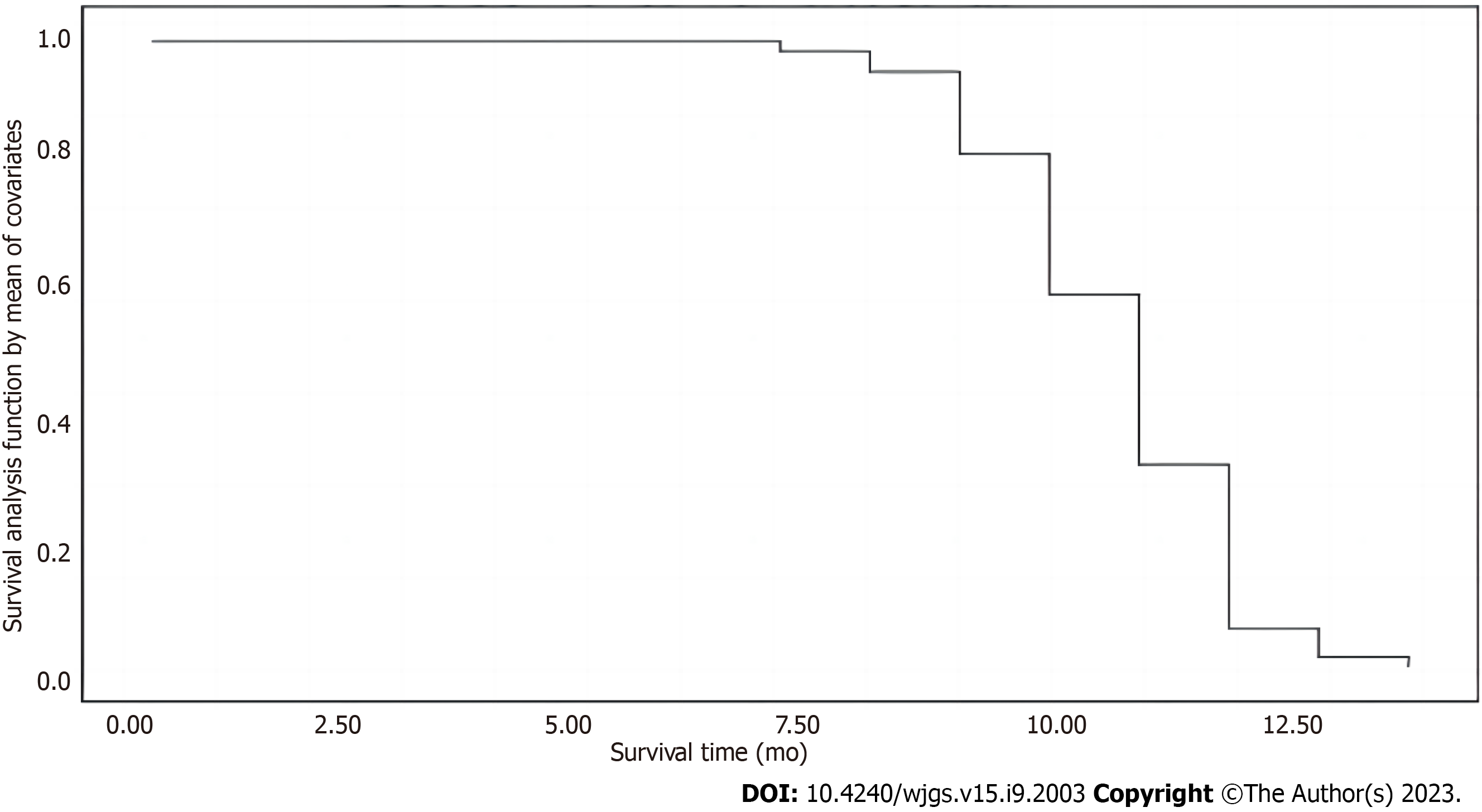
The data were assessed before performing Cox regression analysis. Patient survival was a dependent variable, whereas other statistical differences were independent variables. Details are shown in Table 2. The Cox regression analysis showed that postoperative adjuvant chemoradiotherapy was the main factor affecting the prognosis of patients (P < 0.05). Details are shown in Table 3. The patient survival function plot is shown in Figure 3. The survival time of the subsisting group (10.78 ± 1.52 mo) was significantly higher than that of the death group (7.40 ± 1.51 mo), and the difference was statistically significant (t = 6.444, P < 0.001) (Figure 3).
| Factor | Factor pattern | The assignment situation |
| Patient | Classified variable | 1: Survival, 2: Death |
| Histological type | Classified variable | 1: Poorly differentiated, 2: Moderately differentiated, 3: Highly differentiated |
| Tumor size | Classified variable | 1: ≤ 5 cm, 2: > 5 cm |
| TNM by stages | Classified variable | 1: I period, 2: About period |
| Vascular invasion | Classified variable | 1: Yes, 2: None |
| Postoperative adjuvant chemoradiation therapy | Classified variable | 1: None, 2: To have |
| Factor | B | SE | Wald | Free degree | Conspicuousness | Exp (B) | A 95.0%CI of exp (B) | |
| Lower limit | Superior limit | |||||||
| Histological type | 0.179 | 0.192 | 0.862 | 1 | 0.353 | 1.196 | 0.82 | 1.743 |
| Tumor size | 0.119 | 0.346 | 0.118 | 1 | 0.732 | 1.126 | 0.571 | 2.221 |
| TNM by stages | 0.618 | 0.335 | 3.404 | 1 | 0.065 | 1.855 | 0.962 | 3.574 |
| Vascular invasion | 0.133 | 0.348 | 0.146 | 1 | 0.702 | 1.142 | 0.578 | 2.26 |
| Postoperative adjuvant chemoradiation therapy | 0.702 | 0.355 | 3.913 | 1 | 0.048 | 2.018 | 1.006 | 4.046 |
Gastric cancer is a malignant tumor occurring in gastric epithelial tissues. The cause of its occurrence has not been thoroughly studied. However, most scholars believe that factors such as curing, smoking, high salt consumption, high-fat consumption, drinking, and eating stale food increase the risk of gastric cancer (history of benign gastric diseases)[6]. Furthermore, chronic atrophic gastritis, gastric polyps, and Helicobacter pylori infection may also increase the probability of gastric cancer occurrence. According to statistics, gastric cancer is one of the most common cancers worldwide; however, its incidence in developed countries has decreased significantly. Conversely, its incidence in Asian countries, such as China, South Korea, and Japan, is still high, which can be attributed to the long-term use of high salt and pickled food[7]. Surgery is a common way to treat gastric cancer. With the development of laparoscopic technology and improve
The perioperative indicators showed in this study, such as operation time (318 ± 43 min), esophageal anastomosis time (110 ± 13 min), lymph node dissection (26 ± 6), incision length (3.4 ± 0.6 cm), intraoperative bleeding (48 ± 15 mL), anal first discharge time (5.3 ± 1.8 d), first feeding time (6.0 ± 1.6 d), hospitalization time (11.8 ± 2.5), treatment cost (5.8 ± 0.7 thousand yuan), and a poor prognosis ratio of about 16.67%, were consistent with the results of Tian et al[11]. The results of the present study indicate that the double muscle valve plasty of esophagogastric stomosis can indeed be combined with the total laparoscopic radical surgery of gastric cancer to achieve a good curative effect in the near future. Laparoscopic surgery combined with gastric anastomosis double muscle valve plasty can retain the function of the upper stomach and lower esophagus and reduce the effect of surgery on the digestive function of the patient. Furthermore, the double muscle valve structure can avoid gastric content reflux into the esophagus and improve surgical safety. Addi
Statistical data show that the one-year survival rate of patients with gastric cancer treated with radical surgery is about 70%-90%[12]. In the present study, after the one-year follow-up of the 60 patients, their one-year survival rate was 83.33%, consistent with the epidemiological statistics. The univariate analysis showed differences in histological type, tumor size, TNM stage, vascular invasion, and postoperative adjuvant chemoradiation between the surviving and dying patients. This is similar to the conclusion of the Tougeron et al[13]. The histological types of gastric cancer usually include adenocarcinoma, papillary adeno
In conclusion, esophagogastric-stapled muscle valvuloplasty showed good results in total abdominal gastric cancer. Postoperative adjuvant chemoradiotherapy was the main factor affecting the prognosis of patients. However, the study has the following limitations: The small number of samples, the single source, and the lack of analysis of the long-term efficacy of patients. Thus, large-sample, multi-center, and long-term studies are needed in the future to confirm the present results.
Esophageal gastric anastomosis is a common surgical technique used in the treatment of gastric cancer patients undergoing total gastrectomy. However, complications such as anastomotic stenosis and ulceration can arise when simple anastomosis techniques are used alone, which may not adequately meet patient needs. To address these issues and improve patient prognosis, the muscle flap reconstruction technique has emerged. Muscle flap reconstruction involves transplanting muscle tissue to enhance gastric-esophageal anastomosis. By covering the anastomotic site with muscle tissue, it not only improves stability but also enhances blood supply, promoting healing and recovery. Therefore, the application of muscle flap reconstruction in esophageal gastric anastomosis during total gastrectomy for gastric cancer is increasingly widespread.
Gastric cancer is a significant health concern, and total gastrectomy is a common surgical treatment for this condition. However, traditional esophagogastric anastomosis techniques have limitations, leading to complications and suboptimal patient outcomes. The emergence of muscle flap reconstruction technique provides a potential solution to overcome these challenges. By transplanting muscle tissue, the technique improves the stability and blood supply of the anastomosis site, promoting healing and recovery.
The objective of this study was to evaluate the effect of esophagogastrostomy with muscle flap reconstruction technique on the prognosis of patients undergoing total gastrectomy for gastric cancer.
This study included 60 patients with gastric cancer who underwent total abdominal gastrectomy with esophagogastric anastomosis using double muscle flap reconstruction technique. Perioperative indicators, such as operation time, formation time of esophageal double muscle flap anastomosis, number of lymph node dissections, incision length, intraoperative bleeding volume, were recorded. Patients were followed up for one year to observe outcomes and classify patients based on different outcomes. Clinicopathological parameters were analyzed to identify factors affecting patient prognosis.
The study involved 60 patients with gastric cancer who underwent total abdominal gastrectomy with esophagogastric anastomosis using double muscle flap reconstruction technique. The operation time averaged (318 ± 43 min), formation time of esophageal double muscle flap anastomosis was (110 ± 13 min), and other perioperative indicators were measured. Three postoperative complications were recorded: 2 cases of pulmonary infection and 1 case of respiratory discomfort. During the one-year follow-up, 50 patients survived while 10 died. Univariate analysis identified histological types, tumor size, tumor-node-metastasis staging, vascular invasion, and postoperative adjuvant radiotherapy and chemotherapy as the main factors affecting prognosis in surviving patients. Cox regression analysis confirmed the significance of postoperative adjuvant therapy on patient prognosis. The survival time of the survival group was signi
The study concludes that esophagogastric anastomosis with muscle flap reconstruction is effective for patients undergoing total abdominal gastrectomy for gastric cancer. The technique improves the stability of the anastomosis site and enhances blood supply, promoting healing and recovery. Esophagogastric anastomosis with muscle flap recon
Future research can focus on optimizing the muscle flap reconstruction technique to further enhance surgical outcomes and minimize complications. Additionally, investigating the long-term effects of postoperative adjuvant radiotherapy and chemotherapy on patient prognosis would provide valuable insights. Furthermore, evaluating the cost-effectiveness of this technique and comparing it with other surgical methods will help guide decision-making in clinical practice.
I would like to express my sincere thanks to all those who participated in the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ikeda H, Japan; Triggi M, United Kingdom S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 654] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 2. | Li Z, Shan F, Ying X, Zhang Y, E JY, Wang Y, Ren H, Su X, Ji J. Assessment of Laparoscopic Distal Gastrectomy After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2019;154:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 3. | Liu X, Wang X, Mao T, Yin X, Wei Z, Fu J, Wu J, Li X. Characteristic analysis of early gastric cancer after Helicobacter pylori eradication: a multicenter retrospective propensity score-matched study. Ann Med. 2023;55:2231852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 4. | Zhang H, Liu X, Zheng Z, Yin J, Zhang J. Safety, efficacy, and selection strategy of laparoscopic local gastrectomy for gastrointestinal stromal tumors in the esophagogastric junction. Front Surg. 2022;9:1015126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Lu J, Wu Z, Liu G, Wang B, Shi L. The clinical effectiveness of establishing a proximal jejunum pouch after laparoscopic total gastrectomy: A propensity score-based analysis. Asian J Surg. 2022;45:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Ecker J, Benedetti E, Kindt ASD, Höring M, Perl M, Machmüller AC, Sichler A, Plagge J, Wang Y, Zeissig S, Shevchenko A, Burkhardt R, Krumsiek J, Liebisch G, Janssen KP. The Colorectal Cancer Lipidome: Identification of a Robust Tumor-Specific Lipid Species Signature. Gastroenterology. 2021;161:910-923.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 7. | Yan S, Li B, Bai ZZ, Wu JQ, Xie DW, Ma YC, Ma XX, Zhao JH, Guo XJ. Clinical epidemiology of gastric cancer in Hehuang valley of China: a 10-year epidemiological study of gastric cancer. World J Gastroenterol. 2014;20:10486-10494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Ueda Y, Shiroshita H, Etoh T, Inomata M, Shiraishi N. Laparoscopic proximal gastrectomy for early gastric cancer. Surg Today. 2017;47:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Liu J, Wang G, Zhao J, Liu X, Zhang K, Gong G, Pan H, Jiang Z. LncRNA H19 Promoted the Epithelial to Mesenchymal Transition and Metastasis in Gastric Cancer via Activating Wnt/β-Catenin Signaling. Dig Dis. 2022;40:436-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Yanni F, Singh P, Tewari N, Parsons SL, Catton JA, Duffy J, Welch NT, Vohra RS. Comparison of Outcomes with Semi-mechanical and Circular Stapled Intrathoracic Esophagogastric Anastomosis following Esophagectomy. World J Surg. 2019;43:2483-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Tian P, Liu Y, Bian S, Li M, Zhang M, Liu J, Jin L, Zhang P, Zhang Z. Laparoscopic Proximal Gastrectomy Versus Laparoscopic Total Gastrectomy for Proximal Gastric Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2020;10:607922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Sun KK, Wu YY. Current status of laparoscopic proximal gastrectomy in proximal gastric cancer: Technical details and oncologic outcomes. Asian J Surg. 2021;44:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Tougeron D, Hamidou H, Scotté M, Di Fiore F, Antonietti M, Paillot B, Michel P. Esophageal cancer in the elderly: an analysis of the factors associated with treatment decisions and outcomes. BMC Cancer. 2010;10:510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Kunisaki C, Makino H, Takagawa R, Oshima T, Nagano Y, Ono HA, Akiyama H, Shimada H. Efficacy of laparoscopy-assisted distal gastrectomy for gastric cancer in the elderly. Surg Endosc. 2009;23:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Zurleni T, Gjoni E, Ballabio A, Casieri R, Ceriani P, Marzoli L, Zurleni F. Sixth and seventh tumor-node-metastasis staging system compared in gastric cancer patients. World J Gastrointest Surg. 2013;5:287-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 16. | Yao C, Wu S, Kong J, Sun Y, Bai Y, Zhu R, Li Z, Sun W, Zheng L. Angiogenesis in hepatocellular carcinoma: mechanisms and anti-angiogenic therapies. Cancer Biol Med. 2023;20:25-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 81] [Reference Citation Analysis (0)] |
| 17. | Liu Z, Liu XW, Fang XD, Ji FJ. [Application of Overlap anastomosis to Billroth I digestive tract reconstruction after laparoscopic distal gastrectomy in gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2019;22:441-445. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Ahn HS, Chang MS, Han DS. Comparing the surgical outcomes of dual-port laparoscopic distal gastrectomy and three-port laparoscopic distal gastrectomy for gastric cancer. Ann Surg Treat Res. 2021;100:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Wei J, Yang X, Gao R, Wang W, Li X, Ji G. Initial experience with triple port laparoscopic distal gastrectomy. Front Oncol. 2022;12:1042314. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |