Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.1919
Peer-review started: April 6, 2023
First decision: May 30, 2023
Revised: June 8, 2023
Accepted: July 11, 2023
Article in press: July 11, 2023
Published online: September 27, 2023
Processing time: 169 Days and 7.3 Hours
In a previous study, autologous bone marrow infusion (ABMI) was performed in patients with decompensated liver cirrhosis (DLC) and acquired immunodeficiency syndrome and achieved good results, but whether splenectomy affected outcome was unclear.
To investigate the efficacy of ABMI combined with splenectomy for treatment of DLC.
Eighty-three patients with DLC were divided into an intervention group (43 cases) and control group (40 cases) according to whether splenectomy was performed. The control group was treated with ABMI through the right omental vein, and the intervention group was additionally treated with splenectomy.
After ABMI, the prothrombin time, serum total bilirubin levels, ascites volume and model for end-stage liver disease score in both groups were significantly lower, while the albumin levels were significantly higher than before ABMI (P < 0.01), but there were no significant differences between the groups (P > 0.05). After ABMI, the white blood cell and platelets counts in both groups were significantly higher than before ABMI (P < 0.01), and the counts in the intervention group were significantly higher than in the control group (P < 0.01). After ABMI the CD4+ and CD8+ T cell counts in both groups were significantly higher than before ABMI (P < 0.01). The CD8+ T cell counts in the intervention group increased continuously and the increase had a shorter duration compared with control group.
ABMI through the portal vein in patients with DLC can significantly improve liver synthetic and secretory functions, and splenectomy promotes improvement of bone marrow hematopoietic and cellular immune functions.
Core Tip: In this study, autologous bone marrow infusion (ABMI) through the portal vein in patients with decompensated liver cirrhosis (DLC) can significantly improve liver synthetic and secretory functions and is effective in patients with DLC. And it is the first attempt to investigate the impact of splenectomy on bone marrow hematopoietic function and cellular immune function after ABMI in patients with DLC.
- Citation: Liu BC, Cheng MR, Lang L, Li L, Si YH, Li AJ, Xu Q, Zhang H. Autologous bone marrow infusion via portal vein combined with splenectomy for decompensated liver cirrhosis: A retrospective study. World J Gastrointest Surg 2023; 15(9): 1919-1931
- URL: https://www.wjgnet.com/1948-9366/full/v15/i9/1919.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i9.1919
Cirrhosis is the end stage of liver fibrosis and has several causes. In the global ranking of causes of death in 2012, cirrhosis ranked 14th[1], and drug treatment was not effective. At present, liver transplantation is still the most effective treatment for advanced liver disease. However, due to the lack of donor livers and high costs, there is an urgent need to find a safe and effective alternative that can be widely used[2]. Cell therapy has achieved outstanding results in basic and clinical research and is a promising new treatment. In animal experiments, bone marrow stem cells (BMSCs) can be transformed into hepatic oval cells, hepatocytes and bile duct cells in the liver, which play an important role in the repair of liver damage[3]. In a clinical experiment, it was found that after transplantation of male bone marrow cells (BMCs) to female patients, Y-chromosome-positive hepatocytes were detected in the liver, and it was confirmed that BMSCs can be transformed into hepatocytes[4]. Liver-derived liver stem cells have a positive contribution to hepatocyte regeneration. Lu et al[5] induced hepatocyte apoptosis by targeted deletion of Mdm2, and then transplanted liver progenitor cells into mouse liver. The transplanted liver progenitor cells differentiated into hepatocytes and bile duct cells, significantly improving the structure and function of the damaged liver.
Several studies have reported positive effects of BMCs transplantation for treatment of decompensated liver cirrhosis (DLC). Most studies used BMCs, peripheral blood hematopoietic stem cells and umbilical cord blood stem cells. BMCs were separated by gradient centrifugation, and the suspension of BMCs was injected into the liver through the hepatic artery by interventional methods. Liver function was repaired, but the specific mechanism is still not clear[6]. The anti-DLC effect of autologous BMSCs has been established in animal models[7]. In addition, clinical trials have shown that autologous BMSC transplantation can quickly improve liver function without obvious side effects. However, there are not many clinical trials about autologous BMSCs for cirrhosis, and there is no unified treatment plan[8,9]. In a previous study, BMSCs were used to treat human immunodeficiency virus (HIV) patients with DLC, and achieved good results. This treatment method has the following advantages: BMSCs do not need to be centrifuged, and are reproducible, nonimmunogenic, and free of graft-versus-host disease[10]. Therefore, autologous BMSCs have received much attention in basic and clinical research, and are increasingly used in clinical treatment of various diseases[11-13]. BMSCs are transplanted to the liver mainly through the hepatic artery, portal vein and peripheral vein. This has a significant effect on DLC, is safe and feasible, and can significantly improve the clinical symptoms and liver function[14-16]. It is readily accepted by DLC patients. Other transplantation routes such as intrasplenic transplantation, intraperitoneal and peripheral vein transplantation are commonly used clinically. In a study of four patients with DLC who were treated with peripheral intravenous injection of autologous mesenchymal stem cells, after 2 years of follow-up, it was found that the scores of the end-stage liver disease model in two patients were significantly improved[17], but the number of cases was too small. The mechanism by which stem cells repair liver cells through systemic blood circulation is still unclear, and no effective induction method has been found to orient stem cells to migrate and home to the target organ. This transplantation method is the most applied method in liver cirrhosis.
Due to the rich blood supply and nutrients in the portal vein, stem cells stay in the liver sinusoids for a long time, with good selectivity distribution, and the portal system contains high concentrations of hepatotropic cytokines, which are important for the survival and growth of BMCs that are returned to the liver. In previous clinical trials surgery was performed to insert an infusion port into the right omental vein (ROV), autologous BMCs were returned through the infusion port, and good results were observed in the treatment of HIV patients with DLC[18]. However, it remains unclear whether splenectomy has an effect on patient outcomes. In this study, the efficacy of autologous bone marrow infusion (ABMI) combined with splenectomy was observed in the treatment of patients with DLC.
This was a retrospective analysis of 83 patients with DLC who received ABMI, including 52 males and 31 females, aged 27-75 years, with an average age of 47.53 ± 8.82 years from January 2016 to December 2018 from Shanghai Public Health Clinical Center Affiliated to Fudan University, Shanghai Dongfang Hospital Affiliated to Tongji University, Shanghai Dongfang Hepatobiliary Surgery Hospital Affiliated to Naval Medical University, and Renji Hospital Affiliated to Shanghai Jiao Tong University. There were 60 cases of hepatitis B cirrhosis, four of alcoholic cirrhosis, 11 of hepatitis C cirrhosis and eight cases of schistosomiasis cirrhosis. Child–Pugh classification was grade B in 77 cases and grade C in six.
Inclusion was in accordance with the diagnostic criteria for DLC[19]: (1) Computed tomography (CT), color Doppler ultrasound, or liver biopsy suggested the formation of liver cirrhosis; (2) liver cirrhosis was diagnosed by liver hardness scan; (3) albumin level < 35 g in liver function; (4) gastroscopy showed signs of esophageal and gastric varices; (5) platelet count < 1011/L; (6) esophageal and gastric varices; (7) prothrombin time was longer than normal for 3 s; and (8) ascites formation. If any three items in (1)-(5) were met and any item in (6)-(8), it was possible to diagnose DLC. Exclusion criteria were: (1) Age < 18 years; (2) pregnant and breastfeeding women; (3) malignant tumors of the liver or other organs; (4) spontaneous peritonitis or active gastrointestinal bleeding; (5) patients who could not tolerate the treatment, such as severe those with heart disease and pulmonary insufficiency; (6) hormone therapy; and (7) intellectual disability or mental illness.
Informed consent was signed by all patients and the study was approved by the Ethics Committee of the Shanghai Public Health Clinical Center (2013-030).
Conventional liver protection and diuresis were used for all patients. In addition, viral liver cirrhosis was treated with anti-hepatitis B or C drugs.
According to whether splenectomy was performed during the operation, the patients were divided into an intervention group (43 cases) and control group (40 cases). The control group was treated with ABMI through the ROV, and the intervention group underwent splenectomy in addition to ABMI.
Under general anesthesia, a midline incision was made in the upper abdomen, the ascites was removed after entering the abdominal cavity, the spleen was fully exposed and then the spleen was removed (in the intervention group). The infusion port was embedded in the ROV. During the operation, 40 mL of bone marrow was extracted from the anterior superior iliac spine by puncture, and 40 mL of ABM (without washing, filtration and concentration) was slowly injected with a syringe through the puncture window of the infusion port, and entered the portal vein through the ROV. Saline (5 mL) was injected into the infusion port to prevent coagulation. At 1 and 3 mo after the operation, 40 mL of ABM was infused once again through the infusion port. Five milliliters of cubital venous blood were drawn before surgery, at 1, 3, 6 and 12 mo after ABMI, placed in an anticoagulation tube, and allowed to stand at room temperature for 30 min. The samples were centrifuged centrifugal radius was 9 cm, centrifugal speed was 3000 rpm, 15 min (Tengying Machinery Manufacturing Co., Ltd., Zhangjiagang, China), and supernatants were collected and stored at -80 °C.
Serum total bilirubin (TB), serum albumin and creatinine levels were detected using an AU5800 automatic biochemical analyzer (Beckman Coulter Co. Ltd., CA, United States). Prothrombin time (PT) and international normalized ratio (INR) were measured by CA-500 automatic coagulation analyzer (Sysmex Corporation, Kobe, Japan) using the coagulation index detection kit. The white blood cell (WBC) and platelet counts and hemoglobin level were detected by automatic blood analyzer (Mindray BC-5000, Shenzhen, China).
FACS Calibur flow cytometer, CD4-FITC/CD8-PE, TruCOUNT absolute counting tube, four-color fluorescent standard microspheres, and FACS hemolysin (10 ×) were the products of BD Company in the United States. Multiset tri-color reagent (20 μL) and 50 μL whole blood were added to a TruCOUNT absolute counting tube, mixed thoroughly, and placed in the dark for 15 min. Then, 450 μL of FACS hemolysin (10 ×) was added, mixed well, and placed in the dark for 15 min. After sample preparation, the Multiset program was run on the computer immediately for detection, and the absolute counts of CD4+ and CD8+ T cells in the total T cells were analyzed.
Inadomi et al[20] reported a method of measuring the volume of ascites with ultrasound in 1996. Two variables were observed: Abdominal circumference and maximum ascites depth. Specific operation: Instruct the patient to lie on his back and lie on the stomach, and we measured the abdominal circumference (C) around the umbilicus, and then the patient changed to the prone position. The ultrasonic probe probed the maximum depth of ascites at the umbilical circumference (d), that is, the maximum vertical distance from the interface of the floating intestinal loop to the probe. We used the following formula to calculate the amount of ascites: r = C/2π; V (volume of ascites) = 1/3 [πd2 (3r-d)] (Note: r is radius, π is constant).
Model for end-stage liver disease (MELD) score is often used as an indicator of liver function. The formula was MELD score[21] = 3.78 × ln[TB (μmol/L)] + 11.2 × ln(INR) + 9.6 × ln[creatinine (mmol/L)] + 6.4 × (etiology: Biliary or alcoholic was 0, and other diseases were 1).
SPSS 19.0 was used for statistical analysis. The normal distribution was tested using the Shapiro-Wilk test. The measurement data conforming to the normal distribution were expressed as mean ± SD. Before and after treatment, the paired t test was used for comparison of the intervention group and control group. The data with non-normal distribution were expressed in M (P25, P75), using the Mann-Whitney test. Numerical data were used to describe the percent, using the χ2 test. P < 0.05 was considered statistically significant. The test standard was α = 0.05.
There was no significant difference in gender, age, etiology of liver cirrhosis and Child–Pugh grading between the intervention group and control group (P > 0.05), and the baseline data of the two groups were comparable (Table 1).
| Group | Sex (M/F) | Age (yr) | Etiology | Child-Pugh grade | ||||
| Hepatitis B cirrhosis | Alcoholic cirrhosis | Hepatitis C cirrhosis | Schistosomiasis cirrhosis | B | C | |||
| Control | 27/13 | 47.53 ± 9.21 | 30 | 1 | 6 | 3 | 36 | 4 |
| Intervention | 25/18 | 47.53 ± 8.51 | 30 | 3 | 5 | 5 | 41 | 2 |
| χ2/t | 0.428 | 0.000 | 1.484 | 0.266 | ||||
| P value | 0.513 | 0.999 | 0.686 | 0.606 | ||||
In the intervention group, 43 patients underwent splenectomy and had an infusion port placed in the right gastroepiploic vein. Three patients (all Child-Pugh grade B before surgery) had liver failure due to oozing blood in the splenectomy wound, and died within 3 d after surgery. The surgery-related fatality rate was 6.98%. In the control group, 40 patients had an infusion port placed in the right gastroepiploic vein, and two patients (1 Child-Pugh grade B and 1 grade C before surgery) died of liver failure caused by gastrointestinal bleeding after surgery, and the fatality rate was 5.0%. There was no significant difference in the fatality rate between the two groups after surgery (χ2 = 0.007, P > 0.05). In the intervention group, two cases were Child-Pugh grade C. Due to emergency surgery for gastrointestinal bleeding, the portal vein pressure was high. If splenectomy is not performed, it may recur to bleed after surgery, so splenectomy was performed. After 1-year follow-up, both patients showed good improvement in liver function, similar to that in the Child–Pugh grade B patients.
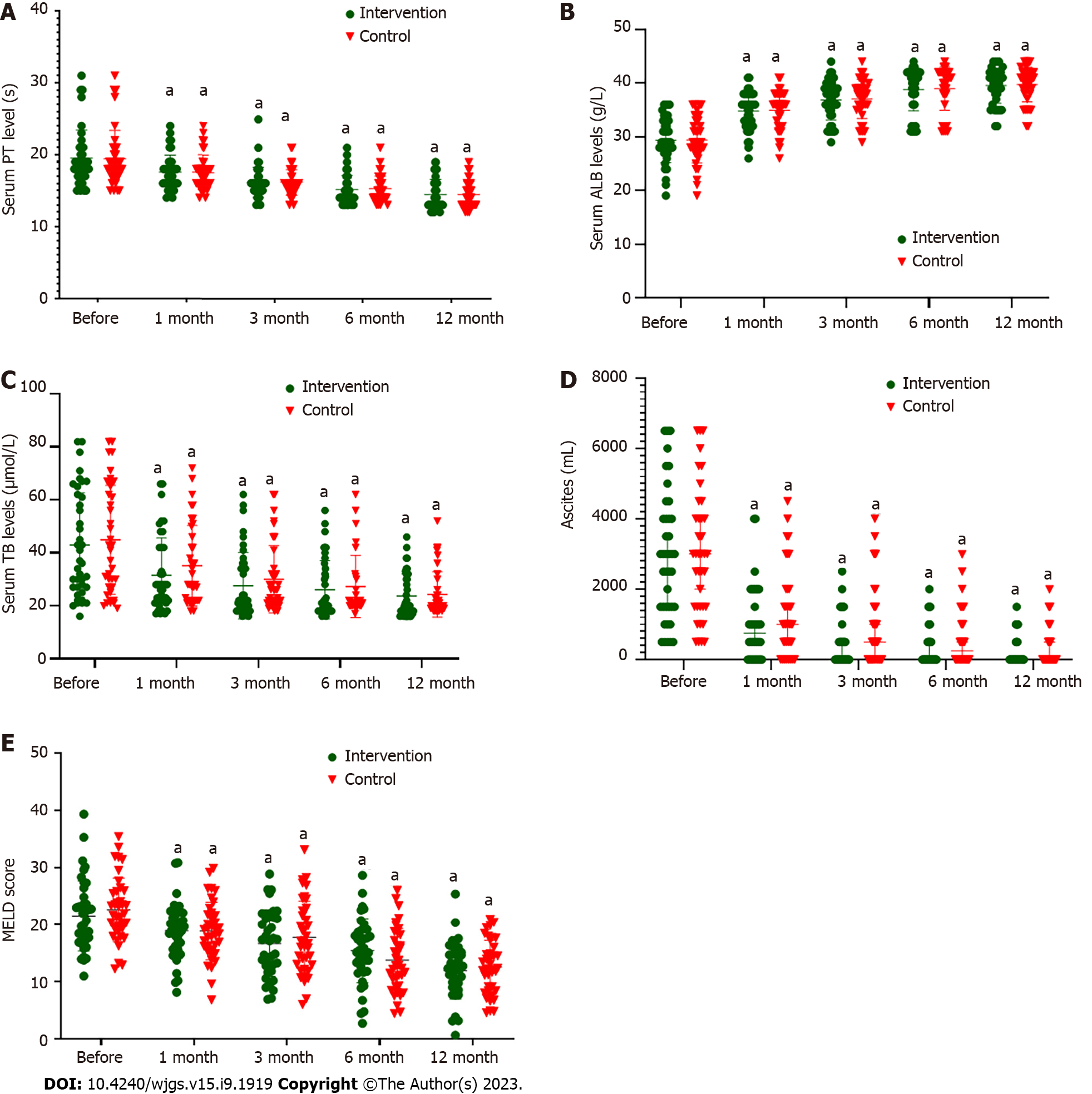
Figure 1A and Table 2 show that there was no significant difference in serum PT levels between the control and intervention groups before ABMI (P > 0.05). After ABMI, serum PT in both groups was significantly lower than before ABMI (P < 0.01), but there was no significant difference between the two groups at each time point (P > 0.05).
| Group | Before | 1 mo | t/P value | 3 mo | t/P value | 6 mo | t/P value | 12 mo | t/P value |
| Control | 19.42 ± 3.95 | 17.55 ± 2.42 | 5.603/0.000 | 16.16 ± 1.82 | 5.811/0.000 | 15.26 ± 1.81 | 8.008/0.000 | 14.42 ± 1.80 | 9.909/0.000 |
| Intervention | 19.50 ± 3.89 | 17.55 ± 2.39 | 6.053/0.000 | 16.15 ± 2.13 | 8.780/0.000 | 15.13 ± 2.03 | 10.528/0.000 | 14.43 ± 2.10 | 11.755/0.000 |
| t | 0.089 | 0.005 | 0.018 | 0.317 | 0.009 | ||||
| P value | 0.929 | 0.996 | 0.986 | 0.752 | 0.993 |
Figure 1B and Table 3 show that there was no significant difference in serum albumin levels between the two groups before ABMI (P > 0.05). After ABMI, albumin level in both groups was significantly higher than before ABMI (P < 0.01), but there was no significant difference between the two groups at each time point (P > 0.05).
| Group | Before | 1 mo | t/P value | 3 mo | t/P value | 6 mo | t/P value | 12 mo | t/P value |
| Control | 29.45 ± 4.26 | 35.05 ± 3.45 | 16.560/0.000 | 37.13 ± 3.20 | 12.024/0.000 | 39.55 ± 2.33 | 14.535/0.000 | 40.24 ± 2.24 | 18.460/0.000 |
| Intervention | 29.38 ± 4.16 | 34.83 ± 3.54 | 17.465/0.000 | 36.85 ± 3.70 | 18.241/0.000 | 38.25 ± 3.97 | 17.519/0.000 | 39.53 ± 3.28 | 19.131/0.000 |
| t | 0.076 | 0.287 | 0.359 | 0.980 | 1.114 | ||||
| P value | 0.940 | 0.775 | 0.721 | 0.330 | 0.369 |
Figure 1C and Table 4 show that there was no significant difference in serum TB level between the two groups before ABMI (P > 0.05). After ABMI, serum TB level in both groups was significantly lower than before ABMI (P < 0.01), but there was no significant difference between the two groups at each time point (P > 0.05).
| Group | Before | 1 mo | t/P value | 3 mo | t/P value | 6 mo | t/P value | 12 mo | t/P value |
| Control | 44.89 ± 20.56 | 35.11 ± 15.20 | 6.536/0.000 | 29.95 ± 12.69 | 7.379/0.000 | 27.26 ± 11.77 | 7.896/0.000 | 24.29 ± 8.57 | 8.255/0.000 |
| Intervention | 42.90 ± 19.77 | 31.48 ± 14.12 | 7.534/0.000 | 27.58 ± 12.55 | 9.014/0.000 | 26.05 ± 11.02 | 9.021/0.000 | 23.65 ± 8.44 | 9.120/0.000 |
| t | 0.437 | 1.093 | 0.830 | 0.470 | 0.332 | ||||
| P value | 0.663 | 0.278 | 0.409 | 0.640 | 0.741 |
Figure 1D and Table 5 showed that there was no significant difference in ascites volume before ABMI between the two groups (P > 0.05). After ABMI, ascites volume in both groups was significantly lower than before ABMI (P < 0.01), but there was no significant difference between the two groups at each time point (P > 0.05).
| Group | Before | 1 mo | Z/P value | 3 mo | Z/P value | 6 mo | Z/P value | 12 mo | Z/P value |
| Control | 3000 (1500-4125) | 1000 (0-2000) | 5.386/0.000 | 500 (0-1500) | 5.388/0.000 | 250 (0-1125) | 5.392/0.000 | 0 (0-1000) | 5.394/0.000 |
| Intervention | 3000 (1500-4000) | 750 (0-1875) | 5.519/0.000 | 0 (0-500) | 5.519/0.000 | 0 (0-500) | 5.525/0.000 | 0 (0-500) | 5.457/0.000 |
| Z | 0.226 | 0.780 | 1.379 | 1.353 | 1.374 | ||||
| P value | 0.790 | 0.436 | 0.168 | 0.176 | 0.170 |
Figure 1E and Table 6 showed that there was no significant difference in MELD score before ABMI between the two groups (P > 0.05). After ABMI, MELD score in both groups was significantly lower than before ABMI (P < 0.01), but there was no significant difference between the two groups at each time point (P > 0.05).
| Group | Before | 1 mo | t/P value | 3 mo | t/P value | 6 mo | t/P value | 12 mo | t/P value |
| Control | 21.53 ± 6.17 | 18.87 ± 5.09 | 2.050/0.044 | 17.73 ± 6.42 | 2.631/0.010 | 13.76 ± 5.51 | 5.790/0.000 | 12.58 ± 4.64 | 7.147/0.000 |
| Intervention | 22.61 ± 5.61 | 18.92 ± 4.85 | 3.147/0.002 | 16.67 ± 5.75 | 4.677/0.000 | 15.42 ± 5.61 | 5.732/0.000 | 11.94 ± 5.00 | 8.980/0.000 |
| t | 0.804 | 0.044 | 0.770 | 1.325 | 0.577 | ||||
| P value | 0.424 | 0.965 | 0.443 | 0.189 | 0.566 |
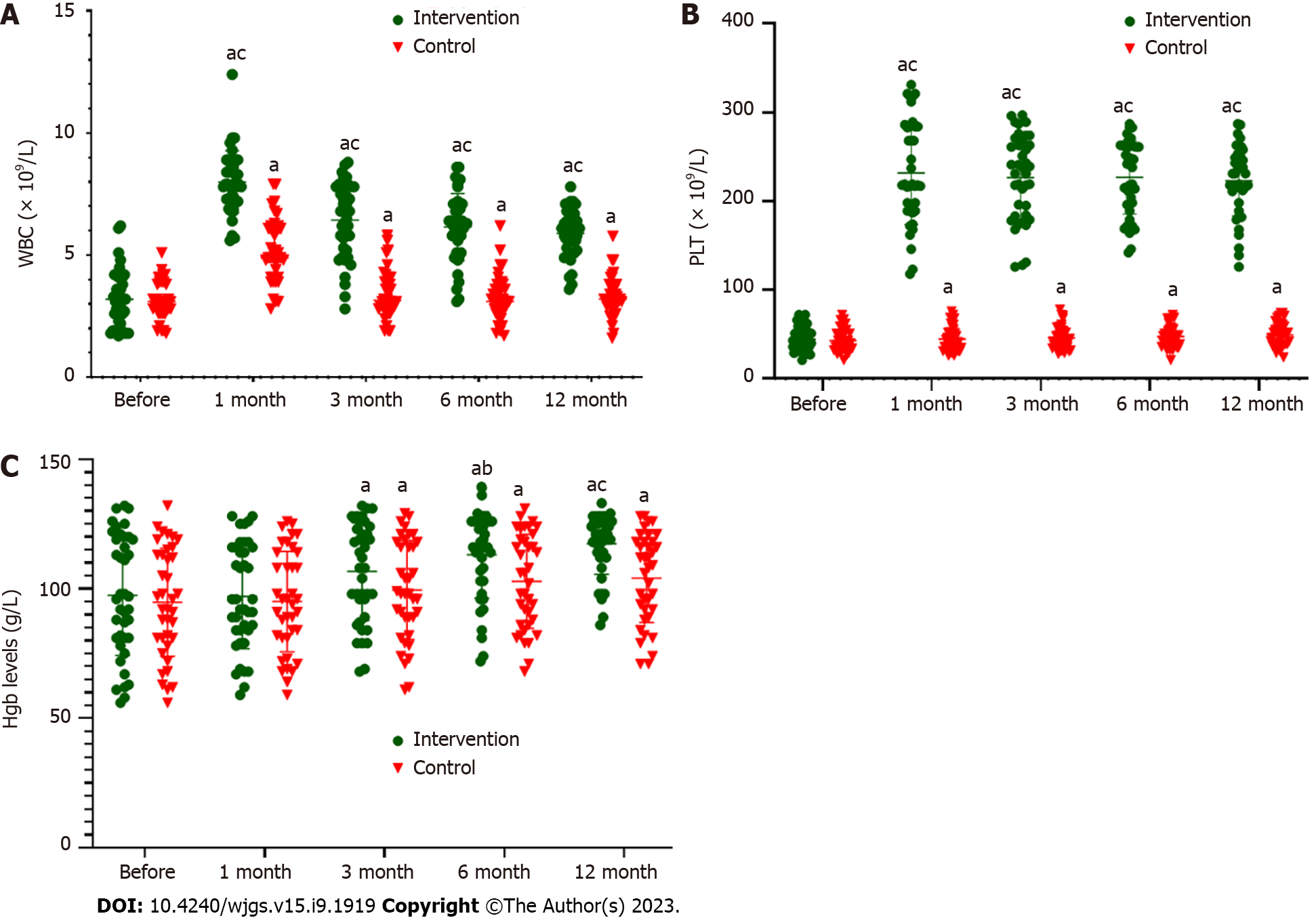
Figure 2A and Table 7 show that there was no significant difference in WBC count between the control and intervention groups before ABMI (P > 0.05). After ABMI, WBC count in both groups was significantly higher than before ABMI (P < 0.01). The increase in the intervention group at each time point was significantly higher than in the control group (P < 0.01).
| Group | Before | 1 mo | t/P value | 3 mo | t/P value | 6 mo | t/P value | 12 mo | t/P value |
| Control | 3.14 ± 0.76 | 5.17 ± 1.32 | 12.975/0.000 | 3.42 ± 0.98 | 3.363/0.002 | 3.27 ± 0.93 | 2.311/0.026 | 3.33 ± 0.82 | 3.505/0.001 |
| Intervention | 3.20 ± 1.17 | 8.00 ± 1.28 | 23.457/0.000 | 6.44 ± 1.54 | 11.474/0.000 | 6.15 ± 1.37 | 13.409/0.000 | 5.89 ± 0.97 | 13.776/0.000 |
| t | 0.258 | 9.625 | 10.211 | 10.768 | 12.531 | ||||
| P value | 0.797 | 0.000 | 0.000 | 0.000 | 0.000 |
Figure 2B and Table 8 show that there was no significant difference in serum platelet count between the two groups before ABMI (P > 0.05). After ABMI, platelet count in both groups was significantly higher than before ABMI (P < 0.01). The increase in the intervention group at each time point was significantly higher than in the control group (P < 0.01).
| Group | Before | 1 mo | t/P value | 3 mo | t/P value | 6 mo | t/P value | 12 mo | t/P value |
| Control | 42.82 ± 12.66 | 44.64 ± 13.08 | 2.776/0.009 | 45.79 ± 12.67 | 3.433/0.001 | 47.45 ± 12.02 | 5.037/0.000 | 49.26 ± 13.37 | 5.093/0.0000 |
| Intervention | 44.30 ± 13.51 | 231.63 ± 57.78 | 22.601/0.000 | 226.50 ± 47.92 | 27.006/0.000 | 226.68 ± 41.32 | 31.942/0.000 | 222.90 ± 39.36 | 32.647/0.000 |
| t | 0.500 | 19.475 | 22.503 | 25.719 | 25.812 | ||||
| P value | 0.618 | 0.000 | 0.000 | 0.000 | 0.000 |
Figure 2C and Table 9 showed that there was no significant difference in serum hemoglobin level between the two groups before ABMI (P > 0.05). Serum hemoglobin level in both groups at 3, 6 and 12 mo after ABMI was significantly higher than before ABMI (P < 0.01). The increase at 6 and 12 mo in the intervention group was significantly higher than in the control group (P < 0.05 and P < 0.01).
| Group | Before | 1 mo | t/P value | 3 mo | t/P value | 6 mo | t/P value | 12 mo | t/P value |
| Control | 94.74 ± 20.86 | 95.03 ± 19.39 | 0.387/0.701 | 99.47 ± 19.12 | 4.211/0.000 | 102.84 ± 18.11 | 6.286/0.000 | 104.13 ± 17.19 | 7.498/0.000 |
| Intervention | 97.48 ± 23.20 | 97.03 ± 20.19 | 0.394/0.696 | 106.70 ± 18.97 | 6.716/0.000 | 113.08 ± 16.69 | 9.445/0.000 | 117.33 ± 11.70 | 8.318/0.000 |
| t | 0.547 | 0.446 | 1.675 | 2.596 | 3.980 | ||||
| P value | 0.586 | 0.657 | 0.098 | 0.011 | 0.000 |
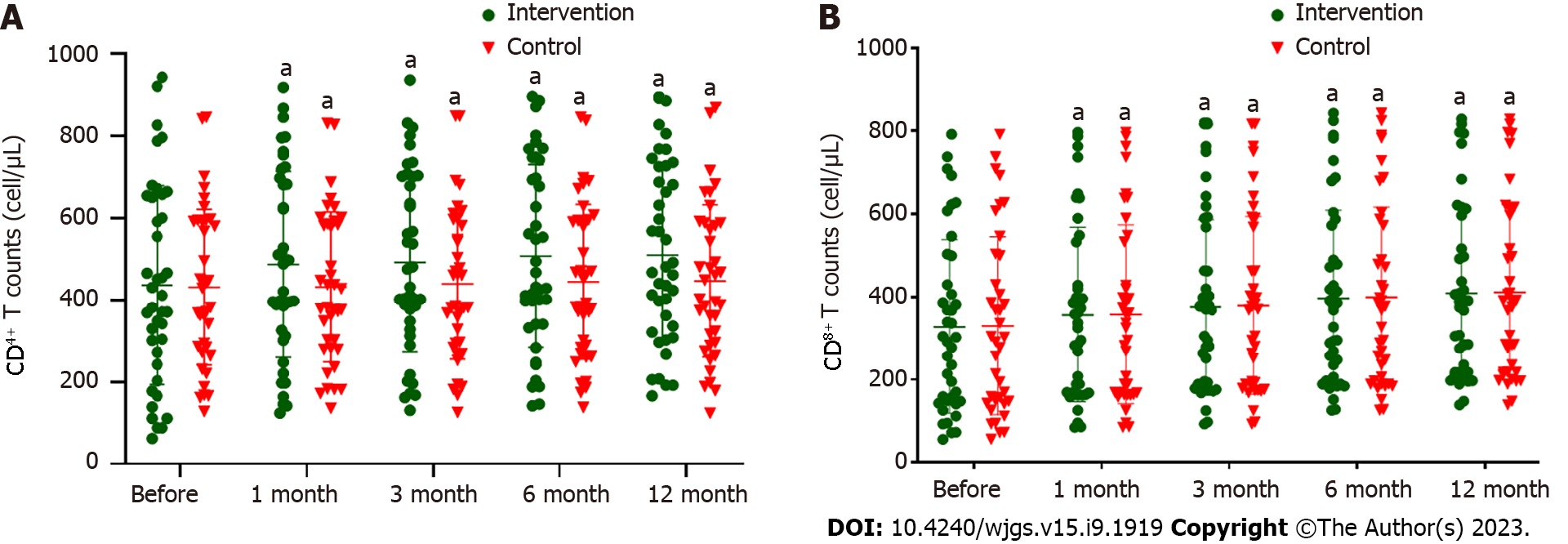
Figure 3A and Table 10 show that there was no significant difference in CD4+ T cell count before ABMI between the control and intervention groups (P > 0.05). After ABMI, CD4+ T cell count in the intervention group at 1, 3, 6 and 12 mo, and in the control group at 3, 6 and 12 mo was significantly higher than before ABMI (P < 0.01), but there was not significant difference between the two groups at each time point (P > 0.05).
| Group | Before | 1 mo | t/P value | 3 mo | t/P value | 6 mo | t/P value | 12 mo | t/P value |
| Control | 432 ± 190 | 432 ± 183 | 0.384/0.703 | 440 ± 184 | 2.805/0.008 | 445 ± 189 | 5.259/0.000 | 447 ± 186 | 4.796/0.000 |
| Intervention | 436 ± 243 | 487 ± 227 | 9.029/0.000 | 492 ± 219 | 8.140/0.000 | 507 ± 223 | 10.150/0.000 | 510 ± 213 | 8.566/0.000 |
| t | 0.106 | 1.177 | 1.148 | 1.329 | 1.400 | ||||
| P value | 0.916 | 0.243 | 0.255 | 0.188 | 0.166 |
Figure 3B and Table 11 showed that there was no significant difference in CD8+ T cell count before ABMI between the two groups (P > 0.05). After ABMI, CD8+ T cell count in the intervention group at 1, 3, 6 and 12 mo, and in the control group at 1, 3 and 6 mo were significantly higher than before ABMI (P < 0.01), but there was no significant difference between the two groups at each time point (P > 0.05).
| Group | Before | 1 mo | t/P value | 3 mo | t/P value | 6 mo | t/P value | 12 mo | t/P value |
| Control | 338 ± 210 | 349 ± 215 | 2.961/0.000 | 361 ± 211 | 4.917/0.000 | 363 ± 213 | 3.432/0.001 | 356 ± 204 | 1.092/0.282 |
| Intervention | 328 ± 210 | 357 ± 211 | 9.594/0.000 | 376 ± 210 | 10.948/0.000 | 396 ± 212 | 12.880/0.000 | 408 ± 207 | 12.709/0.000 |
| t | 0.206 | 0.169 | 0.328 | 0.684 | 1.119 | ||||
| P value | 0.837 | 0.866 | 0.744 | 0.496 | 0.267 |
Stem cells are not highly differentiated and have the potential to regenerate various tissues and organs in the human body. They are called universal cells in the medical field. Under certain conditions, they can differentiate into multiple functional cells. According to different differentiation potentials, they can be divided into four categories[22]: (1) Totipotent stem cells that can develop into independent individuals; (2) multipotent stem cells that are the descendants of universal stem cells; they cannot develop into individuals, but they can develop into multiple tissues; (3) pluripotent stem cells that can only differentiate into cells of specific groups, such as specific tissues or organs; and (4) unipotent stem cells that can only produce one cell type and have a self-updating property. BMSCs are a type of stem cells with multidirectional differentiation potential and self-renewal. They can differentiate into specific tissues under special circumstances, including liver cells and cardiomyocytes. BMSC transplantation, as a new technology for repairing regenerated damaged organs, has become a research hotspot for stem cell transplantation due to the advantages of convenient collection, low rejection, safety and reliability, and low cost[23,24]. BMSCs can replace damaged hepatocytes by inducing differentiation into hepatocytes in severe liver disease, improve function of the damaged liver, and bring new hope for the treatment of cirrhosis[25,26]. In our previous study, ABMI for patients with DLC and acquired immunodeficiency syndrome significantly prolonged survival. ABMI has the following advantages over traditional treatment: (1) It is simple and easy to collect autologous bone marrow, and there is no shortage of donors; (2) there is usually no immune rejection; (3) it is safe, with almost no adverse reactions, exogenous pollution, and disease transmission; and (4) it has remarkable efficacy at a low cost. Whether splenectomy has an impact on the efficacy of patients with DLC remains unclear. In this study, regardless of whether the spleen was removed, serum PT, TB, and ascites volume after ABMI were significantly lower than before ABMI, and albumin levels were significantly higher. TB is an index for evaluating liver reserve function, albumin is an important index for evaluating liver synthetic function, and PT is an index for evaluating the degree of liver cell necrosis. If these indexes increase or decrease, it indicates that liver function is damaged. The possible mechanism is that after ABMI through the ROV, BMSCs can secrete a large number of different growth factors themselves, stimulate damaged liver cells, promote production of hepatocyte growth factors, promote BMSC differentiation, and exert anti-apoptosis, so that the fibrotic liver regenerates and repairs liver function[27,28]. Liver function improvement also improves coagulation function and reduces the risk of spontaneous bleeding. Most DLC patients still have bleeding in the digestive tract after endoscopic ligation and compression of the three-lumen two-balloon tube in other hospitals, and it is often difficult to undergo further treatment. For patients with gastrointestinal bleeding, conservative treatment such as blood transfusion, hemostasis, and hepatoprotective diuresis can alleviate the condition. We adopt elective splenectomy + infusion port placement. For patients who fail conservative treatment, emergency surgery is required. If the liver function is above Child-Pugh grade B, splenectomy and infusion port placement can be selected. Child-Pugh grade C increases the risk of postoperative complications. It is necessary to consider the comprehensive conditions and the pros and cons of splenectomy in patients with DLC. If conditions permit, splenectomy can be considered. In the intervention group, there were two DLC patients with acute gastrointestinal hemorrhage with Child-Pugh grade C. If splenectomy is not performed, postoperative bleeding may occur. Therefore, splenectomy was still selected during the operation. After 1-year follow-up, the Child-Pugh grade C patients achieved the same curative effect as the patients with grade B, so liver function classification is not a barrier for choosing splenectomy, and we will expand the number of DLC patients in our future clinical work. In DLC patients, splenectomy can be selected if one of the following three indications was met: (1) Giant spleen, which affects the daily life of the patient; (2) the hypersplenism is serious; and (3) preventing bleeding caused by portal vein pressure. This study showed that splenectomy does not increase the complications caused by surgery, and can improve the symptoms caused by hypersplenism. We found that there was no significant difference between the surgical mortality of the intervention and control groups. The intervention group mainly suffered from bleeding on the wound surface due to splenectomy, while the control group mainly suffered from gastrointestinal bleeding after surgery. There was no obvious relationship between gastrointestinal bleeding and ABMI. The infusion volume in this group was approximately 40 mL, the infusion speed was slow, and the possibility of gastrointestinal bleeding was small. So the bleeding is mainly related to the stress of surgical trauma.
In this study, the surgical complications in both groups had some relationship with coagulation dysfunction, and after ABMI, the coagulation function gradually improved. Ascites is an important indicator of liver dysfunction. After ABMI, the levels of ascites in both groups were significantly less than before ABMI, indicating that both treatments had a significant effect on improving liver function. There was no significant difference in PT, albumin, ascites and MELD score between the two groups at each time point after ABMI, indicating that ABMI improved liver synthetic and secretory functions and this was not related to splenectomy. The MELD score estimated liver disease severity according to the three parameters of INR, TB and creatinine reflecting not only liver injury but also kidney function. Fung et al[21] studied patients with acute onset of chronic hepatitis B and found that MELD score accurately predicted short-term mortality of patients. When MELD score was ≥ 10.51, the risk of death increased 3.057 times, which has clinical significance for guiding assessment of later follow-up and prognosis.
There are multiple reasons for anemia in patients with liver cirrhosis. The deposition of fibrous tissue in the liver leads to impaired portal vein blood flow, thereby limiting liver hyperplasia[29,30]. Long-term anorexia leads to malnutrition, which makes the intake of iron inadequate, and patients generally have portal hypertension gastrointestinal disease, which reduces iron absorption. Chronic blood loss in turn causes the loss of iron to exceed the amount of iron supplementation, and the stored iron decreases. The possible mechanisms are: (1) Hepatitis B virus causes bone marrow hematopoietic stem cells and hematopoietic regulatory factors to fail to function normally[31]; (2) hematopoietic stem cells proliferate, maintain stemness, or are blocked for differentiated into myeloid stem cells and lymphoid stem cells[32]; (3) hepatitis B virus infection causes immune damage, resulting in hematopoietic stem cell apoptosis, leading to bone marrow hematopoietic failure[33]; and (4) virus-mediated autoimmune abnormalities cause liver dysfunction, reduce degradation of toxic metabolites, resulting in ischemia and necrosis of pluripotent stem cells, proliferation of hematopoietic stem cells is inhibited, and peripheral blood cell production is reduced from the source[34]. Cirrhosis causes portal hypertension, increased splenic vein pressure, splenic congestion and hypersplenism, leading to increased destruction of peripheral blood cells. In this study, after ABMI through the ROV in two groups, liver function was improved, and the levels of WBC count, hemoglobin, and platelet count were higher than before ABMI, but the increases in the intervention group were more obvious than in the control group, indicating that splenectomy can relieve hypersplenism caused by liver cirrhosis. Peripheral blood T cell subsets mediate the adaptive cellular immune response, which is often regarded as an indicator of immune function in clinical practice. In particular, the immune response of virus-specific T cells has an important effect and regulation in liver pathology and virus clearance[35]. CD4+ T cells are helper T cells, CD8+ T cells are cytotoxic T cells, and they secrete different cytokines to exert immune effects[36]. In a previous study, after ABMI through the ROV in the treatment of HIV patients with DLC[18], the CD8+ and CD4+ T cells showed obvious changes, and we found that CD8+ and CD4+ also changed in non-HIV patients with DLC, so we found that ABMI had an impact on immune function, so we retained these two indicators in the present study. We found that after ABMI, the CD4+ T cell count at each time point in the control and intervention groups was significantly higher than before ABMI, but there was no significant difference between the two groups. The CD8+ T cell count in the control group showed a significant increase after ABMI at 1 and 6 mo, but decreased at 12 mo, which was not significantly different from that before ABMI. In the intervention group, the CD8+ T cell count continued to increase after ABMI at 3, 6 and 12 mo, and there was no obvious decrease in CD8+ T cell count, indicating that splenectomy can promote continuous increase of CD8+ T cell count. Without removing the spleen, there was a transient increase in CD8+ T cell count, and after 12 mo of ABMI the CD8+ T cell count was continuously decreased in the spleen because of hypersplenism.
This study had some limitations. The follow-up time was too short to analyze the 5-year survival of patients. The specific mechanism of nucleated cells in treatment of DLC is still unclear. In order to address the above shortcomings, we will further follow up the patients, extend the follow-up time to > 5 years. We will also carry out animal experiments to further explore the mechanism of nucleated cells in treatment of DLC.
ABMI through the ROV in patients with DLC can significantly improve liver synthetic and secretory functions but cannot relieve hypersplenism. ABMI combined with splenectomy can improve liver function and alleviate hypersplenism. However, splenectomy in patients with DLC has a higher risk of surgery-related complications.
Autologous bone marrow infusion (ABMI) was performed in patients with decompensated liver cirrhosis (DLC), with good results, but whether splenectomy affects outcome is still unclear.
The main purpose of this study was to determine the efficacy of ABMI combined with splenectomy in the treatment of DLC, to clarify the impact of splenectomy on liver and bone marrow function, and to provide a basis for routine splenectomy in patients with DLC.
To clarify the efficacy of ABMI combined with splenectomy in the treatment of DLC, and the impact of splenectomy on liver and bone marrow function, so as to provide basis for rational treatment of DLC.
In this study, ABMI combined with splenectomy was used to treat DLC, and the impact of splenectomy on liver and bone marrow function was observed. These common clinical indicators (such as the prothrombin time, serum total bilirubin, ascites volume, white blood cell and platelets counts and so on.) were used to evaluate liver and bone marrow function, which were easy to be popularized in clinic.
This study shows that ABMI combined with splenectomy is effective in the treatment of DLC, which can help to recover liver and bone marrow function, and alleviate hypersplenism. However, the sample size of this study is small and the follow-up time is short, which needs to be further improved in future studies.
ABMI combined with splenectomy is a new method for the treatment of DLC, which provides a theoretical basis for the treatment of other chronic diseases.
Whether ABMI is suitable for other diseases such as osteoarthropathy, cerebral infarction sequelae, diabetes and other chronic diseases, they still need further study.
We would like to thank Chinese Academy of Sciences Experimental Platform for providing technological support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kumar R, India; Kupeli S, Turkey S-Editor: Yan JP L-Editor: A P-Editor: Yu HG
| 1. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9583] [Article Influence: 737.2] [Reference Citation Analysis (0)] |
| 2. | Marques HP, Barros I, Li J, Murad SD, di Benedetto F. Current update in domino liver transplantation. Int J Surg. 2020;82S:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Sun T, Li H, Bai Y, Bai M, Gao F, Yu J, Wu R, Du L, Li F. Ultrasound-targeted microbubble destruction optimized HGF-overexpressing bone marrow stem cells to repair fibrotic liver in rats. Stem Cell Res Ther. 2020;11:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 749] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 5. | Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, Wojtacha D, Man TY, Mackinnon A, Ridgway RA, Kendall T, Williams MJ, Jamieson T, Raven A, Hay DC, Iredale JP, Clarke AR, Sansom OJ, Forbes SJ. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 373] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 6. | Pai M, Spalding D, Xi F, Habib N. Autologous bone marrow stem cells in the treatment of chronic liver disease. Int J Hepatol. 2012;2012:307165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Takami T, Terai S, Sakaida I. Novel findings for the development of drug therapy for various liver diseases: Current state and future prospects for our liver regeneration therapy using autologous bone marrow cells for decompensated liver cirrhosis patients. J Pharmacol Sci. 2011;115:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, Hegazy M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Liu B, Chen X, Wang Y, Shi Y. Curative effect of hepatic portal venous administration of autologous bone marrow in AIDS patients with decompensated liver cirrhosis. Cell Death Dis. 2013;4:e739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Giannotti S, Parchi PD, Colasanti GB, Agostini G, Moreschini F, Cataldi C, Ferrata P, Capanna R. Use of autologous bone marrow cells concentrate enriched with platelet-fibrin on extensor mechanism allograft reconstruction for extensor mechanism failure following total knee arthroplasty. J Biol Regul Homeost Agents. 2017;31:107-111. [PubMed] |
| 12. | Lamirault G, de Bock E, Sébille V, Delasalle B, Roncalli J, Susen S, Piot C, Trochu JN, Teiger E, Neuder Y, Le Tourneau T, Manrique A, Hardouin JB, Lemarchand P. Sustained quality of life improvement after intracoronary injection of autologous bone marrow cells in the setting of acute myocardial infarction: results from the BONAMI trial. Qual Life Res. 2017;26:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Rice CM, Marks DI, Walsh P, Kane NM, Guttridge MG, Redondo J, Sarkar P, Owen D, Wilkins A, Scolding NJ. Repeat infusion of autologous bone marrow cells in multiple sclerosis: protocol for a phase I extension study (SIAMMS-II). BMJ Open. 2015;5:e009090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Abdel Aziz M, Atta H, Roshdy N, Rashed L, Sabry D, Hassouna A, Aboul Fotouh G, Hasan N, Younis R, Chowdhury J. Amelioration of Murine Schistosoma mansoni Induced Liver Fibrosis by Mesenchymal Stem Cells. J Stem Cells Regen Med. 2012;8:28-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Matsumoto T, Takami T, Sakaida I. Cell transplantation as a non-invasive strategy for treating liver fibrosis. Expert Rev Gastroenterol Hepatol. 2016;10:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Takami T, Terai S, Sakaida I. Stem cell therapy in chronic liver disease. Curr Opin Gastroenterol. 2012;28:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459-466. [PubMed] |
| 18. | Liu B, Cheng M, Chen X, Li L, Si Y, Wang S, Wang Y, Shi Y. Autologous bone marrow cell transplantation in the treatment of HIV patients with compensated cirrhosis. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, Kawaguchi T, Kurosaki M, Sakaida I, Shimizu M, Taniai M, Terai S, Nishikawa H, Hiasa Y, Hidaka H, Miwa H, Chayama K, Enomoto N, Shimosegawa T, Takehara T, Koike K. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol. 2021;56:593-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 20. | Inadomi J, Cello JP, Koch J. Ultrasonographic determination of ascitic volume. Hepatology. 1996;24:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Fung J, Mak LY, Chan AC, Chok KS, Wong TC, Cheung TT, Dai WC, Sin SL, She WH, Ma KW, Seto WK, Lai CL, Lo CM, Yuen MF. Model for End-Stage Liver Disease With Additional Criteria to Predict Short-Term Mortality in Severe Flares of Chronic Hepatitis B. Hepatology. 2020;72:818-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Poliwoda S, Noor N, Downs E, Schaaf A, Cantwell A, Ganti L, Kaye AD, Mosel LI, Carroll CB, Viswanath O, Urits I. Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthop Rev (Pavia). 2022;14:37498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 23. | Philips CA, Augustine P, Rajesh S, Ahamed R, George T, Padsalgi G, Paramaguru R, Valiathan G, John SK. Granulocyte Colony-Stimulating Factor Use in Decompensated Cirrhosis: Lack of Survival Benefit. J Clin Exp Hepatol. 2020;10:124-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Anand L, Bihari C, Kedarisetty CK, Rooge SB, Kumar D, Shubham S, Kumar G, Sahney A, Sharma MK, Maiwall R, Kumar A, Sarin SK. Early cirrhosis and a preserved bone marrow niche favour regenerative response to growth factors in decompensated cirrhosis. Liver Int. 2019;39:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Latorre R, Vaquero J, Rincón D, Puerto M, Ponce MD, Sarnago F, Matamoros JA, Ramón E, Elizaga J, Bañares R, Ripoll C. Determinants of platelet count are different in patients with compensated and decompensated cirrhosis. Liver Int. 2016;36:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Mohamadnejad M, Vosough M, Moossavi S, Nikfam S, Mardpour S, Akhlaghpoor S, Ashrafi M, Azimian V, Jarughi N, Hosseini SE, Moeininia F, Bagheri M, Sharafkhah M, Aghdami N, Malekzadeh R, Baharvand H. Intraportal Infusion of Bone Marrow Mononuclear or CD133+ Cells in Patients With Decompensated Cirrhosis: A Double-Blind Randomized Controlled Trial. Stem Cells Transl Med. 2016;5:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Chen B, Pang L, Cao H, Wu D, Wang Y, Tao Y, Wang M, Chen E. Autologous stem cell transplantation for patients with viral hepatitis-induced liver cirrhosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Wu CX, Wang D, Cai Y, Luo AR, Sun H. Effect of Autologous Bone Marrow Stem Cell Therapy in Patients with Liver Cirrhosis: A Meta-analysis. J Clin Transl Hepatol. 2019;7:238-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Paternostro R, Kapzan L, Mandorfer M, Schwarzer R, Benedikt S, Viveiros A, Bauer D, Ferlitsch M, Zoller H, Trauner M, Ferlitsch A. Anemia and iron deficiency in compensated and decompensated cirrhosis: Prevalence and impact on clinical outcomes. J Gastroenterol Hepatol. 2020;35:1619-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Yang J, Yan B, Yang L, Li H, Fan Y, Zhu F, Zheng J, Ma X. Macrocytic anemia is associated with the severity of liver impairment in patients with hepatitis B virus-related decompensated cirrhosis: a retrospective cross-sectional study. BMC Gastroenterol. 2018;18:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY, Weng WZ, Peng YW, Cao HJ, Xie JQ, Xie SB, Xiang AP, Gao ZL. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 32. | Sun A, Gao W, Xiao T. Autologous bone marrow stem cell transplantation via the hepatic artery for the treatment of hepatitis B virus-related cirrhosis: a PRISMA-compliant meta-analysis based on the Chinese population. Stem Cell Res Ther. 2020;11:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Nadelson J, Satapathy SK, Nair S. Glycated Hemoglobin Levels in Patients with Decompensated Cirrhosis. Int J Endocrinol. 2016;2016:8390210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Wang Z, Sheng L, Yang Y, Yang F, Xiao X, Hua J, Guo C, Wei Y, Tang R, Miao Q, Zhang J, Li Y, Fang J, Qiu D, Krawitt EL, Bowlus CL, Gershwin ME, Wang Q, Ma X. The Management of Autoimmune Hepatitis Patients with Decompensated Cirrhosis: Real-World Experience and a Comprehensive Review. Clin Rev Allergy Immunol. 2017;52:424-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Shi Y, Wu W, Yang Y, Yang Q, Song G, Wu Y, Wei L, Chen Z. Decreased Tim-3 expression is associated with functional abnormalities of monocytes in decompensated cirrhosis without overt bacterial infection. J Hepatol. 2015;63:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |