Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.1901
Peer-review started: May 20, 2023
First decision: July 8, 2023
Revised: July 20, 2023
Accepted: August 4, 2023
Article in press: August 4, 2023
Published online: September 27, 2023
Processing time: 125 Days and 1.3 Hours
Pancreatoduodenectomy (PD) is the most effective surgical procedure to remove a pancreatic tumor, but the prevalent postoperative complications, including postoperative pancreatic fistula (POPF), can be life-threatening. Thus far, there is no consensus about the prevention of POPF.
To determine possible prognostic factors and investigate the clinical effects of modified duct-to-mucosa pancreaticojejunostomy (PJ) on POPF development.
We retrospectively collected and analyzed the data of 215 patients who under
A total of 108 patients received traditional end-to-side invagination PJ, and 107 received modified duct-to-mucosa PJ. Overall, 58.6% of patients had various complications, and 0.9% of patients died after PD. Univariate and multivariate logistic regression analyses showed that anastomotic approaches, main pancreatic duct (MPD) diameter and pancreatic texture were significantly associated with the incidence of POPF. Additionally, the POPF incidence and operation time in patients receiving modified duct-to-mucosa PJ were 11.2% and 283.4 min, respectively, which were significantly lower than those in patients receiving traditional end-to-side invagination PJ (27.8% and 333.2 minutes).
Anastomotic approach, MPD diameter and pancreatic texture are major risk factors for POPF development. Compared with traditional end-to-side invagination PJ, modified duct-to-mucosa PJ is a simpler and more efficient technique that results in a lower incidence of POPF. Further studies are needed to validate our findings and explore the clinical applicability of our technique for laparoscopic and robotic PD.
Core Tip: We evaluated the safety and feasibility of modified duct-to-mucosa pancreaticojejunostomy (PJ) during pancreatoduodenectomy (PD) by analyzing the data of 215 patients who underwent PD in our surgery center. Compared with traditional end-to-side invagination PJ, modified duct-to-mucosa PJ was a simpler and more efficient technique that resulted in a lower incidence of postoperative pancreatic fistula (11.2%). Meanwhile, we found that anastomotic approach, main pancreatic duct diameter and pancreatic texture were major risk factors for postoperative pancreatic fistula development.
- Citation: Sun Y, Yu XF, Yao H, Xu S, Ma YQ, Chai C. Safety and feasibility of modified duct-to-mucosa pancreaticojejunostomy during pancreatoduodenectomy: A retrospective cohort study. World J Gastrointest Surg 2023; 15(9): 1901-1909
- URL: https://www.wjgnet.com/1948-9366/full/v15/i9/1901.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i9.1901
Pancreatoduodenectomy (PD) is widely performed as the standard treatment for resectable tumors in the pancreas and periampullary region. Despite recent advances in surgical techniques and perioperative management, the incidence of postoperative complications and overall mortality remain high[1]. Specifically, a postoperative pancreatic fistula (POPF), the most common and potentially deadly postoperative complication, develops in 5% to 26% of patients[2]. To improve the operation efficacy, effective prevention of POPF can be crucial. Therefore, proper assessment of relevant risk factors for POPF is necessary, and anastomosis has proven to be an effective treatment approach[3]. The intention of this retrospective, single-center study is to explore the risk factors for POPF and further determine the effects of modified duct-to-mucosa pancreaticojejunostomy (PJ) on POPF prevention.
The data of a series of 215 consecutive patients who underwent elective PD for benign or malignant pathologies in our center between January 2017 and February 2022 were analyzed. Patients were then stratified into two groups according to the anastomotic method for further analysis. Patients with a pathological diagnosis of periampullary lesions, with an American Society of Anesthesiologists score I-III, and who provided informed consent were included in the study. Patients with incomplete medical records, who underwent neoadjuvant treatment preoperatively, who had undergone emergency surgery, or with synchronous cancer were excluded from the study. The primary outcome measure was the POPF rate, and the secondary outcome measures were mortality rates, operative time, blood loss and length of hospital stay. Other outcomes of interest included demographic characteristics (age, sex, anamnesis, concomitant disease, biochemical indices) and intraoperative data (main pancreatic duct (MPD) diameter, pancreas texture, type of anastomosis). According to the International Study Group on Pancreatic Surgery 2016 consensus statement, POPD was strictly defined as “any measurable volume of drained fluid on or after postoperative Day 3 with an amylase level more than 3 times the upper limit of the normal amylase range and having an impact on clinical outcome”[4]. A grade A pancreatic fistula was defined as a "biochemical leak", a grade B fistula required changes in postoperative management, and a grade C fistula needed reoperation or led to organ failure and/or mortality[4]. Mortality specifically referred to the death of inpatients within 3 mo after surgery.
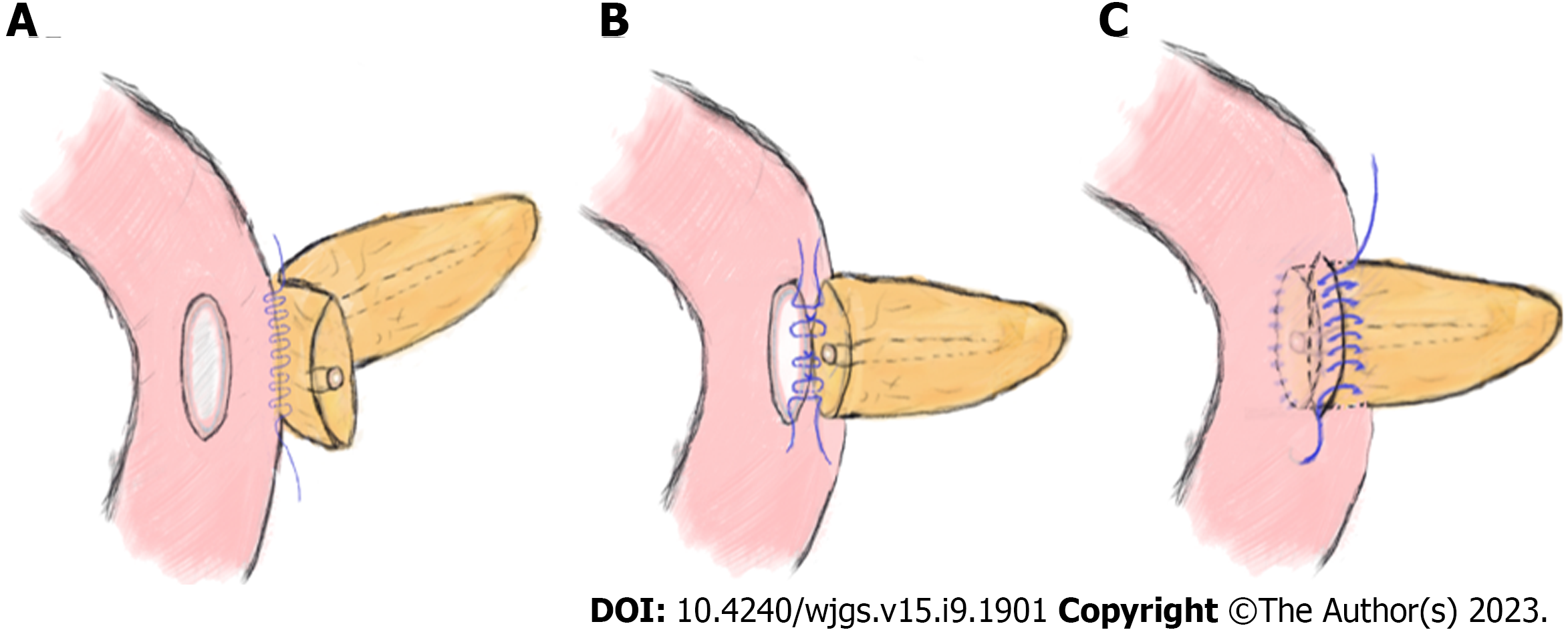
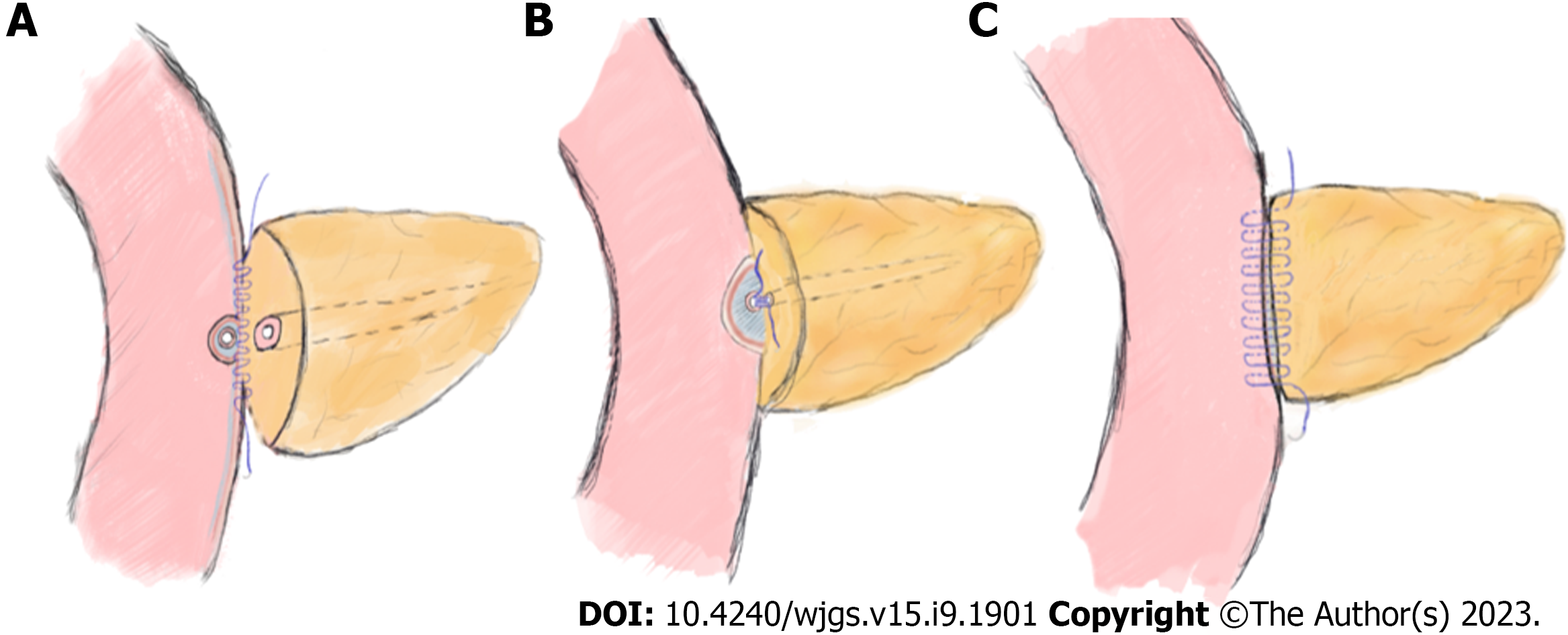
Experienced hepato-bilio-pancreatic surgeons performed standard PD (Child's procedure) on all the patients, and there were no differences between the two groups except for the PJ procedure. The routine procedures for placing the pancreatic stent tube were as follows: After suturing the posterior wall of the pancreatic stump, a right-sized stent tube (8-10 cm in length) with side holes was inserted 3-5 cm into the pancreatic duct, and the other end was placed approximately 5 cm into the small intestine. Then, a stitch was placed to suture and fix the stent tube on the posterior side of the pancreas. Classic end-to-side invagination PJ was implemented as previously reported[5], and the key steps are shown in Figure 1. The procedures of modified duct-to-mucosa PJ were as follows: (1) After enterotomy was performed according to the MPD diameter, the rear edge of the pancreatic stump and the posterior jejunal seromuscular layer were continuously sutured with 3-0 Prolene sutures. The needle was inserted vertically into the pancreas 1.5 cm from the rear edge of the pancreatic stump and passed through the posterior wall of the jejunum after passing through its seromuscular layers. The spacing was approximately 8–10 mm, and the margin was greater than 10 mm (Figure 2A); (2) The posterior wall of the pancreatic duct and the jejunal mucosa were continuously sutured with three to four 4-0 Prolene sutures. The spacing and margin were adjusted according to the MPD diameter (Figure 2B); and (3) After the stent was inserted, the front edges of the pancreatic stump and whole-layer of the jejunum were anastomosed with 3-0 Prolene running sutures. The spacing and margin were similar to those of the first stitch. Although the depth of needle entry was controlled at approximately 1 cm to avoid damaging the MPD on the pancreatic side, it was deeper on the jejunal side to ensure suturing of the whole layer (Figure 2C). In our modified method, tension-free sutures were applied, and no dead space was left between the pancreatic stump and jejunum.
During the perioperative period, most treatment measures were the same for each patient. The preoperative management included smoking and drinking cessation, weight control, skin preparation and antibiotic prophylaxis. Epidural analgesia and gastrointestinal decompression were administered during the operation. Drain amylase levels were routinely measured on the 1st, 3rd and 5th days after surgery, while octreotide was used simultaneously for 7-10 d. Other postoperative management included thromboprophylaxis, nutritional support and controlled fluid infusion. The patients were followed up for 3 mo after discharge.
SPSS 21.0 statistical software was used for data description and analysis. Continuous variables are expressed as the mean ± SD, and Student’s t test was used for comparisons where appropriate. Categorical variables were analyzed by using Fisher’s exact test and the χ2 test. Univariate analysis was used to evaluate the factors associated with POPF development, and multivariate regression analysis was performed to determine the independent risk factors.
Of the 215 patients with an average age of 54.5±13.3 years, 112 patients were male and 103 were female. The percentages of patients with diabetes mellitus, smoking history and previous abdominal surgery were 23.7%, 42.8% and 22.3%, respectively. Preoperative blood tests showed that the respective values of total bilirubin and albumin were 186.9 (µmol/L) and 35.4 (g/L). More than half of the patients (57.2%) had undergone biliary drainage preoperatively. According to the pathological results, the most prevalent conditions were ampullary carcinoma, pancreatic head carcinoma and distal cholangiocarcinoma. The average total operative time was 308.4 min, while the average intraoperative blood loss was 555.1 mL. The overall complication rate was 53.5% (115/215), and the mortality rate was 0.9% (2/215). Specifically, POPF was the most common complication (19.5%), followed by peritoneal infection (13%), abdominal bleeding (11.6%) and bile leakage (9.3%). Additionally, the two cases of death were due to abdominal bleeding associated with POPF development (Table 1).
| Variables | Total patients, n = 215 |
| Gender (male/female) | 112/103 |
| Age (yr) | 54.5 ± 13.3 |
| Diabetes mellitus | 51 (23.7) |
| Smoking history | 92 (42.8) |
| History of abdominal operation | 48 (22.3) |
| Preoperative total bilirubin (µmol/L) | 186.9 ± 74.4 |
| Preoperative biliary drainage | 123 (57.2) |
| Albumin (g/L) | 35.4 ± 4.8 |
| Pathological types | |
| Ampullary carcinoma | 102 (47.4) |
| Pancreatic head carcinoma | 51 (23.7) |
| Distal cholangiocarcinoma | 35 (16.3) |
| Duodenal papillary carcinoma | 22 (10.2) |
| Ampullary benign diseases | 2 (0.9) |
| Other rare diseases | 3 (1.4) |
| Anastomotic method | |
| End-to-side invagination pancreatoduodenectomy | 108 (50.2) |
| Modified duct-to-mucosa pancreatoduodenectomy | 107 (47.8) |
| Main pancreatic duct diameter | |
| ≤ 3 mm | 121 (56.3) |
| > 3 mm | 94 (43.7) |
| Pancreatic texture | |
| Hard | 112 (52.1) |
| Soft | 103 (47.9) |
| Postoperative complications | |
| Postoperative pancreatic fistula | 42 (19.5) |
| Grade A | 27 (12.6) |
| Grade B | 11 (5.1) |
| Grade C | 4 (1.9) |
| Operative time (min) | 308.4 ± 57.3 |
| Blood loss (mL) | 555.1 ± 228.7 |
| Peritoneal infection | 28 (13) |
| Intra-abdominal hemorrhage | 25 (11.6) |
| Biliary fistula | 20 (9.3) |
| Re-operation | 4 (1.9) |
| Mortality | 2 (0.9) |
| Length of stay (d) | 15.7 ± 2.7 |
As the most common postoperative complication, POPF can also increase the risks of abdominal infection and hemorrhage[6]. Consequently, we further explored possible factors correlated with POPF development through univariate analysis. As shown in Table 2, POPF development had no significant correlation with the following factors: Age, sex, smoking history, preoperative bilirubin and albumin, preoperative biliary drainage, previous abdominal surgery, blood loss, or operative time. Anastomotic techniques (P = 0.0015), MPD diameter (P = 0.0015) and pancreatic texture (P = 0.0386) were significantly correlated with POPF development in the multivariate logistic regression analysis.
| Univariate | Multivariate | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (≤ 60 vs > 60 yr) | 1.603 (0.800-3.203) | 0.188 | ||
| Gender (male vs female) | 1.143 (0.594-2.266) | 0.7 | ||
| Diabetes mellitus | 0.711 (0.324-1.585) | 0.427 | ||
| Smoking history | 1.275 (0.649-2.470) | 0.481 | ||
| History of abdominal operation | 0.782 (0.355-1.757) | 0.57 | ||
| Preoperative total bilirubin (≤ 171 vs > 171 µmol/L) | 1.295 (0.654-2.700) | 0.475 | ||
| Preoperative biliary drainage (yes vs no) | 1.444 (0.740-2.894) | 0.385 | ||
| Serum albumin (≤ 35 vs > 35 g/L) | 0.665 (0.322-1.359) | 0.275 | ||
| Anastomotic method (End-to-side invagination pancreaticojejunostomy vs Modified duct-to-mucosa pancreaticojejunostomy) | 3.045 (1.500-6.248) | 0.002a | 0.288 (0.129-0.606) | 0.0015a |
| Main pancreatic duct diameter (≤ 3 vs > 3 mm) | 2.599 (1.255-5.676) | 0.011a | 3.608 (1.678-8.302) | 0.0015a |
| Operative time (≤ 300 vs > 300 min) | 1.125 (0.583-2.181) | 0.735 | ||
| Blood loss (≤ 600 vs > 600 mL) | 0.855 (0.431-1.645) | 0.651 | ||
| Pancreatic texture (Hard vs Soft) | 0.494 (0.253-0.956) | 0.043a | 2.171 (1.051-4.602) | 0.0386a |
Table 3 shows the differences between traditional end-to-side invagination PJ and modified duct-to-mucosa PJ. Of these patients, 108 received traditional end-to-side invagination PJ, and 107 received modified duct-to-mucosa PJ. The results indicated no difference between the groups in terms of age, sex, pancreatic texture, postoperative hospital stay or mortality. However, patients subjected to modified duct-to-mucosa PJ had a lower incidence of POPF (11.2%) than the other group (27.8%). Further analysis indicated that there were 7 cases of grade A POPFs, 4 cases of grade B POPFs, and 1 case of grade C POPF in the modified PJ group. However, in the traditional group, the number of cases at each grade was 20, 7 and 3, respectively. Obviously, modified PJ might attenuate POPF severity based on the comparison results. Similarly, the modified anastomotic method demonstrated its superiority in terms of operative time (end-to-side invagination PJ: modified duct-to-mucosa PJ: 333.2 min vs. 283.4 min). Contrary to expectations, there were more patients with MPD diameters less than 3 mm in the modified method group, a factor that was previously found to be significantly correlated with POPF development.
| End-to-side invagination pancreatoduodenectomy (n = 108) | Modified duct-to-mucosa pancreatoduodenectomy (n = 107) | P value | |
| Age (yr) | 53.6 ± 13.7 | 55.3 ± 12.8 | 0.336 |
| Male | 50 (46.3) | 62 (57.9) | 0.088 |
| Pancreatic texture | 0.152 | ||
| Hard | 51 (47.2) | 61 (57.0) | |
| Soft | 57 (52.8) | 46 (43.0) | |
| Main pancreatic duct diameter | 0.033a | ||
| ≤ 3 mm | 53 (49.1) | 68 (63.6) | |
| > 3 mm | 55 (50.9) | 39 (36.4) | |
| Operative time (min) | 333.2 ± 48.9 | 283.4 ± 54.2 | < 0.0001a |
| Blood loss (mL) | 571.4 ± 257.3 | 538.7 ± 195.4 | 0.295 |
| Postoperative complications | |||
| Postoperative pancreatic fistula | 30 (27.8) | 12 (11.2) | 0.002a |
| Grade A | 20 (18.5) | 7 (15.9) | |
| Grade B | 7 (6.5) | 4 (3.7) | |
| Grade C | 3 (2.8) | 1 (0.9) | |
| Peritoneal infection | 13 (12.0) | 15 (13.9) | 0.38 |
| Intra-abdominal hemorrhage | 15 (13.9) | 10 (9.3) | 0.299 |
| Biliary fistula | 11 (10.2) | 9 (8.3) | 0.654 |
| Re-operation | 3 (2.8) | 1 (0.9) | 0.622 |
| Mortality | 2 (1.9) | 0 (0) | 0.498 |
| Length of stay (d) | 16 ± 2.6 | 15.5 ± 2.8 | 0.187 |
With the advancements in surgical techniques and perioperative care, the mortality of patients subjected to PD has gradually decreased, while the incidence of POPF remains high[7,8]. As the most frequent lethal complication, POPF has been heavily discussed to reach a consensus on its prevention. Our research preliminarily found that the independent risk factors for POPF included PJ method, MPD diameter and pancreatic texture. Our result was partially consistent with the result of a recent meta-analysis evaluating pancreatic texture and MPD size as risk factors for POPF development[9]. Other factors, including sex, body mass index, anastomotic techniques, intraoperative blood loss, operative time and drain fluid amylase, have also been reported to be related to POPF development[10-12]. Obviously, numerous studies on the risk factors for POPF have indicated seemingly conflicting and perplexing results. Ecker et al[13] believed that attempting to create a reliable prediction model based on the risk factors for POPF development seemed to be unrealistic and had limited effectiveness. Nevertheless, we believe that the abovementioned factors are valuable references that can help surgeons improve the therapeutic efficacy during the perioperative period.
In clinical practice, various surgical techniques have been applied to prevent POPF development, such as reconstruction methods [PJ, pancreaticogastrostomy (PG)], anastomotic techniques (Blumgart’s method[14], Kakita’s method[15], Peng’s binding PJ[16] and end-to-side invagination anastomosis) and stent placement. Debates about the pros and cons of the various surgical techniques are ongoing. A multicenter randomized trial conducted between June 2009 and August 2012 showed that PG was more efficient than PJ in reducing the incidence of POPF development[17]. Conversely, in another single-center, phase 3, randomized clinical trial, researchers recommended PJ for patients at high risk for POPF development[18]. In the present study, all the patients were subjected to PJ because surgeons were more skilled and experienced in performing this surgical technique. Two PJ anastomotic techniques were used here: end-to-side invagination anastomosis and modified duct-to-mucosa anastomosis. The operation time (283.4 minutes) and POPF (11.2%) incidence of the modified method group were significantly lower than those of the comparison group. Our results were roughly consistent with some other surgical center reports[19,20]. Classic invagination PJ can completely drain pancreatic juice from the main pancreatic duct and pancreatic stump into the intestinal cavity, but there are risks of pancreatic stump hemorrhage, pancreatic duct obstruction, and pancreatitis[16,21]. Many scholars have conducted comparative studies of various anastomosis methods. Wang et al[22] found no significant differences among duct-to-mucosa PJ, invagination PJ and binding PJ in the prevention of postoperative complications and death. While Ratnayake’s research favored duct-to-mucosa PG[23], Peng’s and Berger’s studies indicated that invagination could reduce the incidence of POPF development more significantly[16,21]. Compared with traditional duct-to-mucosa PJ, our technique used double-layer continuous suturing of posterior tissues and single-layer continuous suturing of anterior tissues, namely, “semicontact continuous anastomosis”. Our modified method had several advantages: first, the procedure better ensured the continuity between the pancreatic duct and the jejunal mucosa; second, tension-free and continuous anastomosis prevented cutting of the pancreas parenchyma; and third, convenient procedures helped reduce the difficulty of PD and the surgeon’s training time. With the popularity of laparoscopic and robotic PD, the advantages of our modified anastomotic approach might better meet the strict requirements of these operations. Although more high-quality evidence is required to demonstrate the benefits of modified duct-to-mucosa anastomosis, our present study indicated that it was a feasible and effective method for reducing the incidence of POPF development.
This study also has some limitations that might weaken the persuasiveness of the evidence. First, our study is a single-center retrospective study with a limited sample size. Second, the limited follow-up time may not accurately reflect the patient’s long-term clinical outcome. Therefore, large-scale randomized studies with long-term follow-up are desperately needed.
In conclusion, we found that anastomotic approaches, MPD diameter and pancreatic texture were major risk factors for POPF development. In addition, modified duct-to-mucosa PJ had advantages of shorter operation time and lower POPF incidence over classic end-to-side invagination PJ. Although the findings need to be further validated with more high-quality evidence, this modified method could be considered for some patients undergoing PD.
Pancreatoduodenectomy (PD) is widely used as an effective surgical treatment for pancreatic tumors, but there is currently no consensus on how to effectively prevent postoperative complications, especially pancreatic fistula. How to prevent postoperative pancreatic fistula (POPF) is a current research hotspot and our research focuses on how to solve this problem by improving surgical methods
To demonstrate the safety and feasibility of modified duct-to-mucosa pancreaticojejunostomy (PJ) during PD, especially in the terms of preventing POPF.
To identify independent risk factors for POPF and evaluate the clinical outcomes of two anastomotic techniques (end-to-side invagination PJ versus modified duct-to-mucosa PJ).
This stud was a retrospective cohort study which collected and analyzed the information of patients undergoing PD in our hospital. Univariate analysis and multivariate logistic regression analysis were used to analyze the risk factors of POPF and subgroup analysis were conducted to compare the different outcomes between end-to-side invagination PJ and modified duct-to-mucosa PJ.
Anastomotic approaches, main pancreatic duct (MPD) diameter and pancreatic texture were proven to be significantly associated with the incidence of POPF. And modified duct-to-mucosa PJ could significantly decrease the POPF incidence (11.2%) and operation time (283.4 min) in patients compared with traditional end-to-side invagination.
Modified duct-to-mucosa PJ had advantages of shorter operation time and lower POPF incidence over classic end-to-side invagination PJ. Additionally, we found that anastomotic approaches, MPD diameter and pancreatic texture were major risk factors for POPF development.
Modified duct-to-mucosa PJ is effective and safe according to preliminary outcomes. It is an innovative anastomotic technique with great application prospects in PD and also has broad application prospects in future robotic or minimally invasive operations of pancreatic tumors.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Isaji S, Japan; Shiryajev YN, Russia S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | Torphy RJ, Fujiwara Y, Schulick RD. Pancreatic cancer treatment: better, but a long way to go. Surg Today. 2020;50:1117-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 2. | Marchegiani G, Bassi C. Prevention, prediction, and mitigation of postoperative pancreatic fistula. Br J Surg. 2021;108:602-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Kawaida H, Kono H, Hosomura N, Amemiya H, Itakura J, Fujii H, Ichikawa D. Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J Gastroenterol. 2019;25:3722-3737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (3)] |
| 4. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2868] [Article Influence: 358.5] [Reference Citation Analysis (35)] |
| 5. | Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA, Salvia R, Pederzoli P. Duct-to-mucosa vs end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Gouillat C, Gigot JF. Pancreatic surgical complications--the case for prophylaxis. Gut. 2001;49 Suppl 4:iv32-iv39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Assifi MM, Lindenmeyer J, Leiby BE, Grunwald Z, Rosato EL, Kennedy EP, Yeo CJ, Berger AC. Surgical Apgar score predicts perioperative morbidity in patients undergoing pancreaticoduodenectomy at a high-volume center. J Gastrointest Surg. 2012;16:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Fu SJ, Shen SL, Li SQ, Hu WJ, Hua YP, Kuang M, Liang LJ, Peng BG. Risk factors and outcomes of postoperative pancreatic fistula after pancreatico-duodenectomy: an audit of 532 consecutive cases. BMC Surg. 2015;15:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Schuh F, Mihaljevic AL, Probst P, Trudeau MT, Müller PC, Marchegiani G, Besselink MG, Uzunoglu F, Izbicki JR, Falconi M, Castillo CF, Adham M, Z'graggen K, Friess H, Werner J, Weitz J, Strobel O, Hackert T, Radenkovic D, Kelemen D, Wolfgang C, Miao YI, Shrikhande SV, Lillemoe KD, Dervenis C, Bassi C, Neoptolemos JP, Diener MK, Vollmer CM Jr, Büchler MW. A Simple Classification of Pancreatic Duct Size and Texture Predicts Postoperative Pancreatic Fistula: A classification of the International Study Group of Pancreatic Surgery. Ann Surg. 2023;277:e597-e608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 114] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 10. | Hu BY, Wan T, Zhang WZ, Dong JH. Risk factors for postoperative pancreatic fistula: Analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. 2016;22:7797-7805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Yang J, Huang Q, Wang C. Postoperative drain amylase predicts pancreatic fistula in pancreatic surgery: A systematic review and meta-analysis. Int J Surg. 2015;22:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Peng YP, Zhu XL, Yin LD, Zhu Y, Wei JS, Wu JL, Miao Y. Risk factors of postoperative pancreatic fistula in patients after distal pancreatectomy: a systematic review and meta-analysis. Sci Rep. 2017;7:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Ecker BL, McMillan MT, Allegrini V, Bassi C, Beane JD, Beckman RM, Behrman SW, Dickson EJ, Callery MP, Christein JD, Drebin JA, Hollis RH, House MG, Jamieson NB, Javed AA, Kent TS, Kluger MD, Kowalsky SJ, Maggino L, Malleo G, Valero V 3rd, Velu LKP, Watkins AA, Wolfgang CL, Zureikat AH, Vollmer CM Jr. Risk Factors and Mitigation Strategies for Pancreatic Fistula After Distal Pancreatectomy: Analysis of 2026 Resections From the International, Multi-institutional Distal Pancreatectomy Study Group. Ann Surg. 2019;269:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 14. | Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg. 2010;210:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Fujii T, Sugimoto H, Yamada S, Kanda M, Suenaga M, Takami H, Hattori M, Inokawa Y, Nomoto S, Fujiwara M, Kodera Y. Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg. 2014;18:1108-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Peng SY, Wang JW, Lau WY, Cai XJ, Mou YP, Liu YB, Li JT. Conventional vs binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007;245:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Topal B, Fieuws S, Aerts R, Weerts J, Feryn T, Roeyen G, Bertrand C, Hubert C, Janssens M, Closset J; Belgian Section of Hepatobiliary and Pancreatic Surgery. Pancreaticojejunostomy vs pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol. 2013;14:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 18. | Andrianello S, Marchegiani G, Malleo G, Masini G, Balduzzi A, Paiella S, Esposito A, Landoni L, Casetti L, Tuveri M, Salvia R, Bassi C. Pancreaticojejunostomy With Externalized Stent vs Pancreaticogastrostomy With Externalized Stent for Patients With High-Risk Pancreatic Anastomosis: A Single-Center, Phase 3, Randomized Clinical Trial. JAMA Surg. 2020;155:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Zhu F, Shen M, Tian R, Shi CJ, Wang X, Jiang JX, Hu J, Wang M, Qin RY. Systematic review and meta-analysis comparing three techniques for pancreatic remnant closure following distal pancreatectomy. Br J Surg. 2015;102:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | El Nakeeb A, El Hemaly M, Askr W, Abd Ellatif M, Hamed H, Elghawalby A, Attia M, Abdallah T, Abd ElWahab M. Comparative study between duct to mucosa and invagination pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized study. Int J Surg. 2015;16:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, Hyslop T, Schmidt CM, Rosato EL, Lavu H, Nakeeb A, Pitt HA, Lillemoe KD, Yeo CJ. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. 2009;208:738-47; discussion 747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Wang W, Zhang Z, Gu C, Liu Q, Liang Z, He W, Chen J, Lai J. The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: A network meta-analysis of randomized control trials. Int J Surg. 2018;57:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Ratnayake CBB, Wells CI, Kamarajah SK, Loveday B, Sen G, French JJ, White S, Pandanaboyana S. Critical appraisal of the techniques of pancreatic anastomosis following pancreaticoduodenectomy: A network meta-analysis. Int J Surg. 2020;73:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |