Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1819
Peer-review started: March 11, 2023
First decision: May 16, 2023
Revised: June 5, 2023
Accepted: July 7, 2023
Article in press: July 7, 2023
Published online: August 27, 2023
Processing time: 167 Days and 10.3 Hours
Hepatocellular carcinoma (HCC) is a highly malignant cancer that often meta
We present an atypical clinical case of a patient with liver, right lung, peritoneal, and colon metastases diagnosed successively following hepatic resection for primary HCC. Comprehensive treatment, including partial liver, lung and colon resection, palliative management such as systemic chemotherapy, trans-arterial chemoembolization, targeted therapy with sorafenib, and cryotherapy were attempted. Despite his early metastases, the patient remained relatively healthy for 8 years after diagnosis.
This case indicates that comprehensive treatment is beneficial for certain patients with metastatic HCC. Clinicians should be alert as to the possibility of rare site metastatic tumors that may be easily misdiagnosed as primary tumors.
Core Tip: Hepatocellular carcinoma (HCC) is a highly malignant cancer worldwide, which often metastasizes, but unusually to the gastrointestinal tract and particularly rare to the colon. We presented an atypical clinical case of a patient with liver, right lung, peritoneal and colon metastases diagnosed successively following hepatic resection for primary HCC. Despite his early metastases, the patient remained relatively healthy for 8 years. This case indicates that comprehensive treatment is beneficial for certain patients with metastatic HCC. Furthermore, clinicians should be alert as to the possibility of rare site metastatic tumors that may be easily misdiagnosed as primary tumors. We believe this article will be very useful to anyone who is interested in this field.
- Citation: Gong YQ, Lu TL, Chen CW. Long-term survival of patients with hepatocellular carcinoma with hepatic, pulmonary, peritoneal and rare colon metastasis: A case report. World J Gastrointest Surg 2023; 15(8): 1819-1824
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1819.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1819
Hepatocellular carcinoma (HCC) is one of the most common malignant cancers, with a high metastatic and invasive potential and a low survival rate[1]. While patients with HCC often present with intrahepatic lung and bone metastasis, digestive tract metastases are rare[2,3]. There are many therapeutic approaches for treating HCC that can be widely classified by their ability to cure or control the tumor. Liver transplant, hepatic resection, and ablative therapies are performed with curative intent, while the majority of locoregional therapies, systemic chemotherapies and sorafenib are considered palliative. Although numerous treatment strategies for metastatic HCC have been evaluated, there has been no significant reduction in HCC mortality. Here we report a case of a long-term survival from primary HCC with early successive liver, lung, peritoneal, and colon metastases following comprehensive treatments.
A 47-year-old male was transferred to our hospital who had received comprehensive treatment for HCC for 4 years and was complaining of hematochezia for 20 d.
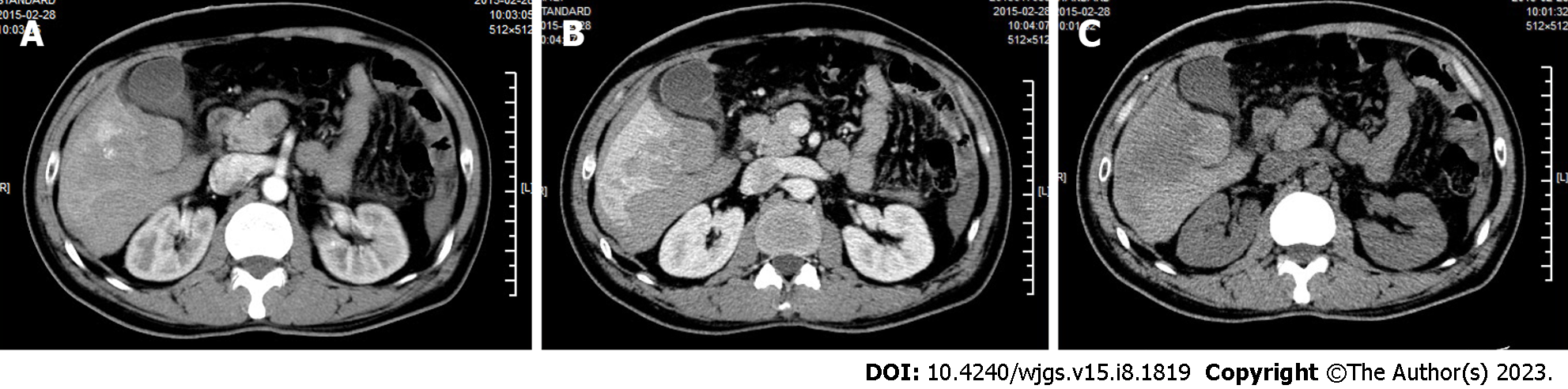
The patient was admitted to a local hospital in February 2015 for upper abdominal pain. Computer tomography (CT) revealed several circular low-density shadows with a maximum size of 4.1 cm × 4.5 cm in the right lobe of the liver (Figure 1). Liver cancer with rupture and hemorrhage was suspected, and no evidence of metachronous metastases were observed. A partial right hepatic lobe resection and cholecystectomy was performed on March 5, 2015. The patient also underwent abdominal and pelvic exploration as well as a priming wash. Operative pathology confirmed a moderately differentiated HCC.
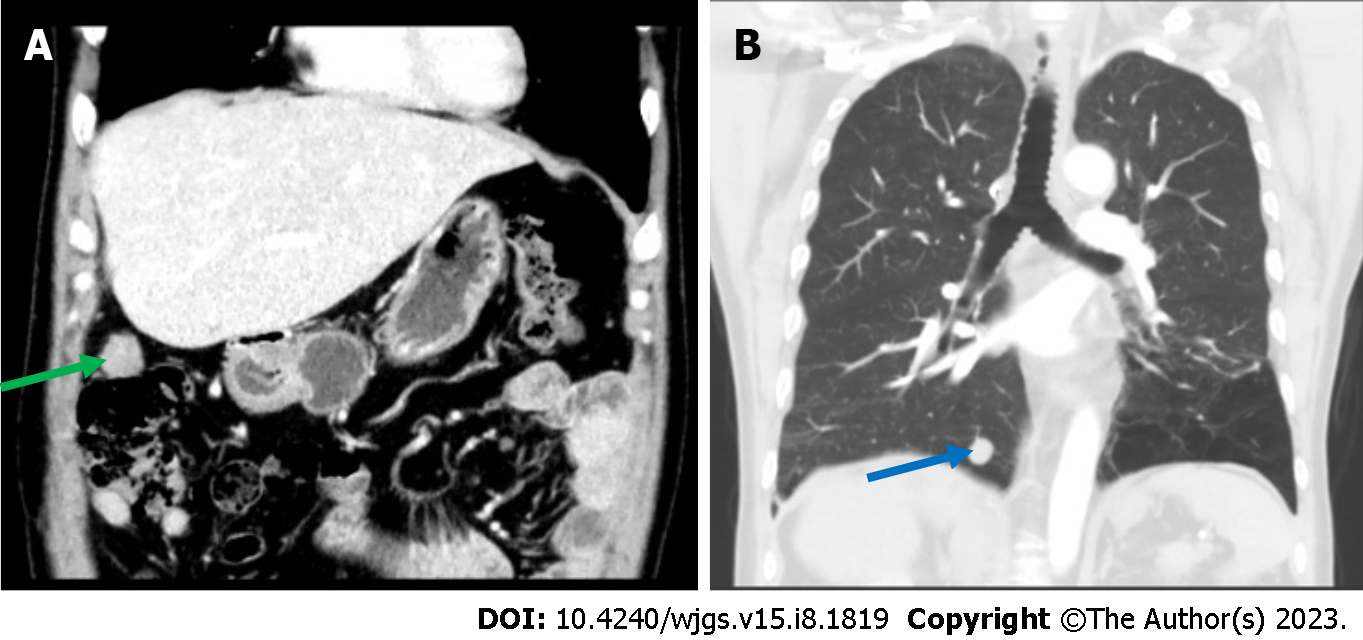
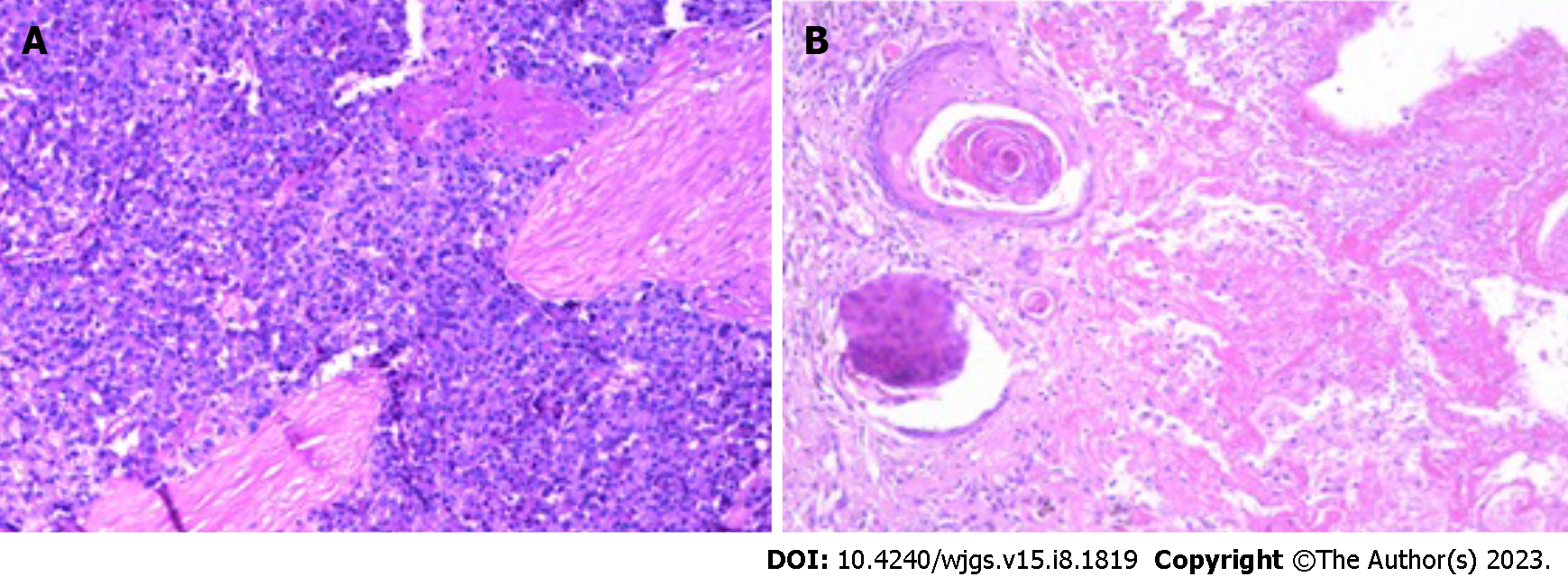
The patient was admitted to our hospital for regular follow up 1 mo later. CT showed a small hepatic nodule in his quadrate lobe, suggesting a possible metastasis. He underwent trans-arterial chemoembolization (TACE) for recurrent HCC in the liver on May 13, 2015. The patient recovered well, and no abnormalities were observed at until 1-year follow up in May 2016, when a CT scan found a metastatic tumor in the lower lobe of his right lung and a 2.5 cm × 2.0 cm mass in his peritoneal soft tissue (Figure 2). Based on the Barcelona Clinic Liver Cancer staging system, the patient was started on palliative sorafenib 0.4 g per os twice daily on May 17, 2016. The patient’s AFP level gradually increased from 2015 to 2017, peaking at 99 ng/ml. The multiple disciplinary team (MDT) suggested the patient undergo surgical resection of his peritoneal and pulmonary metastases. The peritoneal metastases were resected on January 23rd, 2017, and a partial lobectomy was performed on March 27, 2017. A postoperative biopsy confirmed pathologic change within the metastatic deposits (Figure 3). The patient’s postoperative AFP decreased to 20-30 ng/mL. His coagulation and liver function remained normal throughout treatment.
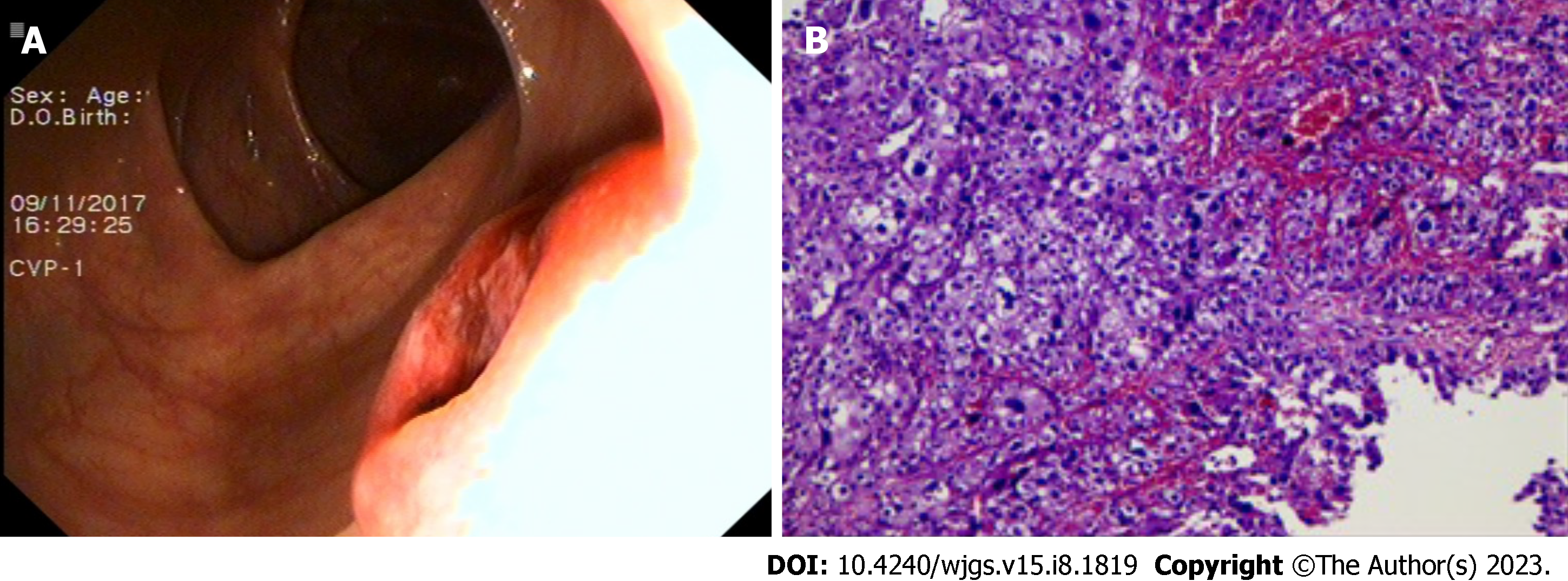
Seven months later, in November 2017, a CT scan observed a lesion in the patient’s ascending colon and multiple small flaky low-density nodules in his liver. The patient underwent a colonoscopy, and biopsy histology was consistent with metastatic HCC (Figure 4). Immunohistochemical results were Hepatocyte (+), Glypican-3 (+), CD34 (indicating vascularization), CDX2 (-), CK7 (-), and CK20 (-). Magnetic resonance imaging and an abdominal ultrasound suggested recurrent HCC. The patient consequently underwent TACE for multifocal HCC recurrence in his liver. Given concern for the patient’s overall condition, a MDT meeting was held and systemic treatment was recommended. The patient was admitted to an anti-programmed death 1 (PD-1) clinical trial in our hospital. The patient received SHR-1210 200mg, i.v. gtt on day 1+ and apatinib mesylate 250 mg, per os on days 1-14, a series that was repeated every 2 wk from July 10, 2018 to March 6, 2019. During this period, the patient’s AFP levels fluctuated between 25 and 100 ng/mL.
The patient complained of hematochezia for over 20 d. Considering the possibility of colon mass rupture and bleeding, the patient was withdrawn from anti-PD-1 immunotherapy until March 17, 2019.
Hepatitis B diagnosed in 2009.
The patient denied any special personal or family history.
A flat and soft abdomen with several visible surgical scars on the epigastrium.
Alpha-fetoprotein (AFP) level was 34 ng/mL (reference range 0-20 ng/mL).
A 2015 CT scan revealed multiple circular low-density shadows with a maximum size of 4.1 cm × 4.5 cm in the right lobe of the liver (Figure 1). A May 2016 CT scan showed a metastatic tumor in right lower lobe of lung (Figure 2A) and a 2.5 cm × 2.0 cm mass in the peritoneal soft tissue (Figure 2B). Operative pathology of the peritoneal mass (Figure 3A) and pulmonary lesion (Figure 3B) confirmed pathologic change in the metastatic HCC. In November 2017, a CT scan found a lesion in the ascending colon and multiple small flaky low-density nodules in the liver. A colonoscopy was performed to biopsy the mass in the ascending colon (Figure 4A), which confirmed metastatic HCC in the ascending colon (Figure 4B).
HCC with hepatic, pulmonary, peritoneal, and colon metastases.
The patient was withdrawn from anti-PD-1 immunotherapy on March 17, 2019, because of hematochezia, which was thought to be from a ruptured mass in his colon. A CT scan revealed increased size of the mass in the ascending colon wall. The MDT recommended a colectomy, and a radical resection of the right colon was performed on May 26, 2019, with pathology consistent with metastatic HCC. The patient recovered well postoperatively, and no abnormalities or metastases were observed over the proceeding 4-years of follow-up.
After comprehensive treatment, including partial surgical resection of his liver, lung, and colon, and palliative management such as systemic chemotherapy, TACE, targeted therapy with sorafenib, and cryotherapy, the patient is still alive and relatively healthy 8 years after being diagnosed with HCC.
HCC, a major subtype of primary liver cancer, is the third most common cause of cancer-related death worldwide, leading to over 600000 deaths annually[4]. While a significant amount of research has been performed into possible HCC treatments, it still carries an extremely dismal prognosis due to its late diagnosis and its high risk of recurrence and drug resistance. HCC always metastasizes via intrahepatic blood vessels, direct infiltration, or the lymphatic system, and thus typically affecting the liver, lung, bone, lymphatics and brain[5]. Metastases to the digestive tract, in particular the colon are very rare. The patient underwent abdominal and pelvic exploration and irrigation for liver cancer with rupture and hemorrhage. It is worth considering whether the colon metastases occurred via normal intestinal metastasis or abdominal implantation. We do not rule out the risk of intraperitoneal implantation, but consider the possibility of metastasis through the normal intestinal pathway to be more likely. First, the patient had successive liver, lung, and peritoneal metastases, with intestinal metastases occurring 2 years later. If the colonic metastases were due to peritoneal implantation, they would have occurred almost simultaneously with the abdominal metastasis. Further, the colonic metastases grew within the intestinal cavity rather than infiltrating it, as shown on imaging and colonoscopy. It is hard to distinguish metastatic carcinoma of the colon from primary colon cancer because the metastases have few specific clinical manifestations. Clinicians should therefore be vigilant to the possibility of rare metastatic tumor sites, such as the colon in the case of our patient, which may avoid misdiagnosis or delayed treatment.
There are many treatments for HCC, which include liver transplantation, surgical resection, locoregional therapy (e.g., TACE), and systemic therapy (e.g., multikinase inhibitor sorafenib). Among the therapies mentioned above, liver transplantation and surgical resection remain the gold standard curative treatments for HCC. Locoregional and systemic therapies are usually considered controlling but not curative, or a means for decreasing tumor size or bridging the patient to surgery. It has been reported that patients treated with TACE have a considerably longer overall survival than the best supportive care in a randomized controlled study[6]. For patients with advanced HCC who are not surgical candidates or who have failed locoregional therapy, the multikinase inhibitor sorafenib may be considered[7]. An expanding body of evidence suggests that cytoreductive surgery can prolong the survival of patients with various metastatic malignancies and improve their quality of life[8,9]. Thus, despite their poor outcomes, the comprehensive treatment of patients with HCC and hepatic or extrahepatic metastases may improve their prognosis[10,11]. The comprehensive use of cytoreductive surgery, regional chemotherapy and other interventions contribute to lowering cancer burden, alleviating the symptoms and improving the quality of life of patients with metastatic HCC. However, patients with advanced HCC require a comprehensive assessment of multiple indicators. A specific preoperative assessment is therefore performed to identify appropriate treatment decisions. The postoperative pathological biopsy of this patient confirmed a moderately differentiated HCC. However, he developed liver, lung, peritoneum, and colon metastases at an early stage, classifying his diagnosis as advanced malignant HCC. According to the National Cancer Institute’s SEER database, the average five-year survival rate of HCC patients in the United States is 19.6%, but can be as low as 2.5% for those with advanced metastatic disease. Although this patient had a moderately differentiated HCC, an 8-year effective survival period should still be considered a longer than expected survival.
There are several deficiencies and omissions in this case. The absence of a well-developed PET-CT to most objectively identify the patient’s systemic lesions and metastases is a major weakness of this work. Further, it is a great pity that postoperative adjuvant therapy was administered earlier to this patient despite his high risk of recurrence.
In conclusion, this case describes a patient with atypical HCC with multiple extrahepatic metastases who survived for 8 years following comprehensive treatment. The primary HCC and metastatic tumors in his liver, lung, peritoneum, and colon were surgically removed. This may indicate that reducing the tumor burden may delay disease progression, thus improving quality of life. This report highlights the role of comprehensive treatment for certain patients with advanced stage HCC. It also supports the early recognition of rare metastatic sites and can provide instruction for the treatment of HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dias E, Portugal; Sun J, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX, Finn RS. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 1006] [Article Influence: 335.3] [Reference Citation Analysis (2)] |
| 2. | Wu W, He X, Andayani D, Yang L, Ye J, Li Y, Chen Y, Li L. Pattern of distant extrahepatic metastases in primary liver cancer: a SEER based study. J Cancer. 2017;8:2312-2318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Hu C, Yang J, Huang Z, Liu C, Lin Y, Tong Y, Fan Z, Chen B, Wang C, Zhao CL. Diagnostic and prognostic nomograms for bone metastasis in hepatocellular carcinoma. BMC Cancer. 2020;20:494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12986] [Article Influence: 1442.9] [Reference Citation Analysis (2)] |
| 5. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13205] [Article Influence: 1467.2] [Reference Citation Analysis (3)] |
| 6. | Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, Zhuang W, Chen X, Chen H, Xu B, Lai J, Guo W. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2022;148:2115-2125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 7. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4092] [Article Influence: 584.6] [Reference Citation Analysis (6)] |
| 8. | Grotz TE, Fournier KF, Mansfield PF. Patient Selection for Cytoreductive Surgery. Surg Oncol Clin N Am. 2018;27:443-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Alzahrani N, Ung L, Valle SJ, Liauw W, Morris DL. Synchronous liver resection with cytoreductive surgery for the treatment of liver and peritoneal metastases from colon cancer: results from an Australian centre. ANZ J Surg. 2017;87:E167-E172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3852] [Article Influence: 963.0] [Reference Citation Analysis (3)] |
| 11. | Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |