Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1799
Peer-review started: April 13, 2023
First decision: May 19, 2023
Revised: May 23, 2023
Accepted: June 11, 2023
Article in press: June 11, 2023
Published online: August 27, 2023
Processing time: 133 Days and 12.4 Hours
Post-operative pancreatic fistula (POPF) is the primary cause of morbidity following pancreaticoduodenectomy. Rates of POPF have remained high despite well known risk factors. The theory that hypoperfusion of the pancreatic stump leads to anastomotic failure has recently gained interest.
To define the published literature with regards to intraoperative pancreas perfusion assessment and its correlation with POPF.
A systematic search of available literature was performed in November 2022. Data extracted included study characteristics, method of assessment of pancreas stump perfusion, POPF and other post-pancreatic surgery specific complications.
Five eligible studies comprised two prospective non-randomised studies and three case reports, total 156 patients. Four studies used indocyanine green fluo
The current published evidence around pancreas perfusion during pancreaticoduodenectomy is of poor quality. It does not support a causative link between hypoperfusion and POPF. Further well-designed prospective studies are required to investigate this.
Core Tip: The pathology of post-op pancreatic fistula remains to be elucidated, however, hypoperfusion of the pancreatic remanent is a suggested mechanism leading to post-operative pancreatitis and failure of the pancreatic jejunal anastomosis. Indocyanine green assessment of the pancreatic remanent is a safe way to visualise perfusion of the stump prior to anastomosis. Whether it can predict post-operative pancreatic fistula requires further studies.
- Citation: Robertson FP, Spiers HVM, Lim WB, Loveday B, Roberts K, Pandanaboyana S. Intraoperative pancreas stump perfusion assessment during pancreaticoduodenectomy: A systematic scoping review. World J Gastrointest Surg 2023; 15(8): 1799-1807
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1799.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1799
Pancreatic resection is a key component in the treatment pathways of various benign and malignant diseases[1]. The postoperative morbidity after pancreatic resection remains high despite centralisation[2,3] and improved surgical techniques[4]. Post-operative pancreatic fistula (POPF), serves as the key risk factor for other post-operative intra-abdominal complications such as delayed gastric emptying (DGE) and intraabdominal collections[5-7], thereby prolonging hospital stay and increasing over all morbidity[8-11]. Furthermore, POPF is the root cause of mortality after pancreaticoduodenectomy[12,13]. Risk factors for POPF are very well defined[14]. Despite numerous trials aimed at reducing the incidence of POPF, its incidence has remained largely unchanged over decades[15]. This is likely due to poor understanding of the underlying pathophysiology, with failure of current interventions to reduce POPF suggesting something more than a mere loss of mechanical anastomotic integrity. One theory is that hypoperfusion of the pancreatic remnant results in pancreas transection margin ischaemia, necrosis and post pancreatectomy pancreatitis with subsequent failure of healing at the pancreatico-enteric anastomosis and resultant pancreatic leak[16-18]. The neck of the pancreas is a watershed area between coeliac and superior mesenteric arterial systems, hence, hypoperfusion in this area may be associated with poor healing and risk of anastomotic leak[15].
There is some evidence from the literature on intraoperative pancreas perfusion assessment and there is a need to identify and map the current evidence for its use in the context of POPF. This will allow identification of techniques used and provide insight into their effectiveness, paving the way for further prospective and randomised studies. Therefore, this scoping review aims to define the current experience with intraoperative pancreas perfusion assessment and highlight the published literature surrounding pancreas stump hypoperfusion as a potential risk factor for POPF.
This scoping review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses Scoping Reviews extension[19]. The study protocol was prospectively registered with the University of York Centre for Review and Dissemination international prospective register of systematic reviews PROSPERO database (2021: CRD42021296863).
Following pilot testing, a systematic search of Medline and EMBASE databases was conducted on 8th November 2022, with screening performed by two independent investigators (HS and FR). The search strategy was conducted using the following search algorithm: ((pancreatic fistula.ti.ab) OR (exp pancreatic fistula) OR (anastomotic leak.ti.ab) OR (exp anastomotic leak)) AND ((pancreatoduodenectomy.ti.ab) OR (exp pancreatoduodenectomy) OR (pancreaticoduodenectomy.ti.ab) OR (exp pancreatoduodenectomy) OR (Whipple’s surgery.ti.ab)) AND ((perfusion.ti.ab) OR (exp perfusion) OR (blood supply.ti.ab) OR (exp blood supply). All studies including patients undergoing pancreaticoduodenectomy were included. Full search strategy and results are presented in Supplementary Table 1. Titles identified following this literature search were entered into the Reference Citation Analysis (RCA) (Baishideng Publishing Group) to search for further studies related to these articles.
Full text studies reporting patients undergoing intraoperative pancreas perfusion assessment, or correlating hypoperfusion with POPF were included regardless of language. Any type of publication reporting primary data on the topic was included. Review articles and studies not reporting primary data were excluded. After excluding duplicates, two researchers (Robertson FP and Spiers HVM) independently reviewed the titles and abstracts of studies identified by the literature search. Where a study was considered relevant to the research question a full copy of the publication was obtained for further review. The reference lists of included articles were hand-searched for any further relevant studies. Any areas of disagreement between the two primary researchers were resolved through discussion with the senior author (Pandanaboyana S).
Data were retrieved from published studies and extraction was performed by an individual author (Spiers HVM) and independently checked by a second author (Robertson FP), with any disagreement resolved by consensus or where necessary with a senior author (Pandanaboyana S). The post-operative outcomes chosen to explore were the development and grade of POPF as defined by the International Study Group of Pancreatic Surgery (ISGPS)[20], the incidence of post-pancreatectomy haemorrhage, grade of DGE as defined by the ISGPS[21,22] and mortality. Study quality was not formally assessed as this is a scoping review.
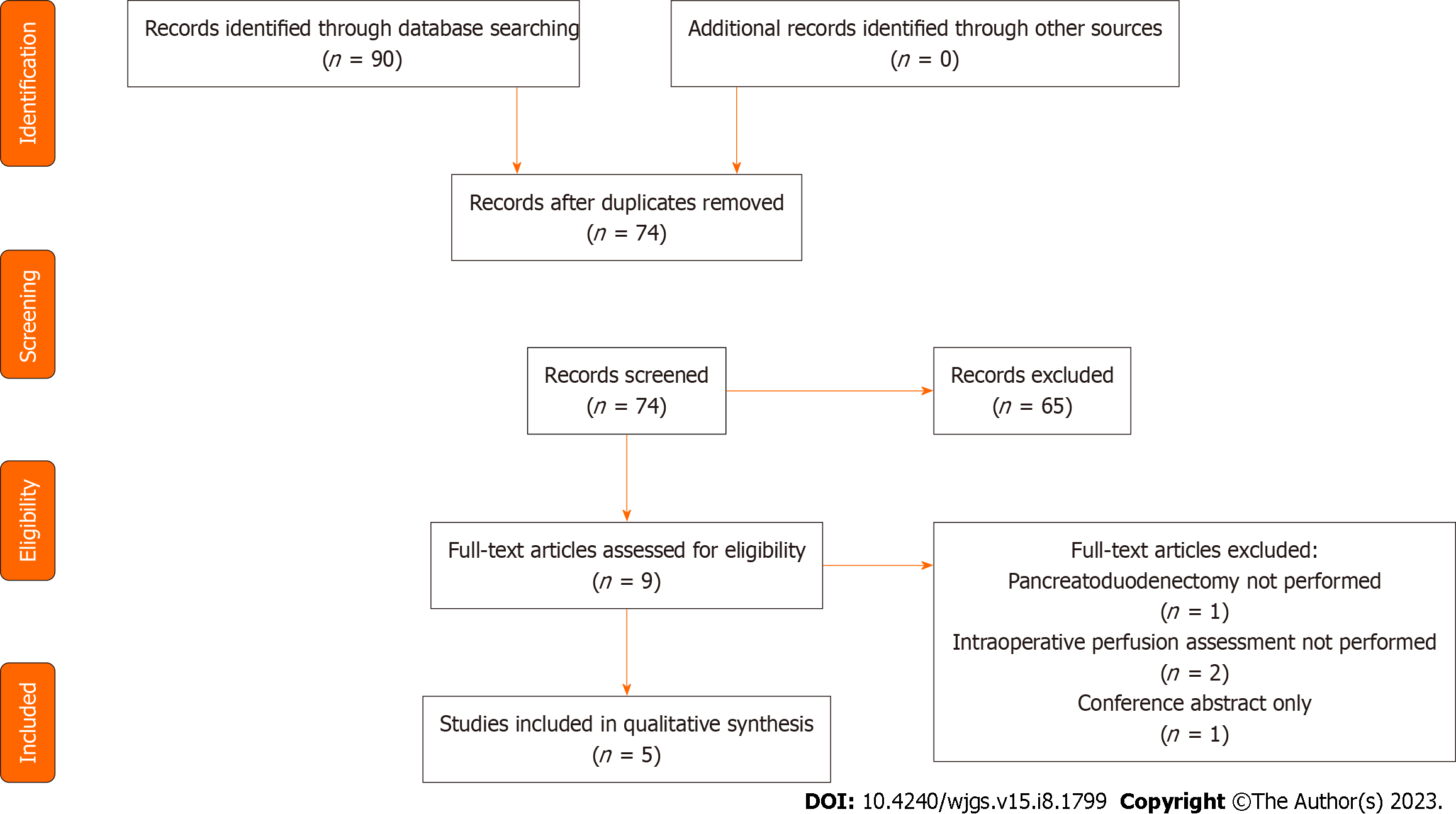
Following the initial search (Figure 1), 90 studies were identified of which 74 remained following removal of duplicates. The 74 titles and abstracts were reviewed and 9 studies were selected for full review. Studies were excluded when not relevant, or non-primary literature. Of the 9 studies reviewed in detail, 4 were excluded because of operative procedure performed not being pancreaticoduodenectomy, conference abstract only or pancreas perfusion assessment not performed intraoperatively. Five studies including 156 patients were included in the final review. The median number of patients included in each study was 30 (1-123). Characteristics of the included studies are shown in Table 1.
| Ref. | Country of origin | Study design | Study population | Disease process |
| Strasberg et al[25], 2002 | United States | Prospective cohort | Patients undergoing pancreatoduodenectomy with pancreatojejunostomy at a single institution 1996 to 2000 | Malignant (n = 107): Pancreatic cancer (n = 48); ampullary cancer (n = 28); NET (n = 6); villous adenoma (n = 6); MCN (n = 6); bile duct cancer (n = 5); duodenal adenocarcinoma (n = 3); serous cystadenoma (n = 3); IPMN (n = 1); GIST (n = 1). Benign (chronic pancreatitis, n = 16) |
| Subar et al[24], 2015 | France | Case report | One patient undergoing laparoscopic pancreatoduodenectomy with pancreatojejunostomy | Malignant (ampullary adenocarcinoma) |
| Rho et al[23], 2019 | Korea | Case report | One patient undergoing laparoscopic pancreatoduodenectomy with pancreatojejunostomy | Malignant (distal cholangiocarcinoma) |
| Doussot et al[27], 2021 | France | Prospective cohort | Consecutive patients undergoing open pancreatoduodenectomy with pancreatojejunostomy at a single institution from January 2020 to November 2020 | Malignant (n = 30, periampullary malignancies) |
| Iguchi et al[26], 2021 | Japan | Case report | One patient undergoing open middle segment-preserving pancreatectomy with pancreatojejunostomy | Malignant (pancreatic ductal adenocarcinoma in head of pancreas with IPMN in the tail of pancreas) |
Studies were published between 2002 and 2021 and included three case reports of a single patient and two prospective non-randomised cohort studies. One hundred and fifty-six patients underwent pancreaticoduodenectomy, two of which were laparoscopic, the rest open. One hundred and forty patients (90%) underwent resection for malignancy and 16 (10%) underwent resection for chronic pancreatitis. Three studies included only patients undergoing pancreaticoduodenectomy with no vascular resection or resection of other organs[23-25]. One study included a patient undergoing open pancreaticoduodenectomy combined with distal pancreatectomy and splenectomy (middle segment-preserving pancreatectomy)[26]. One study included 10 patients undergoing vascular resection[27], 3 patients undergoing simultaneous vascular and arterial resection and 3 patients undergoing synchronous resection of other organs [partial splenectomy (n = 1), partial nephrectomy (n = 1), minor hepatectomy (n = 2)].
In 4 studies pancreas perfusion was measured by intravenous injection of indocyanine green (ICG) into a peripheral vein allowing intra-operative fluorescence angiography under near infrared light[23,24,26,27] (Table 2). Adequate pancreatic perfusion was classified by Doussot et al[27] as homogonous perfusion of the pancreatic stump. Time to achieve this was also measured and divided into 3 groups (< 30 s, 30-60 s and > 60 s). One study assessed pancreas perfusion by visual inspection of arterial bleeding of the pancreatic stump following transection of the pancreatic neck[25]. Perfusion was classified as adequate when brisk arterial pulsatile bleeding was visualised superiorly and inferiorly to the pancreatic duct that required sutures to control the bleeding.
| Ref. | Measurement of hypoperfusion | Description of technique | Total number of patients | Number with hypoperfusion | Management of hypoperfusion |
| Strasberg et al[25] | Visual assessment by surgeon | Blood supply was considered adequate when pulsatile arterial bleeding was present both superior and inferior to the pancreatic duct on the cut surface of the pancreas. The bleeding was required to be brisk (of a level that required sutures to stop the bleeding). If there was no bleeding, or if the bleeding points were of an oozing type that could be controlled without sutures, the blood supply was considered inadequate | 123 | 47 | Further 1.5-2 cm of pancreas transected |
| Subar et al[24] | ICG | Peripheral injection of 2 mL (0.5 mg) of Infracyanine™ (concentration was 0.25 mg/mL). The infrared camera is then focused on the transected margin of the pancreas | 1 | 1 | Ischaemic segment resected further |
| Rho et al[23] | ICG | ICG in jVR 25.0 mg (Doingin-dang Pharmaceutical Company, Siheung, Gyeonggi, Republic of Korea) given via peripheral IV injection at least three minutes before confirmation of pancreatic perfusion. Waited at least 30 s to determine perfusion with IMAGE1 STM H3-LINK and D-LIGHT P system (KARL STORZ SE & Co.KR, Tuttlingen, Germany) | 1 | 1 | Reinforcement using surgical glue (Greenplast QVR 2 mL, GREEN CROSS Corp., Yongin, Gyeonggi, Republic of Korea) |
| Doussot et al[27] | ICG | Pancreas stump was inspected after ICG IV injection (INFRACYANINE 0.1 mg/kg; Serb, Paris, France) using a microscope with near-infrared light source allowing real-time ICG perfusion assessment with near-infrared light images | 30 | 6 | One patient had further 3 cm pancreatic stump resection |
| Iguchi et al[26] | ICG | 10 mg of ICG was administered IV. The presence of fluorescence in the pancreatic remnant was definitively confirmed with a fluorescence camera | 1 | 0 | NA |
When pancreatic hypoperfusion was identified in the study by Strasberg et al[25], the pancreatic margin was cut back further by 1.5-2.0 cm until improved perfusion was visualised. Similarly, when hypoperfusion was identified in the study by Subar et al[24], further cut back of the margin was performed. In the study by Doussot et al[27], the pancreatic stump was only further cut back in one patient. The results of pancreatic perfusion in the case study by Rho et al[23] did not alter the operative strategy. Prophylactic octreotide was administered variably throughout the studies.
Perfusion of the pancreatic stump was assessed successfully in all patients recruited to the studies. Perfusion of the pancreatic stump was assessed by ICG angiography in 33 patients and visual inspection of bleeding from the cut surface in 123 patients. Hypoperfusion of the pancreatic stump was identified in 55 (35%) of patients (Subar et al[24] n = 1, Doussot et al[27] n = 6, Rho et al[23] n = 1, Strasberg et al[25] n = 47). Of these patients 49 (89%) underwent further cutback of the pancreatic stump prior to formation of the pancreatico-jejunostomy. Two patients in the study of Strasberg et al[25] who had their stump cut back were found to still exhibit signs of hypoperfusion within their criteria.
The definition of POPF was heterogeneously defined between the studies. Strasberg et al[25], which was published in 2002 prior to the ISGPS publication of the consensus definition of POPF in 2016, defined POPF as drainage of > 50 mL of pancreatic fluid (> 500 IU/L) for 3 consecutive days as long as it included post-operative day 10. There was no subclassification of clinically relevant POPF vs biochemical leak in the study by Strasberg et al[25]. All other studies defined POPF according to the ISPG classification. DGE was defined in the study by Rho et al[23] according to the ISGPS definition. Neither Doussot et al[27] nor Strasberg et al[25] clarified their definition of DGE. Post-pancreatectomy haemorrhage was measured in the study by Doussot et al[27] and was classified according to the ISGPS definition.
POPF occurred in 18 (12%) of patients (Table 3). No analysis of clinically relevant POPF has been performed as this was not defined in the study by Strasberg et al[25] which contributes the majority of patients to the review. In the study by Doussot et al[27], 2 of the 3 (67%) clinically relevant POPF occurred in patients with documented hypoperfusion of the pancreatic stump. Rho et al[23] identified hypoperfusion in their patient who developed a clinically relevant POPF. In the study by Strasberg et al[25], all POPFs were identified in patients with hypoperfusion of the pancreatic stump, however, this should be interpreted with caution as all patients identified as having hypoperfusion underwent further resection of the pancreas prior to anastomosis. In the study by Iguchi et al[26], no hypoperfusion of the pancreatic stump was identified however they identified a leak from the distal end of the stump following distal pancreatectomy.
| Ref. | Total number developing POPF | Number hypoperfused developing POPF | Delayed gastric emptying | Post-pancreatectomy haemorrhage | 90-d mortality |
| Strasberg et al[25] | 4 | 2 (1.6) | 11 | 0 | 1 |
| Subar et al[24] | 0 | 0 (0) | 0 | 0 | NR |
| Rho et al[23] | 1 | 1 grade A | 1 grade B | 0 | NR |
| Doussot et al[27] | 12 (9 grade A and 3 grade B) | 3 (1 grade A and 2 grade B) | 5 (17) | 2 (1 grade B and 1 grade C) | 0 |
| Iguchi et al[26] | 1 grade B | 1 grade B | 0 | 0 | Alive at 2 mo |
DGE was seen in 17 (11%) patients (Table 3). The incidence of DGE in patients with and without hypoperfusion of the pancreatic stump was not provided in either of the studies by Doussot et al[27] or Strasberg et al[25]. The only patient in the case series by Rho et al[23] experienced grade B DGE. They were found to have hypoperfusion of the pancreatic stump.
Post pancreatectomy haemorrhage (PPH) was seen in 2 (1%) patients (Table 3). The incidence of PPH in patients with and without hypoperfusion of the pancreatic stump was not provided.
Post-operative mortality was seen in 1 (0.6%) patient. It was not documented whether they had hypoperfusion of the pancreatic stump.
This scoping review mapped studies that assessed hypoperfusion of the pancreatic remnant during pancreaticoduodenectomy and its relationship with POPF. The five primary studies, including two prospective non-randomised studies and three case reports, identified utilisation of intraoperative assessment of perfusion using a range of techniques and variable resultant change in surgical management of the pancreatic remnant after confirmation of hypoperfusion. There was significant heterogeneity in the definition of POPF as the largest study in this series was published prior to the publication of the ISPGS definition. Our findings illustrate the safety and feasibility of intraoperative pancreas perfusion assessment and highlight its apparent limited usage since the first report twenty years ago. Variation in practice related to some patients having their pancreas remnant trimmed short if deemed to be hypoperfused whilst other patients were not.
The studies identified in this review have a combined POPF rate of 12%. This is lower than the published rate of 20% for clinically relevant POPF in recent randomised controlled trials comparing laparoscopic and open pancreaticoduodenectomies[28]. This is likely related to the Strasberg et al[25] study which used a different definition for POPF as it was published pre ISPGS. Intraoperative pancreas perfusion assessment revealed that hypoperfusion was present in 39% of patients who developed POPF. The rate of POPF was 11% in patients with no evidence of hypoperfusion and 13% in those with evidence of hypoperfusion, suggesting that not all hypoperfusion gives rise to POPF and further analysis is required to analyse if there is a clinically relevant cut off. From this review, conclusive incidence of PPH and DGE in patients with hypoperfusion of pancreatic stump is not possible given the limited reporting of these complications. Non-invasive perfusion assessment modalities such as infra-red spectroscopy have been investigated in other surgical specialties and have been shown to accurately identify hypoperfusion, but their role in pancreatic surgery is yet to be investigated[29,30].
The link between hypoperfusion of the pancreatic stump and POPF remains inconclusive. In the large 123 patient study by Strasberg et al[25] perfusion was assessed intraoperatively by subjective assessment of bleeding from the pancreatic stump with further resection in those deemed to be hypo-perfused. It demonstrated that 50% of clinically relevant POPFs occurred in patients with hypoperfusion that underwent further resection of the pancreas to well perfused tissue. This suggests that perfusion status of the stump intraoperatively, i.e., local perfusion, may only be one component of the pathophysiology. Hyperlactataemia, a well-recognised hallmark of inadequate tissue perfusion and microcirculatory abnormalities[31], in the early post-operative period has been shown to be predictive of POPF. Hyperlactataemic patients (blood lactate ≥ 2.5 mmol/L) being 4.36 (1.70-11.15; P = 0.002) and 3.58 (1.22-10.48; P = 0.02) times as likely to develop POPF on uni- and multivariate analyses respectively[32].
Whilst several risk factors for POPF have been identified including consistency of the pancreatic parenchyma, size of the pancreatic duct and blood loss and have been combined to create the validated tool to predict POPF - the pancreatic fistula risk score[33], no study included this data and compared between the groups.
Post-operative acute pancreatitis (POAP) of the pancreatic remnant is an emerging entity in pancreas surgery[16], with inadequate tissue perfusion being key to its pathogenesis[34]. POAP exacerbates existing hypoperfusion, which may be why patients with higher vessel density (mm2) at histology show reduced POPF[35,36]. Intraoperative perfusion assessment may allow surgical optimisation of the pancreatic remnant, reducing the incidence and severity of POAP, in turn reducing POPF. Additionally, overall haemodynamic and perfusion status may contribute to local changes causing hypoperfusion, POAP and subsequent POPF, particularly in patients who are high-risk for POPF. Therefore, intraoperative perfusion assessment, coupled with goal directed therapy may improve pancreas perfusion and improve outcomes.
The use of ICG imaging for perfusion assessment is well established in gastrointestinal surgery, specifically in assessing tissues prior to anastomosis[37], yet it is not widely used in pancreas surgery. Four studies identified in this review demonstrated the feasibility of ICG usage to assess the pancreatic remnant, with an advantage over subjective visual assessment being clear identification and demarcation of hypoperfusion confirmed by a lack of fluorescence over affected areas. Moving forward, it is essential to determine the optimum dosage of ICG, timing of its measurement and distance the camera should be held from the anastomosis at time of imaging, to allow widespread reproducible use of this technique in pancreatic surgery. An objective scoring system would also need to be developed to allow reproducible results.
The findings of this review must be set in the context of its limitations. Firstly, data is only available from a small number of publications and it is plausible that the total number of patients who have had intraoperative perfusion assessment is much higher. Secondly, there is a likely publication bias, with only select centres who have experience with ICG and perfusion assessment publishing their results. Thirdly, there may be confirmation bias in those studies using subjective visualisation methods of perfusion assessment. Finally, current methods to assess perfusion of the stump only allow for assessment of the surface perfusion and not the deep tissues. However as robotic surgery develops further advances may allow for more detailed perfusion assessments using the firefly mode.
In conclusion, intraoperative perfusion assessment is technically feasible and appears safe. The quality of the current published literature is poor with the majority of publications included being either case reports or limited case series. The largest study was published prior to the publication of the ISPGS definition of POPF and clinically relevant POPF and their definition of POPF differed from the current accepted definition. There is insufficient evidence currently to evaluate whether poor perfusion of the pancreatic stump during pancreatico-duodenectomy is associated with an increased incidence of POPF. Moving forward further prospective studies are required to confirm the external validity of the studies identified in this review, ideally with creation of objective scoring systems allowing standardisation and improved analysis of data in future. Importantly, identifying the degree of hypoperfusion that is associated with, or predictive of, POPF and how this is best managed is a key priority.
Despite centralization of pancreatic surgery, post-operative pancreatic fistula (POPF) rates remain high. The pathogenesis of the development of POPF remains poorly understood but there is some evidence to support poor perfusion of the pancreatic remanent in the development of this complications.
This research project was designed to identify the current published literature regarding the use of intra-operative perfusion assessment to help guide whether this can be incorporated into clinical use.
The aim of this study was to review the current evidence for assessment of perfusion of the pancreatic remanent prior to anastomosis in patients undergoing pancreatoduodenectomy.
The medical literature was searched for studies assessing the perfusion of the pancreatic remanent intra-operatively. Studies were identified and data was extracted by 2 independent authors. A meta-analysis could not be performed and therefore a systematic scoping review was carried out.
The POPF rate in all studies was 12%. Intraoperative perfusion assessment revealed hypoperfusion was present in 39% of patients who developed POPF. The rate of POPF was 11% in patients with no evidence of hypoperfusion and 13% in those with evidence of hypoperfusion.
This study has shown that indocyanine green can safely assess pancreatic perfusion intraoperatively. There was insufficient evidence to link poor perfusion of the pancreatic remanent with POPF and further well designed studies are required.
The results of this study have not changed our clinical practice but ha highlights further areas of clinical research to make pancreatic surgery safer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Sperti C, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Ju JL
| 1. | Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, Gullerud RE, Donohue JH, Nagorney DM, Farnell MB. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 377] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 2. | de Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH, Porte RJ, Gouma DJ, Busch OR, Molenaar IQ; Dutch Pancreatic Cancer Group. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 3. | Nymo LS, Kleive D, Waardal K, Bringeland EA, Søreide JA, Labori KJ, Mortensen KE, Søreide K, Lassen K. Centralizing a national pancreatoduodenectomy service: striking the right balance. BJS Open. 2020;4:904-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Søreide JA, Sandvik OM, Søreide K. Improving pancreas surgery over time: Performance factors related to transition of care and patient volume. Int J Surg. 2016;32:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Chen JY, Feng J, Wang XQ, Cai SW, Dong JH, Chen YL. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol. 2015;21:5926-5933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Molinari E, Bassi C, Salvia R, Butturini G, Crippa S, Talamini G, Falconi M, Pederzoli P. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg. 2007;246:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Roberts KJ, Hodson J, Mehrzad H, Marudanayagam R, Sutcliffe RP, Muiesan P, Isaac J, Bramhall SR, Mirza DF. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB (Oxford). 2014;16:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Fu SJ, Shen SL, Li SQ, Hu WJ, Hua YP, Kuang M, Liang LJ, Peng BG. Risk factors and outcomes of postoperative pancreatic fistula after pancreatico-duodenectomy: an audit of 532 consecutive cases. BMC Surg. 2015;15:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | van der Gaag NA, Harmsen K, Eshuis WJ, Busch ORC, van Gulik TM, Gouma DJ. Pancreatoduodenectomy associated complications influence cancer recurrence and time interval to death. Eur J Surg Oncol. 2014;40:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): A systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore). 2017;96:e6858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 11. | Dhayat SA, Tamim ANJ, Jacob M, Ebeling G, Kerschke L, Kabar I, Senninger N. Postoperative pancreatic fistula affects recurrence-free survival of pancreatic cancer patients. PLoS One. 2021;16:e0252727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Vollmer CM Jr, Sanchez N, Gondek S, McAuliffe J, Kent TS, Christein JD, Callery MP; Pancreatic Surgery Mortality Study Group. A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg. 2012;16:89-102; discussion 102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 13. | Smits FJ, Verweij ME, Daamen LA, van Werkhoven CH, Goense L, Besselink MG, Bonsing BA, Busch OR, van Dam RM, van Eijck CHJ, Festen S, Koerkamp BG, van der Harst E, de Hingh IH, Kazemier G, Klaase JM, van der Kolk M, Liem M, Luyer MDP, Meerdink M, Mieog JSD, Nieuwenhuijs VB, Roos D, Schreinemakers JM, Stommel MW, Wit F, Zonderhuis BM, de Meijer VE, van Santvoort HC, Molenaar IQ; Dutch Pancreatic Cancer Group. Impact of Complications After Pancreatoduodenectomy on Mortality, Organ Failure, Hospital Stay, and Readmission: Analysis of a Nationwide Audit. Ann Surg. 2022;275:e222-e228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 14. | Kamarajah SK, Bundred JR, Lin A, Halle-Smith J, Pande R, Sutcliffe R, Harrison EM, Roberts KJ; PARANOIA Study Group. Systematic review and meta-analysis of factors associated with post-operative pancreatic fistula following pancreatoduodenectomy. ANZ J Surg. 2021;91:810-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Søreide K, Labori KJ. Risk factors and preventive strategies for post-operative pancreatic fistula after pancreatic surgery: a comprehensive review. Scand J Gastroenterol. 2016;51:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Connor S. Defining post-operative pancreatitis as a new pancreatic specific complication following pancreatic resection. HPB (Oxford). 2016;18:642-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Bannone E, Andrianello S, Marchegiani G, Masini G, Malleo G, Bassi C, Salvia R. Postoperative Acute Pancreatitis Following Pancreaticoduodenectomy: A Determinant of Fistula Potentially Driven by the Intraoperative Fluid Management. Ann Surg. 2018;268:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 18. | Chen H, Wang W, Ying X, Deng X, Peng C, Cheng D, Shen B. Predictive factors for postoperative pancreatitis after pancreaticoduodenectomy: A single-center retrospective analysis of 1465 patients. Pancreatology. 2020;20:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22118] [Cited by in RCA: 18389] [Article Influence: 2627.0] [Reference Citation Analysis (1)] |
| 20. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2951] [Article Influence: 368.9] [Reference Citation Analysis (35)] |
| 21. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2324] [Article Influence: 129.1] [Reference Citation Analysis (0)] |
| 22. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1943] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 23. | Rho SY, Kim SH, Kang CM, Lee WJ. Is ICG-enhanced image able to help predicting pancreatic fistula in laparoscopic pancreaticoduodenectomy? Minim Invasive Ther Allied Technol. 2019;28:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Subar D, Pietrasz D, Fuks D, Gayet B. A novel technique for reducing pancreatic fistulas after pancreaticojejunostomy. J Surg Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Strasberg SM, Drebin JA, Mokadam NA, Green DW, Jones KL, Ehlers JP, Linehan D. Prospective trial of a blood supply-based technique of pancreaticojejunostomy: effect on anastomotic failure in the Whipple procedure. J Am Coll Surg. 2002;194:746-58; discussion 759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 26. | Iguchi T, Iseda N, Hirose K, Ninomiya M, Honboh T, Maeda T, Sawada F, Tachibana YI, Akashi T, Sekiguchi N, Sadanaga N, Matsuura H. Indocyanine green fluorescence to ensure perfusion in middle segment-preserving pancreatectomy: a case report. Surg Case Rep. 2021;7:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | Doussot A, Decrock M, Calame P, Georges P, Turco C, Lakkis Z, Heyd B. Fluorescence-based pancreas stump perfusion is associated with postoperative acute pancreatitis after pancreatoduodenectomy a prospective cohort study. Pancreatology. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Ausania F, Landi F, Martínez-Pérez A, Fondevila C. A meta-analysis of randomized controlled trials comparing laparoscopic vs open pancreaticoduodenectomy. HPB (Oxford). 2019;21:1613-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Levy PT, Pellicer A, Schwarz CE, Neunhoeffer F, Schuhmann MU, Breindahl M, Fumagelli M, Mintzer J, de Boode W; ESPR Special Interest Group “Near InfraRed Spectroscopy” (NIRS). Near-infrared spectroscopy for perioperative assessment and neonatal interventions. Pediatr Res. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Varela JE, Cohn SM, Giannotti GD, Dolich MO, Ramon H, Wiseberg JA, McKenney M. Near-infrared spectroscopy reflects changes in mesenteric and systemic perfusion during abdominal compartment syndrome. Surgery. 2001;129:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Tripodaki ES, Tasoulis A, Koliopoulou A, Vasileiadis I, Vastardis L, Giannis G, Argiriou M, Charitos C, Nanas S. Microcirculation and macrocirculation in cardiac surgical patients. Crit Care Res Pract. 2012;2012:654381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | De Schryver N, Wittebole X, Hubert C, Gigot JF, Laterre PF, Castanares-Zapatero D. Early hyperlactatemia predicts pancreatic fistula after surgery. BMC Anesthesiol. 2015;15:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 918] [Article Influence: 70.6] [Reference Citation Analysis (2)] |
| 34. | Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 35. | Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 36. | Sugimoto M, Takahashi S, Kobayashi T, Kojima M, Gotohda N, Satake M, Ochiai A, Konishi M. Pancreatic perfusion data and post-pancreaticoduodenectomy outcomes. J Surg Res. 2015;194:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Slooter MD, Mansvelders MSE, Bloemen PR, Gisbertz SS, Bemelman WA, Tanis PJ, Hompes R, van Berge Henegouwen MI, de Bruin DM. Defining indocyanine green fluorescence to assess anastomotic perfusion during gastrointestinal surgery: systematic review. BJS Open. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |