Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1719
Peer-review started: May 11, 2023
First decision: May 31, 2023
Revised: June 8, 2023
Accepted: June 19, 2023
Article in press: June 19, 2023
Published online: August 27, 2023
Processing time: 106 Days and 11.1 Hours
Monitoring of gastric residual is an important approach for assessing gastric emptying in patients with mechanical ventilation. By monitoring gastric contents, the enteral nutrition scheme can be adjusted in time to ensure feeding safety.
To investigate the effects of ultrasound monitoring on the incidence of feeding complications, daily caloric intake and prognosis of patients with severe mechanical ventilation. To analyze the clinical significance of ultrasound monitoring of gastric residual volume (GRV) up to 250 mL to provide a theoretical basis for clinical practice.
Patients admitted to the department of emergency medicine of the Affiliated Hospital of Nantong University from January 2018 to June 2022 who received invasive mechanical ventilation and continuous enteral nutrition support within 24-48 h after admission were enrolled in this study. Medical records for patients within 7 d of hospitalization were retrospectively analyzed to compare the incidence of feeding complications, daily caloric intake and clinical prognosis between patients with gastric residual ≥ 250 mL and < 250 mL, as monitored by ultrasound on the third day.
A total of 513 patients were enrolled in this study. Incidences of abdominal distension, diarrhea, and vomiting in the < 250 mL and ≥ 250 mL groups were: 18.4% vs 21.0%, 23.9% vs 32.3% and 4.0% vs 6.5%, respectively; mortality rates were 20.8% vs 22.65%; mechanical ventilation durations were 18.30 d vs 17.56 d while lengths of stay in the intensive care units (ICU) were 19.87 d vs 19.19 ± 5.19 d. Differences in the above factors between groups were not significant. Gastric residual ≥ 250 mL was not an independent risk factor for death and prolonged ICU stay. However, target feeding time of patients in the ≥ 250 mL group was longer than that of patients in the ≥ 250 mL group, and caloric intake (22.0, 23.6, 24.8, 25.3 kcal/kg/d) for patients in the ≥ 250 mL group from the 4th day to the 7th day of hospitalization was lower than that of patients in the ≥ 250 mL group (23.2, 24.8, 25.7, 25.8 kcal/kg/d). On the 4th day (Z = 4.324, P = 0.013), on the 5th day (Z = 3.376, P = 0.033), while on the 6th day (Z = 3.098, P = 0.04), the differences were statistically significant.
The use of ultrasound to monitor GRV and undertaking clinical interventions when the monitoring value is ≥ 250 mL has no significant effects on incidences of feeding complications and clinical prognostic outcomes, however, it significantly prolongs the time to reach target feeding, reduces the daily intake of calories during ICU hospitalization, and increases the risk of insufficient nutrition of patients. The accuracy and necessity of monitoring gastric remnants and monitoring frequencies should be investigated further.
Core Tip: Gastric residue is only one of the indicators of feeding intolerance and cannot predict whether a patient will experience feeding intolerance. It is not recommended for evaluating the patient's feeding tolerance or prognosis solely based on gastric residue.
- Citation: Xu XY, Xue HP, Yuan MJ, Jin YR, Huang CX. Effects of ultrasound monitoring of gastric residual volume on feeding complications, caloric intake and prognosis of patients with severe mechanical ventilation. World J Gastrointest Surg 2023; 15(8): 1719-1727
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1719.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1719
Patients with invasive mechanical ventilation in intensive care units (ICU) are in a high catabolic state and are prone to malnutrition, resulting in intestinal ischemia and reperfusion injury and affecting intestinal immune functions[1]. As one of the important therapeutic nutritional support interventions for severe patients, enteral nutrition can maintain the normal physiological functions of the gastrointestinal tract, prevent intestinal villus atrophy, and guarantee intestinal barrier functions[2]. The nutrition guidelines recommend that if there is no contraindication, enteral nutrition support can be started at 24-48 h after ICU admission[3]. To reduce the mortality rates, infection incidences, as well as hospitalization time and improve the prognostic outcomes of patients, early implementation of enteral nutrition should conform to the physiological needs of the gastrointestinal tract of patients[4]. However, for ICU patients, their gastrointestinal functions are impaired, and there are feeding intolerance (FI) risks during enteral nutrition implementation. There is no unified standard definition for FI. Currently, the definitions proposed by the European Society of Intensive Care Medicine in 2012[5] are widely used, including gastrointestinal adverse reactions, low rate of energy requirements and termination of enteral nutrition. Incidence of FI during early enteral nutrition have been reported to be between 30.5%-67.5%. Therefore, timely and accurate evaluation of gastrointestinal functions is particularly important. Monitoring of gastric residual is an important approach for evaluating gastric emptying of patients with mechanical ventilation. By monitoring gastric contents, the enteral nutrition scheme can be adjusted in time to ensure feeding safety[6,7]. Various methods for monitoring gastric residual volume (GRV) in clinics have been proposed. The most traditional and common method is aspiration, which involves using a syringe to extract gastric contents through the gastric tube. Even though this method is simple to operate, its measurement results are affected by many factors, such as position of the tip of the gastric tube and suction force degree. The extracted gastric contents are exposed to the air and are easily contaminated[8]. Moreover, when the gastric contents are discarded, it is easy to lose the nutrient solution and the digestive fluid in the stomach, and when target feeding amount cannot be attained, it increases the malnutrition risk in patients. Gastric ultrasound can provide information about the nature and volume of gastric contents at the bedside[9]. The accuracy and repeatability of gastric ultrasound has been reported in previous studies. Although it cannot fully assess the gastric functions and state (such as pH value), it can provide important and useful information, such as volume and nature of gastric contents (transparent liquid, solid or not)[9-11]. The accuracy of ultrasonic monitoring of GRV is also high, and there is no need to withdraw gastric contents, which reduces body fluid exposure risks[12]. However, the correlation between gastric residual and poor prognostic outcomes, such as aspiration, ventilator-related pneumonia and FI has not been fully elucidated[13-15]. The guidelines[16] issued by the critical illness Association and the American Association for parenteral and enteral nutrition in 2016 do not recommend monitoring of gastric residual amounts in clinical routine or assessing the feeding tolerance of patients by only relying on gastric residual amounts. However, a previous survey[6,17-19] revealed that 97.1% of nurses judge whether patients have FI by monitoring gastric residual amounts because the monitoring method is simple and convenient.
The aim of this study was to investigate the effects of ultrasound monitoring on incidence of feeding complications, daily caloric intake and clinical prognosis of patients with severe mechanical ventilation. Moreover, we analyzed its clinical significance to provide a theoretical basis for guiding clinical practice.
Patients admitted to the department of emergency medicine of the Affiliated Hospital of Nantong University from January 2018 to June 2022, and who received invasive mechanical ventilation and continuous enteral nutrition support within 24-48 h after admission were enrolled in this study. Medical records of the patients within 7 d of hospitalization were retrospectively analyzed to compare incidences of feeding complications, daily caloric intake and clinical prognosis between patients with gastric residual ≥ 250 mL and those with < 250 mL, as monitored by ultrasound on the third day of admission.
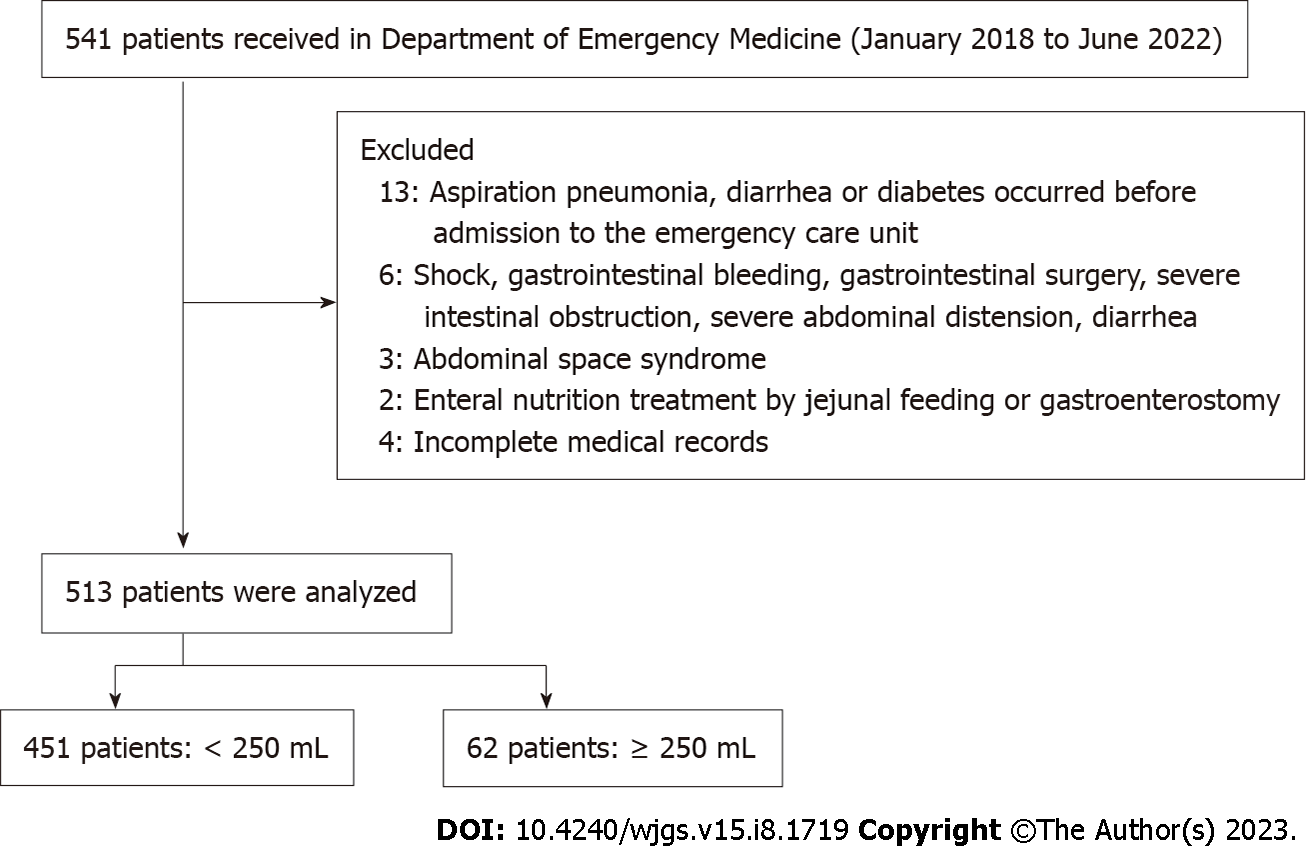
Patient data were retrospectively collected from the electronic medical records system of the intensive care units. Screening of study participants and data collation were performed as shown in Figure 1.
The inclusion criteria were: (1) No previous gastrointestinal dysfunction and enteral nutrition for 3 d; (2) Aged ≥ 18 years; and (3) Patients or family members who agreed to sign the informed consent form.
The exclusion criteria were: (1) Presence of aspiration pneumonia, diarrhea or diabetes before admission to intensive care units; (2) Shock, gastrointestinal bleeding, gastrointestinal surgery, severe intestinal obstruction, severe abdominal distension and diarrhea; (3) Abdominal space syndrome; (4) Enteral nutrition treatment via jejunum feeding or gastroenterostomy; and (5) Patients with incomplete case data records.
The general data and clinical characteristics of study participants, including age, sex, body mass index (BMI), acute physiology and chronic health evaluation II (APACHE II), sequential organ failure assessment (SOFA), and disease diagnosis among others were collected.
Vomiting: Stomach contents flow out of the mouth and nose through the esophagus. Diarrhea: The number of daily defecations is more than 3 times, feces are thin, the water content is high, and the daily defecation volume is more than 200 g. Abdominal distension: Discomfort caused by abdominal swelling or fullness.
Data on time of mechanical ventilation, daily caloric intake from day 3 to day 7 after hospitalization in the ICU, the time to reach the feeding target, ICU hospitalization days and mortality were collected. The time to reach the feeding target: the number of days to reach 25 kcal/kg/D in gastrointestinal nutrition.
Daily caloric intake: Obtained by multiplying the volume of nutrient solution (mL) taken by the patient every day by the energy density of the nutrient solution (kcal/mL) divided by body weight.
The monitoring frequency of gastric remnants was once every 4 h. Briefly, patients were placed in supine positions (the head of the bed was raised by 30°-45°), the portable color ultrasound diagnostic instrument was selected, the probe frequency was set at 2-5 mhz, and the single section of the antrum selected, that is, the ultrasound probe was placed under the xiphoid process of the patient and perpendicular to the abdomen angle. The antrum, the superior mesenteric artery, the left lobe of the liver and the abdominal aorta were examined to locate the position of the antrum, and ultrasound used to determine the size of the antrum. The area of the antrum was calculated by measuring the transverse and anterior posterior diameters of the antrum, after which the gastric residual was obtained by comparing the area of the antrum with age. When residual amount of the stomach exceeded 250 mL, enteral nutrition was stopped and further monitoring performed after 2-4 h. If < 250 mL, enteral nutrition was continued. If the gastric residual was still high, the jejunal nutrition tube or drug treatment was reserved according to patient's conditions, and if necessary, it was changed to parenteral nutrition support. Since some patients were hospitalized for 24-48 h, continuous enteral nutrition was not given until the condition was relatively stable. The GRV of patients was collected on the third day of ICU hospitalization, and the patients were assigned into ≥ 250 mL and < 250 mL groups.
The results for each scale were input into the computer for score conversion. The SPSS 24.0 software (IBM Corp., Armonk, NY, United States) was used for statistical analyses. Measurement data are expressed as means ± SD, while the counting data are expressed as frequencies and percentages. t-tests, analysis of variance, and chi square tests were used for inter-group statistical analyses. Logistic regression models were established for multivariate analyses. Bilateral P < 0.05 was set as the threshold for statistical significance.
A total of 513 patients (451 in the < 250 mL group and 62 in the ≥ 250 mL group) were enrolled in this study. There were 267 (59.2%) males in the 250 mL group, with age (53.04 ± 3.9 years), BMI (20.39 ± 2.5), APACHE II scores (6.39 ± 2.44), and SOFA (3.51 ± 0.53). There were 33 (53.2%) males in the ≥ 250 mL group, with age (53.92 ± 4.29 years), BMI (20.87 ± 2.49), APACHE II scores (16.71 ± 2.41), and SOFA (3.47 ± 0.5). Differences in general data between the groups were insignificant (Table 1).
| Item | < 250 mL (n = 451) | ≥ 250 mL (n = 62) | t/χ2 | P value |
| Gender | 0.8022 | 0.371 | ||
| Female | 184 (40.8) | 29 (46.8) | ||
| Male | 267 (59.2) | 33 (53.2) | ||
| Age (yr) | 53.04 ± 3.9 | 53.92 ± 4.29 | 1.6521 | 0.099 |
| BMI | 20.39 ± 2.5 | 20.87 ± 2.49 | 1.4201 | 0.156 |
| APACHE II | 16.39 ± 2.44 | 16.71 ± 2.41 | 0.9821 | 0.327 |
| SOFA | 3.51 ± 0.53 | 3.47 ± 0.5 | 0.5871 | 0.557 |
| Acute cerebrovascular accident | 133 (29.5) | 23 (37.1) | 1.4902 | 0.222 |
| Acute pneumonia | 84 (18.6) | 9 (14.5) | 0.6202 | 0.431 |
| Acute heart failure | 122 (27.1) | 14 (22.6) | 0.5592 | 0.455 |
| Craniocerebral injury | 112 (24.8) | 16 (25.8) | 0.0282 | 0.868 |
| Other | 20 (4.4) | 3 (4.8) | 0.0212 | 0.885 |
| Hypertension | 271 (60.1) | 45 (72.6) | 3.5961 | 0.058 |
| Diabetes | 106 (23.5) | 12 (19.4) | 0.5302 | 0.467 |
| Coronary heart disease | 161 (35.7) | 23 (37.1) | 0.0462 | 0.830 |
Results showed that 29.9% and 25.1% of patients in the < 250 mL group used sedatives or sedatives, compared to 48.4% and 38.7% in the ≥ 250 mL group (P < 0.05). The probabilities of abdominal distension, diarrhea and vomiting in the < 250 mL group were 18.4%, 23.9% and 4.0%, compared with 21.0%, 32.3% and 6.5% in the ≥ 250 mL group (P > 0.05; Table 2).
| Item | < 250 mL (n = 451) | ≥ 250 mL (n = 62) | χ2 | P value |
| Sedative drug use rate | 135 (29.9) | 30 (48.4) | 8.507 | 0.004 |
| Analgesic drug use rate | 113 (25.1) | 24 (38.7) | 5.192 | 0.023 |
| Abdominal distention | 83 (18.4) | 13 (21.0) | 0.236 | 0.627 |
| Diarrhea | 108 (23.9) | 20 (32.3) | 2.011 | 1.156 |
| Vomit | 18 (4.0) | 4 (6.5) | 2.382 | 0.336 |
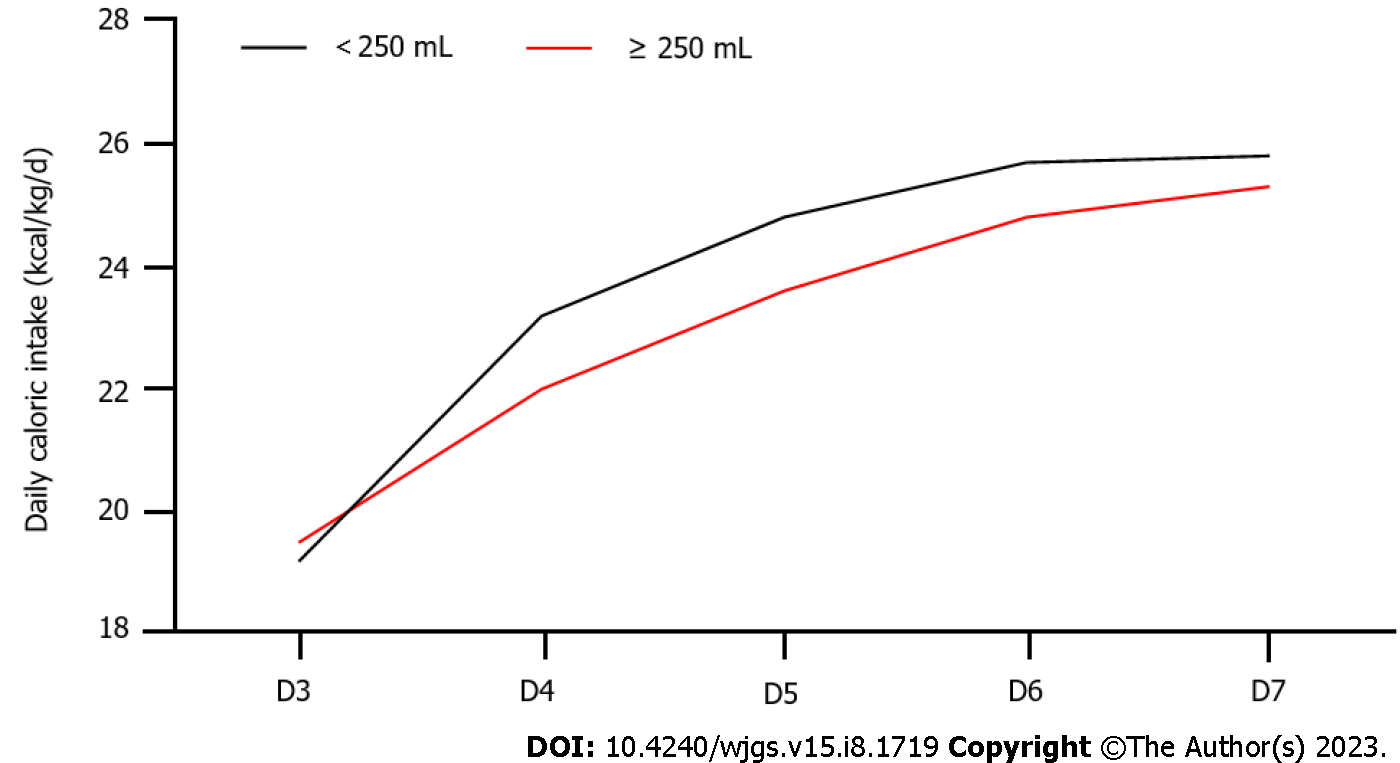
The time to reach the feeding target was significantly shorter for the ≥ 250 mL group, compared to that of the < 250 mL group (P < 0.05). Differences in mechanical ventilation time, ICU hospitalization days and mortality rates between the two groups were not significant (P > 0.05). Caloric intake (22.0, 23.6, 24.8, 25.3 kcal/kg/d) for patients in the < 250 mL group was lower compared with that of patients in the < 250 mL group (23.2, 24.8, 25.7, 25.8 kcal/kg/d). Caloric intakes on the 4th day (Z = 4.324, P = 0.013), 5th day (Z = 3.376, P = 0.033) and 6th day (Z = 3.098, P = 0.04) were significant (Figure 2 and Table 3).
When residual gastric volume > 250 mL, sedative drugs, analgesics, vomiting, and time to reach the feeding target were taken as independent variables and respectively introduced into the logistic regression model for analysis, it was found that the time to reach the target feeding was an independent risk factor influencing the prognosis and extension of ICU stay. However, GRV > 250 mL had no significant effects on patient death and ICU stay outcomes (Tables 4 and 5).
| Related factor | β | SE | χ2 | P value | OR | 95%CI |
| ≥ 250 mL | 0.031 | 0.338 | 0.008 | 0.928 | 1.031 | 0.532-2.000 |
| Sedatives | 0.082 | 0.678 | 0.015 | 0.903 | 0.921 | 0.244-3.481 |
| Analgesics | 0.229 | 0.231 | 0.984 | 0.321 | 0.795 | 0.505-1.251 |
| Time to reach feeding target | 1.186 | 0.311 | 5.659 | 0.039 | 1.205 | 0.655-2.217 |
| Constant | -1.240 | 1.042 | 1.417 | 0.234 | 0.289 |
| Related factor | β | SE | t | P value |
| ≥ 250 mL | -0.634 | 0.659 | -0.963 | 0.336 |
| Sedatives | -0.307 | 1.340 | -0.229 | 0.819 |
| Analgesics | 0.324 | 0.452 | 0.717 | 0.474 |
| Time to reach feeding target | -1.393 | 0.613 | -3.641 | 0.034 |
| Constant | 20.608 | 1.268 | 16.252 | 0.000 |
The 2016 guidelines of the American Society of critical care medicine and the society of enteral and parenteral nutrition recommend monitoring of tolerance of enteral tube feeding (ETF) for critically ill patients in combination with radiological images, physical examination, flatulence and defecation[20]. The ETF intolerance is mainly manifested by nasal feeding tube withdrawal, abnormal imaging, vomiting, abdominal distension or diarrhea, which can occur in up to one third of hospitalized patients. The TF intolerance is associated with poor prognostic outcomes[21]. The 2021 international guidelines for management of sepsis and gastric shock recommend that GRV should be routinely measured for patients with FI or high risk of aspiration[22]. Currently, the definition of GRV has not been standardized. A meta-analysis[23] involving 72 articles showed that the definition of FI includes one or all of the three aspects: large gastric residues (average 250 mL), gastrointestinal symptoms, and insufficient intake of calories. A previous study[24] revealed that the degree of influence of FI on poor prognostic outcomes is associated with definition of FI, and that the definition of high GRV (more than 500 mL for 24 h) and gastrointestinal symptoms is strongly correlated with 90-day mortality. The 2017 European Society of critical care clinical practice guidelines recommend delayed gastrointestinal nutrition for critically ill patients with GRV > 500 mL/6 h[25]. In 2021, expert consensus recommendation in China reported that residual gastric residue ≥ 250 mL suggest FI, and intervention treatments should be started as soon as possible [26]. This is why 250 mL was selected as the grouping standard in this study. Studies[23,27-30] have confirmed that FI increases mortality outcomes and prolongs the ICU hospitalization as well as mechanical ventilation times. Currently, there is no unified definition standard for FI. Abdominal distension, diarrhea and vomiting are regarded as the signs of FI and increased aspiration risk. In this study, it was found that when gastric residues of patients > 250 mL, clinical interventions did not significantly increase the incidences of abdominal distension, diarrhea and vomiting. Regarding the relationship between gastric residual allowance and enteral nutrition complications, studies[13-15] have confirmed that occurrences of vomiting, diarrhea, aspiration, pneumonia and other complications in ICU patients are not directly related to setting of critical values of gastric residual allowance, and that increasing the critical value of gastric residual allowance has no significant impact on enteral nutrition complications. In 2016, the Association for critical illness and the American Association for parenteral and enteral nutrition proposed the nutrition treatment guidelines[16]: They recommend monitoring gastric residual allowance in an irregular manner in clinical practice. For ICU patients, when the gastric residual allowance is less than 500 mL and if the patient has no abdominal symptoms such as vomiting and diarrhea, enteral nutrition should not be stopped. Therefore, we do not recommend clinical interventions to prevent vomiting when the patient's gastric residue exceeds 250 mL, unless the patient has abdominal symptoms or the gastric residue exceeds 500 mL. We found that > 250 mL gastric remnants for ICU patients had no significant effects on mortality outcomes and ICU hospitalization time. Therefore, we postulate that gastric residue is only one of the signs of FI, and it cannot predict whether the patient has FI, thus, it will not have a significant impact on prognostic outcomes. Assessment of feeding tolerance or estimating its impact on prognostic outcomes should not be based on gastric residues only.
We also found that food intake for ICU patients with gastric residual > 250 mL from the 4th to the 7th day was lower than that of patients with gastric residual < 250 mL, and that differences between the groups from the 4th to the 6th day were significant. This may have been because enteral nutrition was stopped for 2-4 h when the GRV exceeded 250 mL. The higher the number of times the patient suspends enteral nutrition, the less calories he consumes on that day. If the GRV cannot accurately reflect the gastrointestinal movement, it causes unnecessary interruption of nutrition supply and increases the mortality as well as complication rates for patients, which is attributed to insufficient energy supply. When monitoring the gastric residual amount, interruption or cessation of enteral nutrition due to high gastric residual amounts leads to insufficient feeding of the patient, which affects the patient's caloric intake, and ultimately increases the mortality outcomes[31,32]. The monitoring frequency of GRV also has an impact on daily caloric intake for patients. A multicenter study involving a large sample size by Reignier et al[33] reported that the proportion of patients who did not routinely monitor GRV and reached the target feeding volume was significantly higher than that of the routine monitoring group. It was 1.77 times that of the routine monitoring group. Wiese et al[15] found that 84.5% of patients who did not routinely monitor gastric residual amounts had their actual enteral nutrition feeding amounts reaching more than 90% of the target feeding amount within 24 h, and that 83.3% of patients had their actual enteral nutrition feeding amount being more than 90% of the target feeding amount during ICU hospitalization, which were significantly higher than those in the routine monitoring group (46.4% in 24 h and 61.9% in ICU hospitalization).
Ultrasound monitoring of gastric residual and clinical interventions when the monitoring value exceeds 250 mL have no significant impacts on complication rates and clinical prognosis of ICU patients, but significantly reduces the intake of calories during ICU hospitalization, prolongs the time to reach the feeding target, increases the risk of insufficient nutrition of patients, and affects the prognostic outcomes of patients. When the gastric residual exceeds 250 mL, clinical interventions that increase the nutritional intake are not recommended. This study has some limitations. As a retrospective single center study, there may be some information bias, therefore, our findings should be further confirmed by prospective and large sample studies.
Gastric residual monitoring is considered an important way to evaluate gastric emptying in mechanically ventilated patients, but its correlation with adverse outcomes such as aspiration, ventilator-associated pneumonia, and feeding intolerance is controversial.
To analyze the impact of intervention with ultrasound monitoring of gastric residual volume (GRV) reaching 250mL on the incidence of feeding complications, daily calorie intake, and clinical prognosis in patients with severe mechanical ventilation.
To provide theoretical basis for clinical practice.
Retrospective analysis method.
The use of ultrasound to monitor gastric residue and clinical intervention at monitoring value ≥ 250ml did not significantly affect the incidence of feeding complications and clinical prognosis of patients.
This study suggests that ultrasound monitoring of gastric residue and clinical intervention when the monitoring value exceeds 250 mL have no significant impact on the incidence of complications and clinical prognosis of intensive care unit (ICU) patients. However, it significantly reduces the calorie intake of patients during ICU hospitalization, prolongs the time to reach feeding goals, increases the risk of insufficient nutrition, and affects patient prognosis.
It is not recommended to judge the patient's feeding tolerance or estimate the impact on the patient's prognosis solely based on GRV in clinical practice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Emergency medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Sayes IA, Egypt; Surve A, United States S-Editor: Yan JP L-Editor: A P-Editor: Wu RR
| 1. | Boeykens K. Nutritional Support in the Intensive Care Unit: Implications for Nursing Care From Evidence-Based Guidelines and Supporting Literature. Dimens Crit Care Nurs. 2021;40:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 2. | Li AY, Rustad KC, Long C, Rivera E, Mendiola M, Schenone M, Karanas YL. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44:1203-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Oczkowski S, Szczeklik W, Bischoff SC. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1527] [Article Influence: 218.1] [Reference Citation Analysis (0)] |
| 4. | Yang S, Guo J, Ni Q, Chen J, Guo X, Xue G, Ye M, Zhang L. Enteral nutrition improves clinical outcome and reduces costs of acute mesenteric ischaemia after recanalisation in the intensive care unit. Clin Nutr. 2019;38:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (1)] |
| 6. | Ozen N, Blot S, Ozen V, Arikan Donmez A, Gurun P, Cinar FI, Labeau S. Gastric residual volume measurement in the intensive care unit: an international survey reporting nursing practice. Nurs Crit Care. 2018;23:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Farsi Z, Kamali M, Butler S, Zareiyan A. The Effect of Semirecumbent and Right Lateral Positions on the Gastric Residual Volume of Mechanically Ventilated, Critically Ill Patients. J Nurs Res. 2020;28:e108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Yamaoka I, Kagawa T, Mizugai K, Ebisu G. Detecting Enteral Nutrition Residues and Microorganism Proliferation in Feeding Tubes via Real-Time Imaging. Nutr Clin Pract. 2017;32:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Segura-Grau E, Segura-Grau A, Ara Jo R, Payeras G, Cabral J, Afreixo V. Reinforcing the valuable role of gastric ultrasound for volume and content assessment: an observational study. Braz J Anesthesiol. 2022;72:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth. 2014;113:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 11. | Gültekin Y, Kılıç Ö, Özçelik Z, Toprak ŞS, Bayram R, Arun O. Can Gastric Volume be Accurately Estimated by Ultrasound? Turk J Anaesthesiol Reanim. 2022;50:194-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Maheshwari K, Bakal O, Cummings KC 3rd, Mao G, Rivas E, Elsharkawy H, Kolli S, Sessler DI, Bhavani S. The effects of diabetes mellitus on gastric emptying: A prospective observational cohort study. J Clin Anesth. 2021;75:110463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Machado LS, Rizzi P, Silva FM. Administration of enteral nutrition in the prone position, gastric residual volume and other clinical outcomes in critically ill patients: a systematic review. Rev Bras Ter Intensiva. 2020;32:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Bruen T, Rawal S, Tomesko J, Byham-Gray L. Elimination of Routine Gastric Residual Volume Monitoring Improves Patient Outcomes in Adult Critically Ill Patients in a Community Hospital Setting. Nutr Clin Pract. 2020;35:522-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wiese AN, Rogers MJ, Way M, Ballard E. The impact of removing gastric residual volume monitoring and enteral nutrition rate titration in adults receiving mechanical ventilation. Aust Crit Care. 2020;33:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Warren M, McCarthy MS, Roberts PR. Practical Application of the Revised Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: A Case Study Approach. Nutr Clin Pract. 2016;31:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Padar M, Uusvel G, Starkopf L, Starkopf J, Reintam Blaser A. Implementation of enteral feeding protocol in an intensive care unit: Before-and-after study. World J Crit Care Med. 2017;6:56-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (2)] |
| 18. | Hammad SM, Al-Hussami M, Darawad MW. Jordanian Critical Care Nurses' Practices Regarding Enteral Nutrition. Gastroenterol Nurs. 2015;38:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Poveda VB, Castilho ACBA, Nogueira LS, Ferretti-Rebustini REL, Silva RCGE. Assessing gastric residual volume: a description of nurses' clinical practice. Rev Esc Enferm USP. 2018;52:e03352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Mifsud S, Schembri EL, Gruppetta M. Stress-induced hyperglycaemia. Br J Hosp Med (Lond). 2018;79:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Skorepa P, Sobotka O, Vanek J, Ticha A, Fortunato J, Manak J, Blaha V, Horacek JM, Sobotka L. The Impact of Glucose-Based or Lipid-Based Total Parenteral Nutrition on the Free Fatty Acids Profile in Critically Ill Patients. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 2264] [Article Influence: 566.0] [Reference Citation Analysis (0)] |
| 23. | Blaser AR, Starkopf J, Kirsimägi Ü, Deane AM. Definition, prevalence, and outcome of feeding intolerance in intensive care: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2014;58:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Reintam Blaser A, Starkopf L, Deane AM, Poeze M, Starkopf J. Comparison of different definitions of feeding intolerance: A retrospective observational study. Clin Nutr. 2015;34:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, Fruhwald S, Hiesmayr M, Ichai C, Jakob SM, Loudet CI, Malbrain ML, Montejo González JC, Paugam-Burtz C, Poeze M, Preiser JC, Singer P, van Zanten AR, De Waele J, Wendon J, Wernerman J, Whitehouse T, Wilmer A, Oudemans-van Straaten HM; ESICM Working Group on Gastrointestinal Function. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43:380-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 465] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 26. | Chinese Abdominal Intensive Care Association; Asia Society for Emergency and Critical Care Medicine. Expert consensus on enteral nutrition for gastrointestinal dysfunction in critically ill patients (2021 edition). Zhonghua Xiaohua Waike Zazhi. 2021;20:1123-1136. |
| 27. | Wang K, McIlroy K, Plank LD, Petrov MS, Windsor JA. Prevalence, Outcomes, and Management of Enteral Tube Feeding Intolerance: A Retrospective Cohort Study in a Tertiary Center. JPEN J Parenter Enteral Nutr. 2017;41:959-967. [PubMed] [DOI] [Full Text] |
| 28. | Gungabissoon U, Hacquoil K, Bains C, Irizarry M, Dukes G, Williamson R, Deane AM, Heyland DK. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr. 2015;39:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 29. | Virani FR, Peery T, Rivas O, Tomasek J, Huerta R, Wade CE, Lee J, Holcomb JB, Uray K. Incidence and Effects of Feeding Intolerance in Trauma Patients. JPEN J Parenter Enteral Nutr. 2019;43:742-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Peng J, Liu G, Li F, Yuan M, Xiang Y, Qin D. The correlation between feeding intolerance and poor prognosis of patients with severe neurological conditions: a case-control study. Expert Rev Neurother. 2020;20:415-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux R N MC, Delarue J, Berger MM. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 590] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 32. | Serón Arbeloa C, Zamora Elson M, Labarta Monzón L, Garrido Ramírez de Arellano I, Lander Azcona A, Marquina Lacueva MI, López Claver JC, Escos Orta J. [Nutritional support outcomes in critical care]. Nutr Hosp. 2011;26:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, Clavel M, Frat JP, Plantefeve G, Quenot JP, Lascarrou JB; Clinical Research in Intensive Care and Sepsis (CRICS) Group. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |