Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1684
Peer-review started: April 6, 2023
First decision: April 19, 2023
Revised: May 16, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: August 27, 2023
Processing time: 141 Days and 2.9 Hours
The liver hemodynamic changes caused by portal hypertension (PH) are closely related to various complications such as gastroesophageal varices and portosys-temic shunts, which may lead to adverse clinical outcomes in these patients, so it is of great clinical significance to find treatment strategies with favorable clinical efficacy and low risk of complications.
To study the clinical efficacy of total laparoscopic splenectomy (TLS) for PH and its influence on hepatic hemodynamics and liver function.
Among the 199 PH patients selected from October 2016 to October 2020, 100 patients [observation group (OG)] were treated with TLS, while the remaining 99 [reference group (RG)] were treated with open splenectomy (OS). We observed and compared the clinical efficacy, operation indexes [operative time (OT) and intraoperative bleeding volume], safety (intraperitoneal hemorrhage, ascitic fluid infection, eating disorders, liver insufficiency, and perioperative death), hepatic hemodynamics (diameter, velocity, and flow volume of the portal vein system), and liver function [serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), and serum total bilirubin (TBil)] of the two groups.
The OT was significantly longer and intraoperative bleeding volume was significantly lesser in the OG than in the RG. Additionally, the overall response rate, postoperative complications rate, and liver function indexes (ALT, AST, and TBil) did not differ significantly between the OG and RG. The hepatic hemodynamics statistics showed that the pre- and postoperative blood vessel diameters in the two cohorts did not differ statistically. Although the postoperative blood velocity and flow volume reduced significantly when compared with the preoperative values, there were no significant inter-group differences.
TLS contributes to comparable clinical efficacy, safety, hepatic hemodynamics, and liver function as those of OS in treating PH, with a longer OT but lesser intraoperative blood loss.
Core Tip: Portal hypertension (PH) can bring adverse effects to patients such as hepatic hemodynamic changes and decreased liver function. We propose and demonstrate that total laparoscopic splenectomy, although comparable to open splenectomy in clinical efficacy, safety, and effects on hepatic hemodynamics and liver function in patients with PH, has the advantage of less intraoperative blood loss.
- Citation: Qi RZ, Li ZW, Chang ZY, Chang WH, Zhao WL, Pang C, Zhang Y, Hu XL, Liang F. Clinical efficacy of total laparoscopic splenectomy for portal hypertension and its influence on hepatic hemodynamics and liver function. World J Gastrointest Surg 2023; 15(8): 1684-1692
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1684.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1684
Portal hypertension (PH) and cirrhosis are considered the endpoints of chronic liver injury[1]. Knowledge regarding the pathophysiology of PH has increased enormously over the past decade[2]. Currently, it is believed that increased visceral and peripheral vasodilators increase portal pressure, leading to changes in hepatic hemodynamics[3]. Furthermore, the negative effects of PH on the portal blood entering the liver will impair the patient’s liver functional reserve, leading to the deterioration of liver function[4]. Additionally, the hepatic hemodynamic changes caused by PH are closely related to several complications, such as gastroesophageal varices and portosystemic shunt, which can affect the clinical outcome in these patients[5]. Therefore, this study aimed to find a treatment strategy with better clinical efficacy and low risk of complications, which is important for optimizing the clinical treatment and improving the prognosis of patients with PH.
Open splenectomy (OS) is the traditional treatment choice for patients with PH. Despite its advantages of a wide field of view and simple technique, it has the disadvantages of a relatively large amount of blood loss, severe trauma, and slow recovery[6]. With the gradual development of medical technology, total laparoscopic splenectomy (TLS), a minimally invasive surgery, is gradually being accepted by patients. The gastric fundus and deep abdominal cavity of the patients can be observed through an endoscope during TLS, with a small incision and a relatively low risk of complications[7,8]. Thus, TLS is a feasible option for PH patients with conditions such as benign splenic lesions and hematological diseases but not for those with deformed or huge spleen, high pressure of portal blood vessels, and peri-splenic adhesion[9]. Recently, due to the continuous maturation and optimization of clinical practice, TLS combined with porta-azygos devascularization has gradually demonstrated its superiority. For instance, Luo et al[10] demonstrated that this method has superior surgical effects and similar long-term effects when compared with those of OS in patients with cirrhotic PH.
This study compares the clinical efficacy of TLS + devascularization and traditional OS + devascularization in patients with PH as well as the influences on the hepatic hemodynamics and liver function to provide a new reference and treatment choice for the management of PH patients.
This study selected 199 PH patients admitted to the Chinese PLA General Hospital between October 2016 and October 2020. Among them, 100 patients were assigned to the observation group (OG) and 99 to the reference group (RG) to undergo TLS and OS, respectively. The OG comprised 54 males and 46 females, with a mean age of 49.53 ± 9.14 years. Based on the Child-Pugh scores for liver function assessment, 72 and 28 cases in the OG were classified as Class A and Class B, respectively. The RG comprised 57 males and 42 females, with a mean age of 49.23 ± 9.84 years; 70 and 29 cases were classified as Child-Pugh Class A and Class B, respectively. The inter-group comparison of the baseline data revealed no obvious differences between them (P > 0.05). Details of the baseline data can be found in Table 1.
| Categories | Observation group (n = 100) | Reference group (n = 99) | χ2 | P value |
| Sex | 0.258 | 0.612 | ||
| Male | 54 (54.00) | 57 (57.58) | ||
| Female | 46 (46.00) | 42 (42.42) | ||
| Age | 49.53 ± 9.14 | 49.23 ± 9.84 | 0.223 | 0.824 |
| Child-Pugh score | 0.041 | 0.840 | ||
| Class A | 72 (72.00) | 70 (70.71) | ||
| Class B | 28 (28.00) | 29 (29.29) | ||
| Place of residence | 1.644 | 0.200 | ||
| Rural | 26 (26.00) | 34 (34.34) | ||
| Urban | 74 (74.00) | 65 (65.66) | ||
| Educational level | 1.537 | 0.215 | ||
| Senior high school or above | 45 (45.00) | 36 (36.36) | ||
| Below high school | 55 (55.00) | 63 (63.64) |
The inclusion criteria of this study were PH diagnosis according to the diagnostic criteria, surgical indications for splenectomy, preoperative liver function classification of Child-Pugh Class A or B, presence of splenomegaly and hypersplenism, availability of complete clinical data, and willingness to cooperate with the research requirements.
On the other hand, the exclusion criteria were as follows: Severe systemic organ function damage contraindicated for surgery; severe hepatatrophy, hepatic encephalopathy, jaundice, refractory ascites, and other liver conditions; malignancies such as carcinoma of the liver, stomach, and/or pancreas; and history of other abdominal operations.
For performing TLS in the OG, the patient was placed in the supine position with the legs spread, and the head and the left side of the body raised by 15°. The surgeon stood on the right side of the patient, the assistant stood on the opposite side, and the laparoscope assistant stood between the legs of the patient. Conventional four-hole laparoscopy was performed. The upper or lower edge of the umbilicus was selected as the first endoscopic hole, where a 10-12 mm trocar was placed. The second hole was located under the xiphoid process or the costal margin of the midline of the right clavicle, and a 5-mm trocar was placed. The right side of the patient's umbilicus was selected as the third hole, and a 12-mm trocar was placed. The fourth hole (assistant operation hole) was located under the left costal margin, and a 5-mm trocar was placed. An ultrasonic scalpel was used to isolate the stomach omentum layer-by-layer against the gastric wall up to the upper splenic pole and gastric vasa brevia. The upper splenic pole was severed if it was easily separated. The splenic artery could be separated and ligated if the pulse was obvious at the superior border of the pancreas, but a forcible separation was not necessary if it was inconvenient to be exposed. The splenocolic ligament and posterior peritoneal connective tissue attached to the spleen at the lower pole were dissociated, and the splenic pedicle tissue was thinned as far as possible. Endo-GIA, a 3.5-mm thick and 6-cm long endoscopic linear stapler, was used to sever the splenic pedicle. After splenectomy, dissection of the varicose vessels around the cardia-esophagus area was performed, and the esophageal branches of the coronary vessels and gastric branches were clamped or separated. After devascularization, the spleen was put into the retrieval bag, crushed, and removed piece by piece through the abdominal wall using the 12-mm trocar. After examination of the wound and achieving proper hemostasis, a drainage tube was placed in the splenic fossa to lead out from the abdominal wall, and the trocar incision was closed.
Patients in the RG underwent OS. An oblique incision under the costal margin was made in the abdomen, the artery was ligated if necessary, and the splenic pedicle vessels were cut off to complete the splenectomy. This was followed by azygo-portal disconnection. After observation and confirmation of no active bleeding, the abdomen was closed, and the operation was completed.
Clinical efficacy: The clinical efficacy was classified as follows: Marked response: Markedly improved liver function and significantly relieved clinical symptoms; Response: Improved liver function and relieved clinical symptoms; Non-response: Barely improved or even deteriorated liver function with no improvement in the clinical symptoms; The overall response rate (ORR) was the percentage of the sum of marked response and response patients from the total number of cases.
Surgical indicators: We mainly observed and recorded the operative time (OT) and intraoperative bleeding volume of the two groups.
Safety: The incidence rates of postoperative abdominal hemorrhage, ascitic fluid infection, eating disorders, liver dysfunction, and perioperative death were observed and recorded.
Hepatic hemodynamics: Color Doppler ultrasound was used to measure the diameter, velocity, and flow volume of the portal vein system in the two groups before and 2 wk after the operation.
Liver function: Fasting venous blood (4 mL) was collected from each patient of both groups before and 2 wk after the operation. The serum was collected after centrifugation to quantify the alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBil) using an automatic biochemical analyzer.
The data in this study were processed using SPSS, version 24.0 (SPSS Inc., Chicago, IL, United States). Perioperative indicators, liver function indicators, portal system hemodynamics, and other measurement data are expressed as means ± SD; the inter-group comparison was conducted using an independent sample t-test. Count data, such as curative effects and postoperative complications, are expressed as numbers (percentages); these data were analyzed using a chi-square test. P values < 0.05 were considered statistically significant.
The RG and OG were similar with respect to the sex, age, Child-Pugh score, place of residence, and educational level of the participants (P > 0.05; Table 1).
Based on the statistical analysis of the number of marked response, response, and non-response cases in the two groups, the ORR of the RG and OG was 85.86% and 90.00%, respectively, with no statistically significant difference between them (P > 0.05; Table 2).
| Categories | Observation group (n = 100) | Reference group (n = 99) | χ2 | P value |
| Marked response | 39 (39.00) | 35 (35.35) | - | - |
| Response | 51 (51.00) | 50 (50.50) | - | - |
| Non-response | 10 (10.00) | 14 (14.14) | - | - |
| Overall response rate | 90 (90.00) | 85 (85.86) | 0.805 | 0.370 |
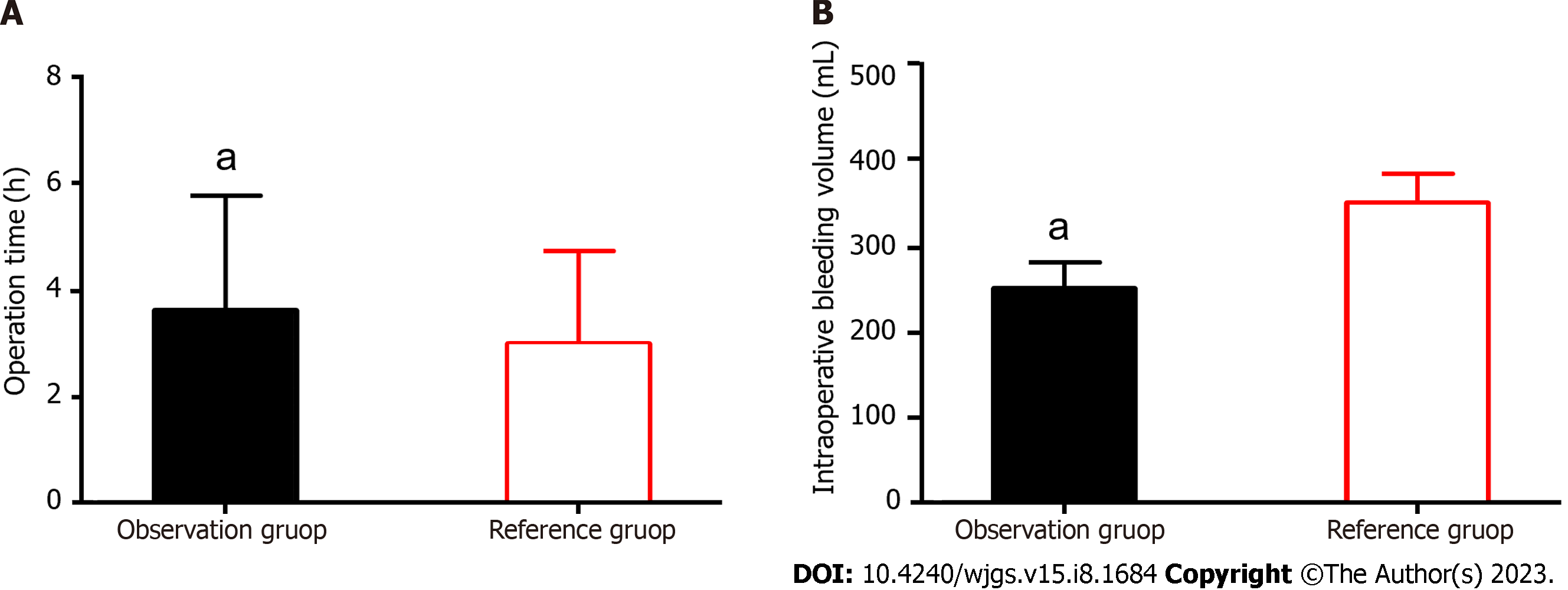
We statistically analyzed the OT and intraoperative bleeding volumes of the two groups. The mean OT and intraoperative bleeding volume were 3.6 ± 2.1 h and 251.9 ± 31.6 mL in the OG and 3.1 ± 2.1 h and 353.3 ± 34.7 mL in the RG, respectively. Longer OT and lesser intraoperative bleeding volume were observed in the OG than in the RG, with a statistically significant difference (P < 0.05; Figure 1).
The number of cases of intraperitoneal hemorrhage, ascitic fluid infection, eating disorders, liver insufficiency, and perioperative death in the two groups was recorded. The main complications in the OG were ascitic fluid infection (3.00%), followed by intraperitoneal hemorrhage (2.00%), eating disorders (1.00%), and liver insufficiency (1.00%). The major complications in the RG were ascitic fluid infection (4.04%), followed by intraperitoneal hemorrhage (3.03%), eating disorders (2.02%), liver insufficiency (1.01%), and perioperative death (1.01%). The incidence of postoperative complications did not differ significantly between the OG and RG (11.11% vs 7.00%, P > 0.05; Table 3).
| Categories | Observation group (n = 100) | Reference group (n = 99) | χ2 | P value |
| Intraperitoneal hemorrhage | 2 (2.00) | 3 (3.03) | - | - |
| Ascitic fluid infection | 3 (3.00) | 4 (4.04) | - | - |
| Eating disorders | 1 (1.00) | 2 (2.02) | - | - |
| Hepatic insufficiency | 1 (1.00) | 1 (1.01) | - | - |
| Perioperative death | 0 (0.00) | 1 (1.01) | - | - |
| Total incidence | 7 (7.00) | 11 (11.11) | 1.022 | 0.312 |
The hemodynamic indexes of the portal vein system, such as blood vessel diameter, blood flow velocity and blood flow volume [blood flow volume = 60 × portal vein velocity × π × (1/2 portal vein diameter)2], were recorded for both the groups.
In the OG, the average blood vessel diameters, blood flow velocities, and blood flow volumes before and after surgery were 12.4 ± 3.2 mm and 11.2 ± 3.0 mm, 11.6 ± 2.5 cm/s and 10.2 ± 2.2 cm/s, and 915.8 ± 519.1 mL/min and 648.5 ± 370.5 mL/min, respectively.
In the RG, the average blood vessel diameters, blood flow velocities, and blood flow volumes before and after surgery were 12.2 ± 2.9 mm and 11.4 ± 3.2 mm, 11.2 ± 2.1 cm/s and 10.3 ± 2.4 cm/s, and 848.3 ± 454.7 mL/min and 685.4 ± 408.3 mL/min, respectively.
There were no significant inter-group or intra-group differences in the blood vessel diameters before and after surgery (P > 0.05). The postoperative blood flow velocities and volumes in both groups decreased significantly when compared with the preoperative values (P < 0.05), but without a significant difference between the groups (P > 0.05; Figure 2).
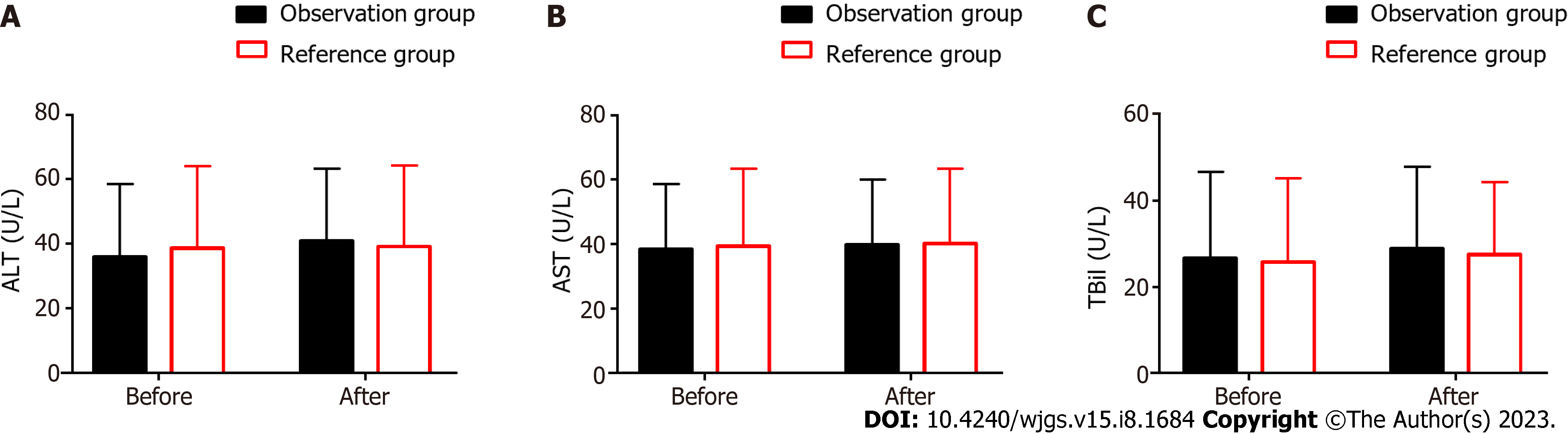
The liver function indexes, such as ALT, AST, and TBil, were recorded (Figure 3). In the OG, the pre- and postoperative ALT levels were 36.0 ± 22.5 U/L and 40.8 ± 22.5 U/L, respectively; AST levels were 38.3 ± 20.2 U/L and 39.6 ± 20.2 U/L, respectively; and TBil levels were 26.6 ± 20.0 U/L and 28.9 ± 18.9 U/L, respectively.
In the RG, the pre- and postoperative ALT levels were 38.7 ± 25.4 U/L and 39.1 ± 25.1 U/L, respectively; AST levels were 39.3 ± 23.9 U/L and 40.0 ± 23.1 U/L, respectively; and TBil levels were 25.7 ± 19.4 U/L and 27.5 ± 16.7 U/L, respectively.
The data revealed no evident inter-group or intra-group differences before and after surgery in the ALT, AST, and TBil levels (P > 0.05).
The most common clinical manifestations of PH are hypersplenism and gastric varices, and it accounts for 24%-80% of the cases. The mortality rate is up to 40% within 1 year after the clinical manifestations worsen[11,12]. Therefore, the debate regarding the best treatment for PH has been ongoing worldwide but remains controversial[13]. It has been suggested previously that conventional treatment and surgery for PH cannot prevent emergencies or pain and that long-acting medications offer the opportunity to improve the patient’s quality of life[14]. However, some evidence suggests that this treatment may compromise hemodynamic stability[15]. Therefore, in many cases, surgery remains the mainstream treatment for PH, and splenectomy + devascularization has become one of the most effective treatments for gastroesophageal varices in patients with PH after years of development[16]. PH patients have also benefited from the advances in laparoscopic technology, which allow surgery to be performed in a less invasive manner[17]. However, based on the relevant literature, information on hepatic hemodynamics and liver function after TLS + devascularization is lacking. Therefore, we conducted a retrospective study to contribute to the existing knowledge on the clinical treatment of PH.
In our study, the ORR of the treatment did not differ significantly between the OG and RG (90.00% vs 85.86%). This indicates that TLS + devascularization had a comparable therapeutic effect as that of OS, which corroborated the research results of Lin et al[18] on the laparoscopic application in PH patients. The approach sequence, layout of the trocar, and prediction of surgical risks pre- and postoperatively are important factors affecting the surgical indexes. According to the statistical analysis of the operation indexes, the OT was significantly prolonged in the OG, and despite the significantly lesser intraoperative bleeding volume, the risk of conversion to laparotomy due to bleeding remains. Another study reported that the laparoscopic group presented significantly less blood loss and a short postoperative hospital stay[19]. Moreover, TLS + devascularization is a minimally invasive procedure, which ensures the therapeutic effect while speeding up the postoperative recovery of the patients. In terms of safety, we compared the incidence of intraperitoneal hemorrhage, ascitic fluid infection, eating disorders, liver insufficiency, and perioperative death between the two groups. The results showed no statistical difference in the total complication rate between the RG and OG (11.11% vs 7.00%), suggesting that the application of TLS + devascularization does not increase the probability of perioperative complications in PH patients. Chen et al’s comparative study on laparoscopic splenectomy plus selective pericardial devascularization (LSSD) and OS showed that laparoscopic splenectomy combined with selective pericardial revascularization could significantly reduce the probability of complications in patients[20]. This has some implications for our study and the reduction of postoperative complications in PH patients. We assessed three hepatic hemodynamic indexes of the patients. Previous studies have shown that abnormally increased blood vessel diameter, velocity, and flow volume of the portal venous system not only increases the risk of PH progression but also causes portal vein thrombosis, which is associated with an increased risk of adverse events such as abdominal distension, hyperthermia, and gastrointestinal bleeding[21,22]. The results showed that the postoperative blood vessel diameters of the two groups did not change significantly from the preoperative values; however, the postoperative blood flow volumes and velocities of both cohorts decreased significantly. This suggests that TLS + devascularization could significantly reduce the portal blood flow; however, whether minimally invasive or not, both procedures have similar effects on the hemodynamics and can relieve gastric varices. Deibert et al[23] suggested that if the positive effect on hepatic hemodynamics can be maintained long-term and in a stable manner, it could prevent patients from having further variceal bleeding, which plays an important role in the long-term survival of patients with PH. However, this study did not assess the long-term hepatic hemodynamics of the patients, which should be explored further in future research. Finally, we tested the liver function indexes of patients. The ALT, AST, and TBil are indicators of pathological changes in liver function. Abnormal elevations in these three markers are related to liver function damage. With effective intervention, the abnormal increase in these three indexes can be effectively reduced to achieve torsion and improvement of the liver tissue lesions[24]. The postoperative ALT, AST, and TBil values in the OG did not differ statistically from the preoperative values. Similarly, no significant differences were observed between the OG and RG after surgery, indicating that TLS + devascularization had a limited effect on liver function recovery in PH patients when compared with OS. Thus, based on our research, we highly recommend performing TLS + devascularization in the following patient populations: (1) Patients with a poor clinical response after treatment with drugs and endoscopy, with poorly controlled clinical symptoms such as variceal bleeding, or at a high risk of variceal bleeding; (2) Patients with obvious abdominal distension that is indicative of compression of splenomegaly on the abdominal organs, with significantly reduced quality of life; and (3) Patients with severe hypersplenism that affects other treatment indications
Nevertheless, the decision on surgical interventions should be made considering both the specific medical conditions and the patient’s needs.
This study confirms that TLS + devascularization in PH patients has the same clinical effect as that of OS, despite having longer OT and lesser intraoperative bleeding volume. In the future, the development of surgical methods should be the goal to further enrich and promote the treatment of PH. Meanwhile, the long-term examinations of liver function and hepatic hemodynamics of postoperative patients in future studies are warranted to understand the long-term effects of these two surgical modalities on them.
In addition to the resulting adverse effects on portal blood entering the liver that lead to decreased liver function in patients, portal hypertension (PH) can also induce liver hemodynamic changes that are closely related to many complications, warranting more clinical attention to this disease.
To help people gain a better understanding of the clinical effect of total laparoscopic splenectomy (TLS) in the treatment of PH.
The clinical effect of TLS on PH and its effect on liver hemodynamics and liver function are analyzed through case discussion and literature review.
The clinical efficacy, surgical indexes, safety, liver hemodynamics, and liver function were compared between the observation group (n = 100) receiving TLS and the reference group (n = 99) receiving open splenectomy.
Although the operation time was significantly longer compared with the reference group, the overall response rate was significantly higher and the intraoperative blood loss and incidence of postoperative complications were significantly lower in the observation group. The detection of liver hemodynamics and liver function revealed significantly lower liver hemodynamics (blood vessel diameter, blood flow velocity and blood flow volume) and liver function indexes in the observation group vs the reference group 2 wk after surgery.
For the treatment of PH, TLS is significantly better than open splenectomy in clinical efficacy, reducing the risk of postoperative complications in patients and improving their liver hemodynamics and liver function.
In addition to clinical efficacy, we believe that future research and exploration of PH could also focus on the influence on liver hemodynamics and liver function, so as to further screen and optimize the clinical treatment of PH and improve patient outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nio M, Japan; Sakamoto E, Brazil S-Editor: Yan JP L-Editor: A P-Editor: Ji MX
| 1. | Huang HC, Tsai MH, Chang CC, Pun CK, Huang YH, Hou MC, Lee FY, Hsu SJ. Microbiota transplants from feces or gut content attenuated portal hypertension and portosystemic collaterals in cirrhotic rats. Clin Sci (Lond). 2021;135:2709-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Kristensen H, Kimer N, Møller S. Indications and methods for measuring portal hypertension in cirrhosis. Scand J Gastroenterol. 2022;57:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Huang HC, Ho HL, Chang CC, Chuang CL, Pun CK, Lee FY, Huang YH, Hou MC, Hsu SJ. Matrix metalloproteinase-9 inhibition or deletion attenuates portal hypertension in rodents. J Cell Mol Med. 2021;25:10073-10087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Ishikawa T. Efficacy of interventional radiology in the management of portal hypertension: A narrative review. Medicine (Baltimore). 2022;101:e30018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 5. | Kimer N, Wiese S, Mo S, Møller S, Bendtsen F. Advances in the treatment of portal hypertension in cirrhosis. Expert Rev Gastroenterol Hepatol. 2016;10:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Zhang XF, Liu Y, Li JH, Lei P, Zhang XY, Wan Z, Lei T, Zhang N, Wu XN, Long ZD, Li ZF, Wang B, Liu XM, Wu Z, Chen X, Wang JX, Yuan P, Li Y, Zhou J, Pawlik M, Lyu Y. [Effect of splenectomy on the risk of hepatocellular carcinoma development among patients with liver cirrhosis and portal hypertension: a multi-institutional cohort study]. Zhonghua Wai Ke Za Zhi. 2021;59:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Dragomir MP, Tudor S, Lacatus M, Stanciulea O, Trandafir B, Diaconu A, Coriu D, Colita A, Droc G, Purnichescu-Purtan R, Calin G, Vasilescu C. TNF-alpha releasing capacity of the whole blood drops after open total splenectomy, but increases after partial/subtotal or minimally invasive splenectomy. Acta Chir Belg. 2022;122:346-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Zheng X, Liu Q, Yao Y. Laparoscopic splenectomy and esophagogastric devascularization is a safe, effective, minimally invasive alternative for the treatment of portal hypertension with refractory variceal bleeding. Surg Innov. 2013;20:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Wang X, Li Y, Crook N, Peng B, Niu T. Laparoscopic splenectomy: a surgeon's experience of 302 patients with analysis of postoperative complications. Surg Endosc. 2013;27:3564-3571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Luo HP, Zhang ZG, Long X, Liu FL, Chen XP, Zhang L, Zhang WG. Combined Laparoscopic Splenectomy and Esophagogastric Devascularization versus Open Splenectomy and Esophagogastric Devascularization for Portal Hypertension due to Liver Cirrhosis. Curr Med Sci. 2020;40:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 638] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 12. | Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery; Chinese Medical Association. [Expert consensus on diagnosis and treatment of esophagogastric variceal bleeding in cirrhotic portal hypertension (2019 edition)]. Zhonghua Wai Ke Za Zhi. 2019;57:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery; Chinese Medical Association. [Expert consensus on clinical diagnosis and treatment of portal hypertension with hepatocellular carcinoma (2022)]. Zhonghua Wai Ke Za Zhi. 2022;60:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Han B, Tang H, Liang Q, Zhu M, Xie Y, Chen J, Li Q, Jia J, Li Y, Ren Z, Cong D, Yu X, Sui D, Pei J. Preparation of long-acting microspheres loaded with octreotide for the treatment of portal hypertensive. Drug Deliv. 2021;28:719-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, Noureddin M, Pyko M, Shiffman M, Sanyal A, Allgood A, Shlevin H, Horton R, Zomer E, Irish W, Goodman Z, Harrison SA, Traber PG; Belapectin (GR-MD-02) Study Investigators. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension. Gastroenterology. 2020;158:1334-1345.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 16. | Cheng Z, Li JW, Chen J, Fan YD, Guo P, Zheng SG. Therapeutic effects of laparoscopic splenectomy and esophagogastric devascularization on liver cirrhosis and portal hypertension in 204 cases. J Laparoendosc Adv Surg Tech A. 2014;24:612-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Qi R, Jin X, Shi H, Wang C, Li H, Shi X. Effect of laparoscopic splenectomy on portal vein thrombosis and serum YKL-40 in patients with cirrhotic portal hypertension. Ann Hepatol. 2019;18:898-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Lin J, Liu Q, Liang Z, He W, Chen J, Ma J, Gu C, Wang W. Laparoscopic selective esophagogastric devascularization and splenectomy for patients with cirrhotic portal hypertension. Wideochir Inne Tech Maloinwazyjne. 2019;14:187-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Zheng S, Sun P, Liu X, Li G, Gong W, Liu J. Efficacy and safety of laparoscopic splenectomy and esophagogastric devascularization for portal hypertension: A single-center experience. Medicine (Baltimore). 2018;97:e13703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Chen H, Yang F, Li TT, Zhang KN, Sun ZG, Yu CZ, Sun Y. Comparison of Efficacy of Laparoscopic and Open Splenectomy Combined With Selective and Nonselective Pericardial Devascularization in Portal Hypertension Patients. Surg Laparosc Endosc Percutan Tech. 2018;28:401-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Turco L, Garcia-Tsao G. Portal Hypertension: Pathogenesis and Diagnosis. Clin Liver Dis. 2019;23:573-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Deibert P, Lazaro A, Stankovic Z, Schaffner D, Rössle M, Kreisel W. Beneficial long term effect of a phosphodiesterase-5-inhibitor in cirrhotic portal hypertension: A case report with 8 years follow-up. World J Gastroenterol. 2018;24:438-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 24. | Li C, Meng M, Guo M, Wang M, Ju A, Wang C. The polysaccharides from Grifola frondosa attenuate CCl(4)-induced hepatic fibrosis in rats via the TGF-β/Smad signaling pathway. RSC Adv. 2019;9:33684-33692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |