Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1673
Peer-review started: May 6, 2023
First decision: May 19, 2023
Revised: May 31, 2023
Accepted: June 21, 2023
Article in press: June 21, 2023
Published online: August 27, 2023
Processing time: 111 Days and 8.2 Hours
The ratio of lymphocytes to monocytes (LMR) has been shown to be an effective predictor of gastric cancer prognosis. However, its predictive accuracy for signet ring gastric cancer is currently not well understood.
To evaluate the prognosis predictive accuracy of preoperative LMR in signet ring gastric cancer.
A total of 212 signet ring gastric cancer patients admitted at the Xiangya Hospital of Central South University, Department of Gastrointestinal Surgery, from January 2012 to December 2016 were enrolled in the study. The prognosis predictive accuracy of preoperative LMR was explored based on the area under the receiver operating characteristic. Factors that significantly affect the survival of patients were identified using single factor analysis, and those that were independently associated with signet ring gastric cancer were identified through multivariate analysis.
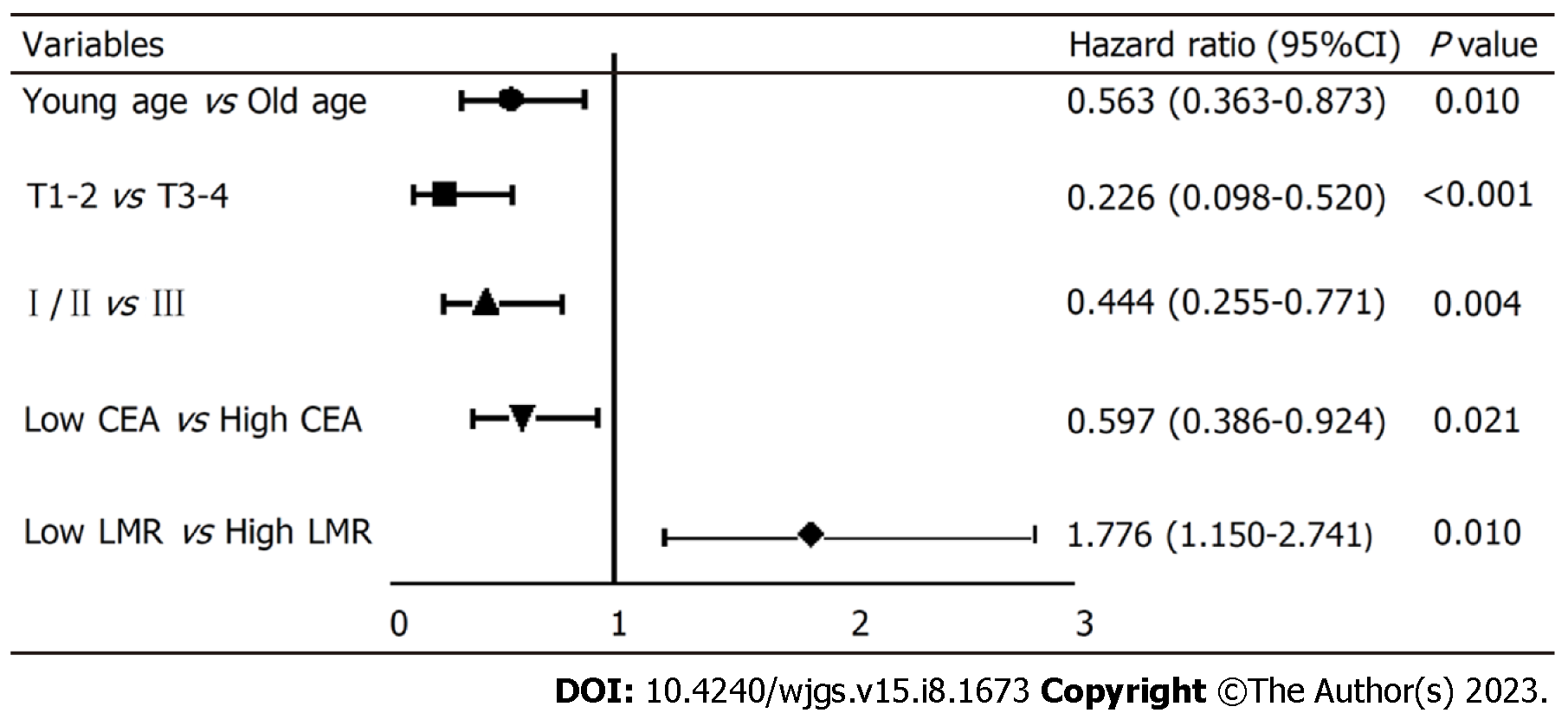
The results of the single factor analysis revealed a strong correlation between the survival of signet ring gastric cancer patients and several factors, including tumor invasion (χ2 = 49.726; P < 0.001), lymph node metastasis (χ2 = 30.269; P < 0.001), pTNM stage (χ2 = 49.322; P < 0.001), surgical approach (χ2 = 8.489; P = 0.004), age (t = -2.213; P < 0.028), carcinoembryonic antigen (CEA) (Z = -3.265; P = 0.001), platelet-to-lymphocyte ratio (Z = -2.196; P = 0.028), LMR (Z = -2.226; P = 0.026), ALB (t = 3.284; P = 0.001), prognostic nutritional index (t = -3.789; P < 0.001) and FIB (Z = -3.065; P = 0.002). Furthermore, the multivariate analysis further demonstrated that age (HR: 0.563, 95%CI: 0.363-0.873), tumor invasion depth (HR: 0.226, 95%CI: 0.098-0.520), pTNM stage (HR: 0.444, 95%CI: 0.255-0.771), preoperative CEA level (HR: 0.597, 95%CI: 0.386-8.790), and preoperative LMR level (HR: 1.776, 95%CI: 1.150-2.741) were independent factors influencing the prognosis of signet ring gastric cancer.
In signet ring gastric cancer patients, a low preoperative LMR level predicts poor prognosis. The death risk ratio of the low LMR group compared to the high LMR group is 1.776.
Core Tip: Low preoperative lymphocytes to monocytes levels -predict poor prognosis of patients with signet ring gastric cancer, making it a valuable prognostic factor.
- Citation: Liu HL, Feng X, Tang MM, Zhou HY, Peng H, Ge J, Liu T. Prognostic significance of preoperative lymphocyte to monocyte ratio in patients with signet ring gastric cancer. World J Gastrointest Surg 2023; 15(8): 1673-1683
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1673.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1673
Signet ring gastric cancer is a type of stomach cancer that is characterized by presence of cells filled with mucus, which push the nucleus to one side of the cell, giving it a ring-like appearance. This type of cancer is highly invasive, progresses rapidly, and has a high degree of malignancy. Although the incidence of gastric cancer has decreased in recent decades, cases of signet-ring cell carcinoma (SRCC) are increasingly being reported. Studies have demonstrated that SRCC accounts for 35 % to 45 % of all cases of gastric adenocarcinoma[1], and its incidence increased by tenfold from 1970 to 2000[2].
Currently, the prognosis of SRCC is not well understood. Given that SRCC is prone to lymph node and peritoneal metastasis, less responsive to chemotherapy, and most patients are diagnosed at an advanced cancer stage, patients with SRCC have a poor prognosis[2].
The occurrence and development of tumors are driven by several factors including inflammatory immune response of the host[3]. Numerous studies have explored the relationship between different inflammatory markers, chemotherapeutic effects, and prognosis in gastric cancer. Among the most easily available inflammatory markers obtained from the whole blood cell count are lymphocyte-to-monocyte ratio (LMR)[4], neutrophil-to-lymphocyte ratio (NLR)[5], platelet-to-lymphocyte ratio (PLR)[6], and systemic immune inflammation (SII)[7]. The prognostic nutritional index (PNI), a simple and easy detection index, has been widely used in clinical practice and shown to be associated with the prognosis of malignant gastric tumors[8,9]. Moreover, the development of tumors is accompanied by changes in the blood coagulation dynamics of the host[10]. Coagulation factor levels, such as platelet count, international standard ratio, fibrin degradation products, fibrinogen, and D-dimer levels, have been associated with tumor stage, metastasis, chemotherapeutic effect, and prognosis of patients with solid tumors[11,12]. Although many scientists have explored the relationship between various indicators and chemotherapy response and prognosis of gastric cancer, few studies have explored the prognostic value of these indicators in SRCC.
Against this background, we explored the relationship between the common inflammatory indicators, nutritional indicators, coagulation indicators and the prognosis of SRCC to identify prognostic predictors of SRCC.
The retrospective study included 212 patients with gastric cancer admitted to the Department of Gastroenterology at Xiangya Hospital of Central South University from January 2012 to December 2016. To be included in the study, patients had to meet the following criteria: (1) Postoperative pathology revealed SRCC components greater than 10%; (2) Accepted to undergo radical gastrectomy; and (3) With complete clinical and follow-up data. Moreover, the exclusion criteria are as follows: (1) Preoperative radiotherapy, chemotherapy, targeted therapy, immunotherapy, and other anti-tumor treatments that may affect the patient's blood routine, liver and kidney function, and coagulation routine; (2) Preoperative examination indicated the presence of distant metastases such as liver, lung, and bone metastases; (3) Intraoperative detection of metastasis; (4) Comorbidity hematological diseases and other systemic malignancies; (5) Combined with severe infections, liver disease, kidney disease, and autoimmune diseases; (6) Emergency surgery due to perforation and bleeding of gastric cancer; and (7) Gastric stump cancer. This study was approved by the Ethics Committee of Xiangya Hospital of Central South University.
Patient-related results, including demographic data, clinic characteristics, and biochemical test results such as carcinoembryonic antigen (CEA), LMR [= lymphocyte count (× 109/L)/monocyte count (× 109/L)], NLR [= Neutrophil count (× 109/L)/Lymphocyte count (× 109/L)], PLR [= platelet count (× 109/L)/Lymphocyte coun (× 109/L)], SII [= Neutrophil count (× 109/L) × Platelet count (× 109/L)/Lymphocyte count (× 109/L)][13], coagulation index [activated partial thromboplastin time (APTT), fibrinogen degradation product (FDP), fibrinogen (FIB), prothrombin time (PT), thrombin time (TT), and international normalized ratio (INR)] were obtained from the medical database of the hospital. Additionally, albumin (ALB), globulin (GLB), albumin to globulin ratio [AGR = serum albumin (g/L)/serum globulin (g/L)], and prognostic nutritional index [PNI = 5 × Lymphocyte count (× 109/L)+serum albumin (g/L)][14] were obtained through peripheral complete blood count and blood biochemistry before the surgery. Cut-off values for each variable were obtained from the receiver operating characteristic (ROC) curves.
Statistical analysis was performed using SPSS 25.0 and GraphPad Prism 8.0. The normality of the data was assessed using the Shapiro-Wilk test, and the data which did not fit the normal distribution was represented by M (P25, P75) and analyzed using the Mann-Whitney U test. Single factor analysis was performed using the independent-sample t-test for normally distributed data, and the counts were presented as percentages (%). The prognostic value of inflammation, blood coagulation and other indicators was evaluated using ROC curves, and the optimal cut-off point of patient survival was determined. The patients were then divided into high and low groups based on the medium levels of the above indicators. Kaplan-Meier survival analysis and the Log-rank tests were used to compare survival rates between the high and low-risk groups. Cox proportional hazards regression analysis was conducted to identify independent predictors of the prognosis of gastric cancer with SRCC by including indicators that significantly affected the survival status. Statistical significance was set at P < 0.05.
The study included 212 patients with SRCC, of whom 87 patients (41.04 %) died, and 125 patients (58.96 %) survived for the 5-year follow-up period. The mean age of the patients was 51.42 ± 11.27 years, 117 were males (55.19 %), and 95 were females (44.81 %). The tumor location was in the upper, middle and lower third of the stomach in 5 (2.36 %), 58 (27.36 %) and 149 (70.28 %) cases, respectively. Distal gastrectomy was performed in 167 (78.78 %) cases, while total gastrectomy was performed in 45 (21.22 %) cases. The tumor infiltration depth was pT1 or pT2 in 79 cases (37.26 %) and pT3 or pT4 in 133 cases (62.74 %). Lymph node metastasis was found in 118 (55.66 %) cases with pN1, pN2 or pN3, while 94 (44.34 %) cases were classified as N0. The pTNM stage was I or II in 117 cases (55.19 %) and III in 95 (44.81 %) cases (Table 1).
| Characteristics | n (%) |
| Gender | |
| Male | 117 (55.19) |
| Female | 95 (44.81) |
| Tumor site | |
| Upper third | 5 (2.36) |
| Middle third | 58 (27.36) |
| Lower third | 149 (70.28) |
| pT | |
| pT1-2 | 79 (37.26) |
| pT3-4 | 133 (62.74) |
| pN | |
| pN0 | 94 (44.34) |
| pN1-3 | 118 (55.66) |
| pTNM stage | |
| Ⅰ/Ⅱ | 117 (55.19) |
| Ⅲ | 95 (44.81) |
| Resection scope | |
| Distal stomach | 167 (78.78) |
| Total stomach | 45 (21.23) |
| Survival status | |
| Survival | 125 (58.96) |
| Dead | 87 (41.04) |
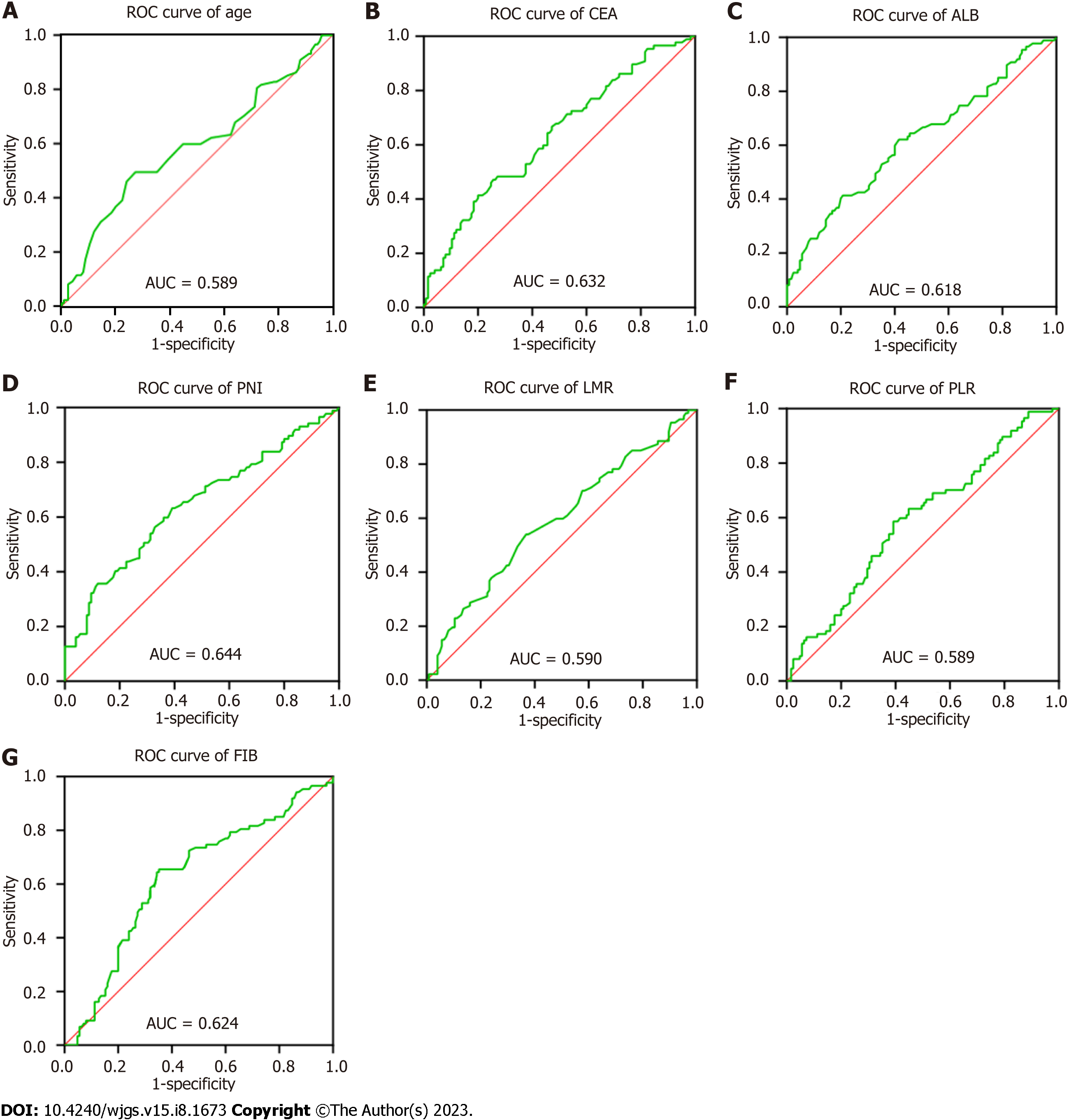
Table 2 shows the cut-off value and area under the curve (AUC) for each index, and Figure 1 shows the ROC curves (AUC, 95%CI) of the predictors, including age, CEA, PLR, LMR, ALB, PNI and FIB. Overall, the results indicate good predictive values of CEA (0.632, 0.556-0.708), LMR (0.590, 0.512-0.669), ALB (0.618, 0.540-0.696), PNI (0.644, 0.567-0.721), and FIB (0.624, 0.547-0.701).
| Variables | AUC (95%CI) | P value | Cut-off value | Sensitivity | Specificity |
| Age | 0.589 (0.509-0.669) | 0.028 | 55.5 | 0.494 | 0.728 |
| PLR | 0.589 (0.511-0.666) | 0.028 | 124.63 | 0.586 | 0.608 |
| LMR | 0.590 (0.512-0.669) | 0.025 | 3.83 | 0.54 | 0.632 |
| ALB | 0.618 (0.540-0.696) | 0.004 | 38.95 | 0.414 | 0.792 |
| PNI | 0.644 (0.567-0.721) | < 0.001 | 49.85 | 0.632 | 0.608 |
| FIB | 0.624 (0.547-0.701) | 0.002 | 3.115 | 0.655 | 0.648 |
| CEA | 0.632 (0.556-0.708) | 0.001 | 1.455 | 0.471 | 0.744 |
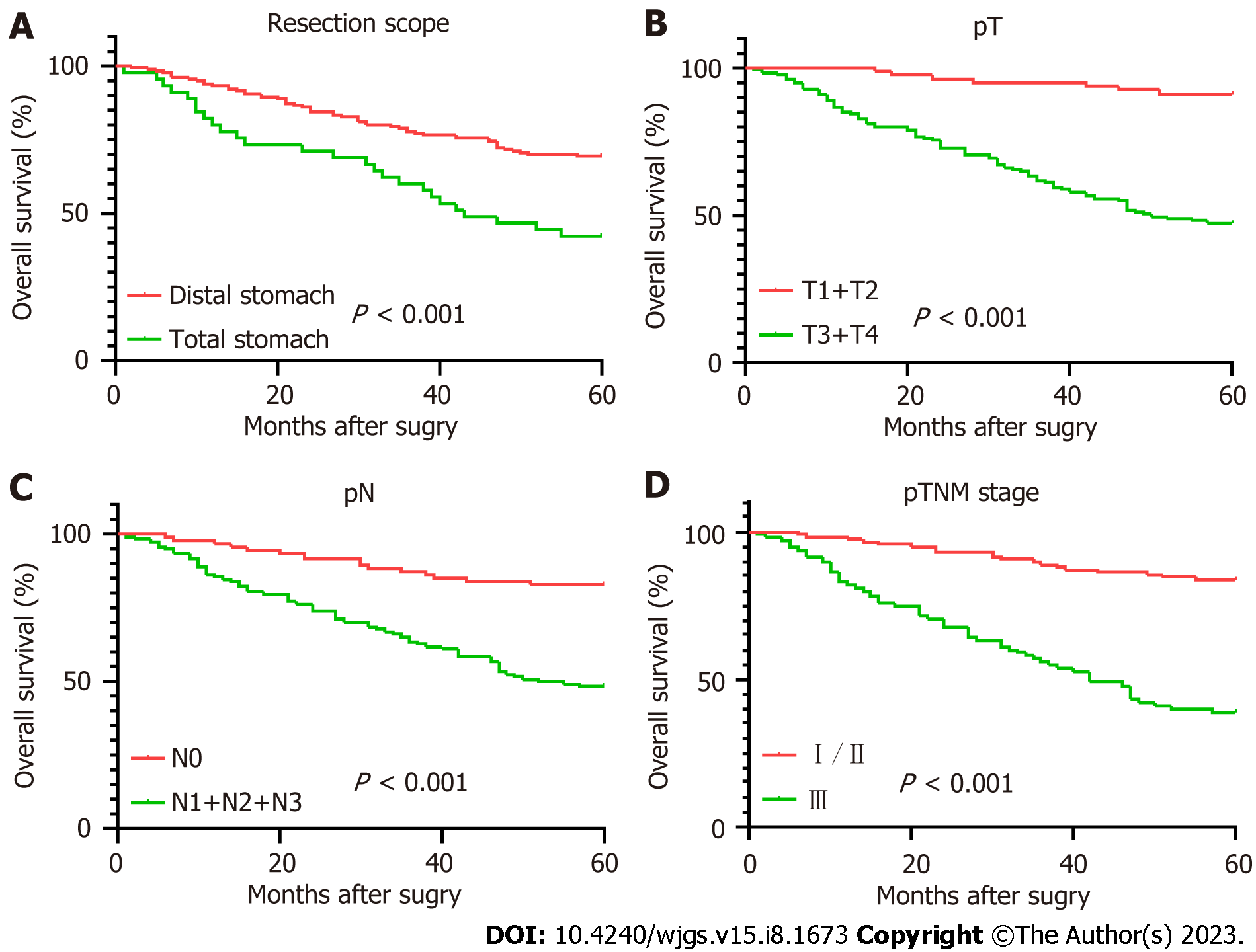
Table 3 displays the results of the pathological features and the 5-year survival rate of the patients. The analysis revealed that depth of tumor invasion, lymph node metastasis, pTNM stage and resection range were associated with the survival rate, while gender and tumor location showed no correlation.
| Group | Survival status | 5-OS (%) | χ2 | P value | |
| Survival (n) | Dead (n) | ||||
| Gender | 1.27 | 0.26 | |||
| Male | 73 | 44 | 62.39 | ||
| Female | 52 | 43 | 54.74 | ||
| Tumor site | 3.556 | 0.169 | |||
| Upper third | 1 | 4 | 20 | ||
| Middle third | 33 | 25 | 56.9 | ||
| Lower third | 91 | 58 | 61.07 | ||
| pT | 49.726 | < 0.001 | |||
| pT1-2 | 71 | 8 | 89.87 | ||
| pT3-4 | 54 | 79 | 40.6 | ||
| pN | 30.269 | < 0.001 | |||
| pN0 | 75 | 19 | 79.79 | ||
| pN1-3 | 50 | 68 | 42.37 | ||
| pTNM stage | 49.322 | < 0.001 | |||
| Ⅰ/Ⅱ | 94 | 23 | 80.34 | ||
| Ⅲ | 31 | 64 | 32.63 | ||
| Resection scope | 8.489 | 0.004 | |||
| Distal stomach | 107 | 60 | 64.07 | ||
| Total stomach | 18 | 27 | 40 | ||
As shown in Table 4, firstly, the single factor analysis demonstrated that younger patients had a better survival rate compared with older patients. The survival rate was also significantly better in the lower CEA level group compared with the higher CEA level group. In addition, SRCC patients in the higher PLR value had poorer survival rates than those in the lower PLR value group, while SRCC patients in the higher LMR value group had a better survival rate than those in the lower LMR value group. Finally, the survival rate of patients with lower FIB was significantly better in those with higher FIB, whereas patients with lower ALB had a significantly lower survival rate compared with higher ALB group.
| Variables | Survival | Dead | t/Z value | P value |
| Age | 50 ± 10.82 | 53 ± 11.65 | -2.213 | 0.028 |
| CEA | 0.90 (0.58, 1.48) | 1.21 (0.75, 2.35) | -3.265 | 0.001 |
| NLR | 1.82 (1.30, 2.43) | 2.05 (1.45, 2.71) | -1.668 | 0.095 |
| PLR | 118.33 (99.41, 150.18) | 130.59 (108.10, 162.00) | -2.196 | 0.028 |
| LMR | 4.20 (3.40, 5.67) | 3.80 (2.80, 5.00) | -2.226 | 0.026 |
| SII | 392.36 (275.38, 565.21) | 448.00 (311.67, 600.71) | -1.391 | 0.164 |
| PT | 12.40 (11.96, 12.90) | 12.30 (12.00, 12.70) | -1.114 | 0.265 |
| INR | 0.97 (0.93, 1.02) | 0.95 (0.93, 0.99) | -1.728 | 0.084 |
| APTT | 32.71 ± 4.16 | 31.90 ± 3.98 | 1.425 | 0.156 |
| TT | 17.55 ± 1.73 | 17.66 ± 1.85 | -0.424 | 0.672 |
| FIB | 2.93 (2.56, 3.42) | 3.29 (2.81, 3.80) | -3.065 | 0.002 |
| ALB (g/L) | 41.79 ± 3.83 | 40.09 ± 4.72 | 2.854 | 0.005 |
| GLB (g/L) | 26.00 (23.80, 29.40) | 25.20 (22.80, 28.50) | -1.356 | 0.175 |
| AGR | 1.58 (1.41, 1.80) | 1.56 (1.40, 1.75) | -0.716 | 0.474 |
| PNI | 51.11 ± 4.95 | 48.21 ± 6.15 | 3.789 | < 0.001 |
The predictors, including age, CEA, ALB, PNI, LMR, PLR, and FIB, were grouped as shown in Table 5.
| Survival (n) | Dead (n) | 5-OS (%) | Total | ||
| Age | < 56 | 91 | 44 | 67.41 | 135 |
| ≥ 56 | 34 | 43 | 44.16 | 77 | |
| CEA (ng/mL) | < 1.455 | 93 | 46 | 66.91 | 139 |
| ≥ 1.455 | 32 | 41 | 43.84 | 73 | |
| PLR | < 124.63 | 76 | 36 | 67.86 | 112 |
| ≥ 124.63 | 49 | 51 | 49 | 100 | |
| LMR | < 3.83 | 81 | 30 | 72.97 | 111 |
| ≥ 3.83 | 44 | 57 | 43.56 | 101 | |
| ALB (g/L) | < 38.95 | 26 | 36 | 41.94 | 62 |
| ≥ 38.95 | 99 | 51 | 66 | 150 | |
| PNI | < 49.85 | 49 | 55 | 47.12 | 104 |
| ≥ 49.85 | 76 | 32 | 70.37 | 108 | |
| FIB (g/L) | < 3.115 | 46 | 47 | 49.46 | 93 |
| ≥ 3.115 | 79 | 40 | 66.39 | 119 |
The Kaplan-Meier survival curves are shown in Figure 2. The Log-rank test indicated no significant difference in survival rate between the low PLR group and the high PLR group (P = 0.147). In contrast, significant differences were observed in survival rate between different resection groups (P < 0.001), depth of invasion (P < 0.001), lymph node metastasis (P < 0.001), pTNM stage (P < 0.001), age (P = 0.004), LMR (P = 0.003), ALB (P = 0.008), PNI (P = 0.002) and FIB (P = 0.001).
To explore the independent factors affecting the prognosis of SRCC, indicators that demonstrated statistical differences by the Log-rank test were included in a Cox proportional hazards regression model for multivariate analysis. The results (HR, 95%CI) are presented in Figure 3. The independent factors for SRCC prognosis were age (0.563, 0.363-0.873, P = 0.010), depth of tumor invasion (0.226, 0.098-0.520), pTNM stage (0.444, 0.255-0.771), preoperative CEA level (0.597, 0.386-8.790), and preoperative LMR level (1.776, 1.150-2.741). Advanced age, high CEA level before surgery, low LMR level before surgery, deep tumor invasion, and late pTNM stage were all indicative of a relatively poor prognosis. Specifically, the risk of death in low LMR group before surgery was 1.776 times higher than that of the high LMR group.
Currently, surgery is the mainstay treatment for gastric cancer patients, especially those with SRCC. However, despite radical resection or adjuvant chemotherapy, the prognosis of SRCC patients, particularly those in advanced stages, is not optimistic. Therefore, it is crucial to elucidate the mechanism of tumor progression and identify independent prognostic factors to evaluate the overall condition of tumor patients and optimize diagnosis and treatment.
The correlation between inflammation and tumors was first proposed by Rudolf Virchow[14]. Research has shown that inflammation participates in tumor development[15,16]. Furthermore, inflammation can influence the prognosis of tumors by altering immune response[17,18].
Lymphocytes and monocytes play a crucial role in anti-tumor immune response[19]. The relationship between LMR and the prognosis of malignant tumors has been widely reported[20-22]. However, few studies have investigated the relationship between inflammatory markers and SRCC. Chengcheng Tong et al[23], reported that derived monocyte-to-lymphocyte ratio (dMLR) could independently predict lymph node metastasis of SRCC. Zhu et al[9] reported the relationship between SII and the prognosis of SRCC, but the relationship between LMR and SRCC has not been investigated.
Numerous studies have shown that high levels of peripheral blood lymphocyte count and TILs are associated with a good prognosis in gastric cancer[24-26]. Li et al[27] reported that patients with smaller tumors (< 5 cm) had higher counts of peripheral blood CD4 + T cells (P = 0.003) and CD8 + T cells (P = 0.002). In addition, patients with well-differentiated gastric cancer showed higher counts of CD4 + T cells (P = 0.029).
NK cells, which possess potent anti-tumor, anti-viral and antibacterial activity, are crucial in activating and regulating adaptive immune responses. In human peripheral blood, NK cells account for approximately 3%-5% of lymphocytes[28,29]. The anti-tumor activity of NK cells is mainly determined by a group of inhibitory and activating receptors[30]. Patients with gastric cancer exhibit lower expression of activating receptors such as NKG2D, NKp30, and NKp46, but higher PD-1 expression. Moreover, NK cells of patients with gastric cancer secrete lower cytokines (IFN-γ, IL-2, TNF-α, IL-12) and impaired ability to release perforin and granzyme. Meanwhile, gastric cancer cells express little MICA/B, ULBP, and B7H6, to evade NK cell-mediated innate immunity. Gastric cancer cells can also produce cytokines such as IL-10, TGF-β, and PGE2, which recruit MDSC and Treg cells to suppress NK cell function[31]. The proportion of apoptotic NK cells in patients with gastric cancer is elevated when receiving gastrectomy[32]. Collectively, these lines of evidence show that the number and function of NK cells decrease sharply with the progression of gastric cancer[31].
Monocytes, particularly those that differentiate into tumor-associated macrophages (TAMs), contribute to the development of gastric cancer[33]. M1 TAMs have anti-tumor effects, while M2 TAMs promote tumor growth[34]. Under the influence of cytokines and extracellular matrix secreted by tumour cells and lymphocytes, M1 TAMs can convert into M2 TAMs. In gastric cancer, M2 TAMs are highly expressed in SRCC, mucinous adenocarcinoma and diffuse gastric cancer[35].
Our study found that the 5-year survival rate of patients with low LMR before surgery was significantly lower than that with high LMR. In summary LMR affects the prognosis of SRCC due to reduction of lymphocytes during inflammatory response and increase of tumor-associated macrophages produced by circulating monocytes. However, this study has some limitations. First, this was a retrospective single-center study and no external validation was performed. In addition, studies have suggested that gastric cancer with different SRCC ratios may have varying biological characteristics, although we demonstrated a relationship between LMR and the prognosis of SRCC. Therefore, it is imperative to explore further the predictive value of various indicators in gastric cancer with different SRCC ratios.
In summary, this study shows that a low preoperative LMR level indicates a poor prognosis of signet ring gastric cancer. Particularly, compared with the high LMR group, the risk of mortality in the low LMR group is 1.776.
The incidence of signet ring gastric carcinoma has increased among the past decades. Several inflammation indexes, including ratio of lymphocytes to monocytes (LMR), have been shown to be effective predictors of gastric cancer prognosis.
The predictive accuracy of ratio of LMR for signet ring gastric cancer is unclear now.
To assess the prognosis predictive accuracy of preoperative LMR for signet ring gastric cancer.
Our research center conducted a retrospective analysis of clinical data from patients diagnosed with signet ring gastric carcinoma over the past 5 years, identifying factors that significantly affect patients’ survival by using single factor analysis, and deciding independent prognostic factors related to signet ring cell gastric cancer by using multivariate analysis.
The results of the single factor analysis indicated a strong correlation between the survival of signet ring gastric cancer patients and several factors, including tumour invasion, lymph node metastasis, pTNM stage, surgical approach, age, carcinoembryonic antigen (CEA), platelet-to-lymphocyte ratio (PLR), LMR, ALB, PNI and FIB. Furthermore, the multivariate analysis revealed that age, tumor invasion depth, pTNM stage, preoperative CEA level, and preoperative LMR level were independent factors related to the prognosis of signet ring gastric cancer.
In signet ring gastric cancer patients, a low preoperative LMR level is indicative of a poor prognosis. The death risk ratio of the low LMR group compared to the high LMR group is 1.776.
The study subjects were followed up for 5 years and divided into survival group and death group. Clinical data, pathological data, and prognosis of the two groups of patients were observed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Toxicology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al Zoubi M, Qatar; Kermansaravi M, Iran S-Editor: Ma YJ L-Editor: A P-Editor: Zhang YL
| 1. | Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, Brennan MF, Coit DG, Strong VE. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 831] [Article Influence: 166.2] [Reference Citation Analysis (0)] |
| 4. | Pan YC, Jia ZF, Cao DH, Wu YH, Jiang J, Wen SM, Zhao D, Zhang SL, Cao XY. Preoperative lymphocyte-to-monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine (Baltimore). 2018;97:e13896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 6. | Zhang X, Zhao W, Yu Y, Qi X, Song L, Zhang C, Li G, Yang L. Clinicopathological and prognostic significance of platelet-lymphocyte ratio (PLR) in gastric cancer: an updated meta-analysis. World J Surg Oncol. 2020;18:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Cao X, Xue J, Yang H, Han X, Zu G. Association of Clinical Parameters and Prognosis with the Pretreatment Systemic Immune-inflammation Index (SII) in Patients with Gastric Cancer. J Coll Physicians Surg Pak. 2021;31:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Ma LX, Taylor K, Espin-Garcia O, Anconina R, Suzuki C, Allen MJ, Honorio M, Bach Y, Allison F, Chen EX, Brar S, Swallow CJ, Yeung J, Darling GE, Wong R, Kalimuthu SN, Jang RW, Veit-Haibach P, Elimova E. Prognostic significance of nutritional markers in metastatic gastric and esophageal adenocarcinoma. Cancer Med. 2021;10:199-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Zhu Y, Fan L, Geng X, Li J. The predictive value of the prognostic nutritional index to postoperative prognosis and nursing intervention measures for colorectal cancer. Am J Transl Res. 2021;13:14096-14101. [PubMed] |
| 10. | Gil-Bernabé AM, Lucotti S, Muschel RJ. Coagulation and metastasis: what does the experimental literature tell us? Br J Haematol. 2013;162:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Kanda M, Tanaka C, Kobayashi D, Mizuno A, Tanaka Y, Takami H, Iwata N, Hayashi M, Niwa Y, Yamada S, Fujii T, Sugimoto H, Murotani K, Fujiwara M, Kodera Y. Proposal of the Coagulation Score as a Predictor for Short-Term and Long-Term Outcomes of Patients with Resectable Gastric Cancer. Ann Surg Oncol. 2017;24:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Jomrich G, Paireder M, Kristo I, Baierl A, Ilhan-Mutlu A, Preusser M, Asari R, Schoppmann SF. High Systemic Immune-Inflammation Index is an Adverse Prognostic Factor for Patients With Gastroesophageal Adenocarcinoma. Ann Surg. 2021;273:532-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 13. | Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001-1005. [PubMed] |
| 14. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5762] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 15. | Mantovani A. Cancer: inflammation by remote control. Nature. 2005;435:752-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47062] [Article Influence: 3361.6] [Reference Citation Analysis (5)] |
| 17. | Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and inflammation: new insights into breast cancer development and progression. Am Soc Clin Oncol Educ Book. 2013;33:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Moore MM, Chua W, Charles KA, Clarke SJ. Inflammation and cancer: causes and consequences. Clin Pharmacol Ther. 2010;87:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, Joehrens K, Warth A, Renner M, Mehrabi A, Hafezi M, Thelen A, Schirmacher P, Weichert W. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013;109:2665-2674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 21. | Trinh H, Dzul SP, Hyder J, Jang H, Kim S, Flowers J, Vaishampayan N, Chen J, Winer I, Miller S. Prognostic value of changes in neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) for patients with cervical cancer undergoing definitive chemoradiotherapy (dCRT). Clin Chim Acta. 2020;510:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Li C, Zhang H, Li S, Zhang D, Li J, Dionigi G, Liang N, Sun H. Prognostic Impact of Inflammatory Markers PLR, LMR, PDW, MPV in Medullary Thyroid Carcinoma. Front Endocrinol (Lausanne). 2022;13:861869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Tong C, Wang W, Xia Y, He C. A potential novel biomarker in predicting lymph node metastasis of gastric signet ring cell carcinoma: A derived monocyte to lymphocyte ratio. Am J Surg. 2022;223:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Zhang D, He W, Wu C, Tan Y, He Y, Xu B, Chen L, Li Q, Jiang J. Scoring System for Tumor-Infiltrating Lymphocytes and Its Prognostic Value for Gastric Cancer. Front Immunol. 2019;10:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 25. | Feng F, Zheng G, Wang Q, Liu S, Liu Z, Xu G, Wang F, Guo M, Lian X, Zhang H. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. 2018;18:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 26. | Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 27. | Li F, Sun Y, Huang J, Xu W, Liu J, Yuan Z. CD4/CD8 + T cells, DC subsets, Foxp3, and IDO expression are predictive indictors of gastric cancer prognosis. Cancer Med. 2019;8:7330-7344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 28. | Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2338] [Cited by in RCA: 2876] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 29. | Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 541] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 30. | Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019;79:4557-4566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 2137] [Article Influence: 356.2] [Reference Citation Analysis (2)] |
| 31. | Du Y, Wei Y. Therapeutic Potential of Natural Killer Cells in Gastric Cancer. Front Immunol. 2018;9:3095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Wang Z, Si X, Xu A, Meng X, Gao S, Qi Y, Zhu L, Li T, Li W, Dong L. Activation of STAT3 in human gastric cancer cells via interleukin (IL)-6-type cytokine signaling correlates with clinical implications. PLoS One. 2013;8:e75788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Rihawi K, Ricci AD, Rizzo A, Brocchi S, Marasco G, Pastore LV, Llimpe FLR, Golfieri R, Renzulli M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 34. | Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2917] [Article Influence: 194.5] [Reference Citation Analysis (0)] |
| 35. | Räihä MR, Puolakkainen PA. Tumor-associated macrophages (TAMs) as biomarkers for gastric cancer: A review. Chronic Dis Transl Med. 2018;4:156-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |