Published online Aug 27, 2023. doi: 10.4240/wjgs.v15.i8.1663
Peer-review started: March 29, 2023
First decision: April 26, 2023
Revised: May 12, 2023
Accepted: June 12, 2023
Article in press: June 12, 2023
Published online: August 27, 2023
Processing time: 148 Days and 21.6 Hours
Pancreatic adenocarcinoma is currently the fourth leading cause of cancer-related deaths in the United States. In patients with “borderline resectable” disease, current National Comprehensive Cancer Center guidelines recommend the use of neoadjuvant chemoradiation prior to a pancreaticoduodenectomy. Although neoadjuvant radiotherapy may improve negative margin resection rate, it is theorized that its administration increases operative times and complexity.
To investigate the association between neoadjuvant radiotherapy and 30-d morbidity and mortality outcomes among patients receiving a pancreaticoduodenectomy for pancreatic adenocarcinoma.
Patients listed in the 2015-2019 National Surgery Quality Improvement Program data set, who received a pancreaticoduodenectomy for pancreatic adenocarcinoma, were divided into two groups based off neoadjuvant radiotherapy status. Multivariable regression was used to determine if there is a significant correlation between neoadjuvant radiotherapy, perioperative blood transfusion status, total operative time, and other perioperative outcomes.
Of the 11458 patients included in the study, 1470 (12.8%) underwent neoadjuvant radiotherapy. Patients who received neoadjuvant radiotherapy were significantly more likely to require a perioperative blood transfusion [adjusted odds ratio (aOR) = 1.58, 95% confidence interval (CI): 1.37-1.82; P < 0.001] and have longer surgeries (insulin receptor-related receptor = 1.14, 95%CI: 1.11-1.16; P < 0.001), while simultaneously having lower rates of organ space infections (aOR = 0.80, 95%CI: 0.66-0.97; P = 0.02) and pancreatic fistula formation (aOR = 0.50, 95%CI: 0.40-0.63; P < 0.001) compared to those who underwent surgery alone.
Neoadjuvant radiotherapy, while not associated with increased mortality, will impact the complexity of surgical resection in patients with pancreatic adenocarcinoma.
Core Tip: In this retrospective study, we used a national database to investigate the impact that neoadjuvant radiotherapy has on intraoperative and 30-d post-operative outcomes among patients undergoing surgical resection for pancreatic adenocarcinoma. We found that neoadjuvant radiotherapy was associated with longer operative times and the more frequent need for perioperative blood transfusions, but not with increased 30-d mortality. Neoadjuvant radiotherapy was also associated with a lower number of organ space infections and post-operative pancreatic fistula formation. Taken together, the results highlight the challenges that surgeons may face when operating in previously irradiated fields.
- Citation: Aploks K, Kim M, Stroever S, Ostapenko A, Sim YB, Sooriyakumar A, Rahimi-Ardabily A, Seshadri R, Dong XD. Radiation therapy prior to a pancreaticoduodenectomy for adenocarcinoma is associated with longer operative times and higher blood loss. World J Gastrointest Surg 2023; 15(8): 1663-1672
- URL: https://www.wjgnet.com/1948-9366/full/v15/i8/1663.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i8.1663
Pancreatic adenocarcinoma currently represents the fourth leading cause of cancer-related deaths in the United States. In patients suffering from the disease, an R0 resection is the primary and preferred method of treatment[1,2]. While this is often obtainable in patients with early-stage pancreatic malignancies, roughly 85% of patients present with disease that is not amenable to cure with surgical resection alone[3]. The tumors in many of these patients are considered borderline resectable, defined by National Comprehensive Cancer Network (NCCN) guidelines as contact with 180 degrees or less of the superior mesenteric artery or the celiac artery, contact with the common hepatic artery, and/or contact with the superior mesenteric vein/portal vein resulting in contour irregularity or vein thrombosis[4]. According to the NCCN guidelines, such cancers may benefit from treatment with neoadjuvant therapy in the form of chemoradiation[5].
When compared to adjuvant therapy, neoadjuvant chemoradiation has the theoretical benefit of testing for che
Despite the purported benefits, neoadjuvant therapy is still regarded with caution and its use remains low in the United States[13]. It is theorized that neoadjuvant therapy increases the rate of gastrointestinal toxicity and impairs post-operative wound healing, which can result in preoperative decompensation and subsequent post-operative morbidities. Neoadjuvant therapy can also theoretically delay definitive surgical resection, which can lead to the development of metastatic disease in non-responders. Radiotherapy, in particular, has been associated with disruption of the pancreatic tissue planes, which is thought to make eventual surgical resection more difficult[14]. Numerous studies have contradicted these ideas, showing that neoadjuvant therapy results in little to no intra- and post-operative increases in morbidity and mortality[9,10,15]. To date, however, many of these prior studies are plagued by single institution analysis with small sample sizes. These samples are even smaller when looking at the number of participants who received neoadjuvant radiotherapy, as chemotherapy remains the lion’s share of neoadjuvant therapy.
The aim of this study was to investigate the effects that neoadjuvant radiation therapy have on both intra-operative and 30-d postoperative morbidities using a nationwide dataset. We specifically hypothesize that among patients receiving a pancreaticoduodenectomy for pancreatic adenocarcinoma, those that also undergo neoadjuvant radiotherapy are more likely to have longer operative times and a perioperative transfusion compared with those who simply receive surgery alone.
We performed a cross sectional study utilizing data from the American College of Surgeons (ACS) National Surgery Quality Improvement Program (NSQIP) database from January 1, 2015 to December 31, 2019. Data from the standard public use file was merged with that from the NSQIP Targeted Pancreatectomy Participant Use Data Files (a separate collection of pancreas-specific variables) using the Case Identification Number (CASEID) variable. Patients older than 18 years old, who had a histological diagnosis of pancreatic adenocarcinoma and underwent a pancreaticoduodenectomy, were included in this study. Patients undergoing resection were identified by one or more of the following additional CPT codes: 48150, 48152, 48153, 48154. This study was exempt from our institutional review board review since the data was de-identified and obtained from a participant use data file.
From the patients included in the study, two groups were formed based on the independent variable of interest: those that received neoadjuvant radiotherapy prior to a pancreaticoduodenectomy, and those that had progressed directly to surgery. For the purposes of this study, neoadjuvant radiotherapy was defined as those receiving treatments within 90 d of the index operation. The primary outcomes of interest were perioperative blood transfusions (defined by the need for a blood transfusion within 72 h of surgery start time; OTHBLEED variable) and total operative time (defined by operative time in minutes; OPTIME variable). The secondary outcomes of interest were 30-d post-operative morbidities and mortality. Specific variables included rate of the following: wound dehiscence, ventilator dependence, stroke, myocardial infarction (MI), deep venous thrombosis (DVT), pulmonary embolism (PE), pneumonia, urinary tract infection, septic shock, superficial surgical site infection (SSI), organ space SSI, 30-d re-operation, 30-d mortality, 30-d readmission, renal failure, hospital length of stay, duration of pancreatic drain, pancreatic fistula, and delayed gastric emptying. Pancreatic fistulae were defined according to the International Study Group for Pancreatic Fistula grading scheme[16].
StataSE was used for the statistical analyses. Descriptive statistics including mean ± standard deviation for normally distributed continuous variables, median/interquartile range for skewed continuous variables, and number/percentage for categorical variables. We assessed bi-variable differences in outcomes between patients with upfront surgery and neoadjuvant radiotherapy with surgery using the χ2 test, univariable logistic regression, and Fisher’s exact test for categorical variables. Two-tailed Student’s t-tests were used for continuous variables. Variables that were statistically associated (α < 0.05) with both the outcome and the independent variable, as well as those that were predicted theoretical confounders, were included in the multivariate regression analyses. Multivariable negative binomial regression was used for the total operative time variable, and multivariable logistic regression was used for the remaining secondary variables. We used stepwise, backward selection, and tested full/reduced models with the likelihood ratio test to determine the most parsimonious model. For 30-d outcome variables occurring less than 5% of the time, multivariable regression was not performed. For variables missing less than 5% of data, the listwise deletion method was used. Variables missing greater than 5% of data were reported as “unknown” in the tables.
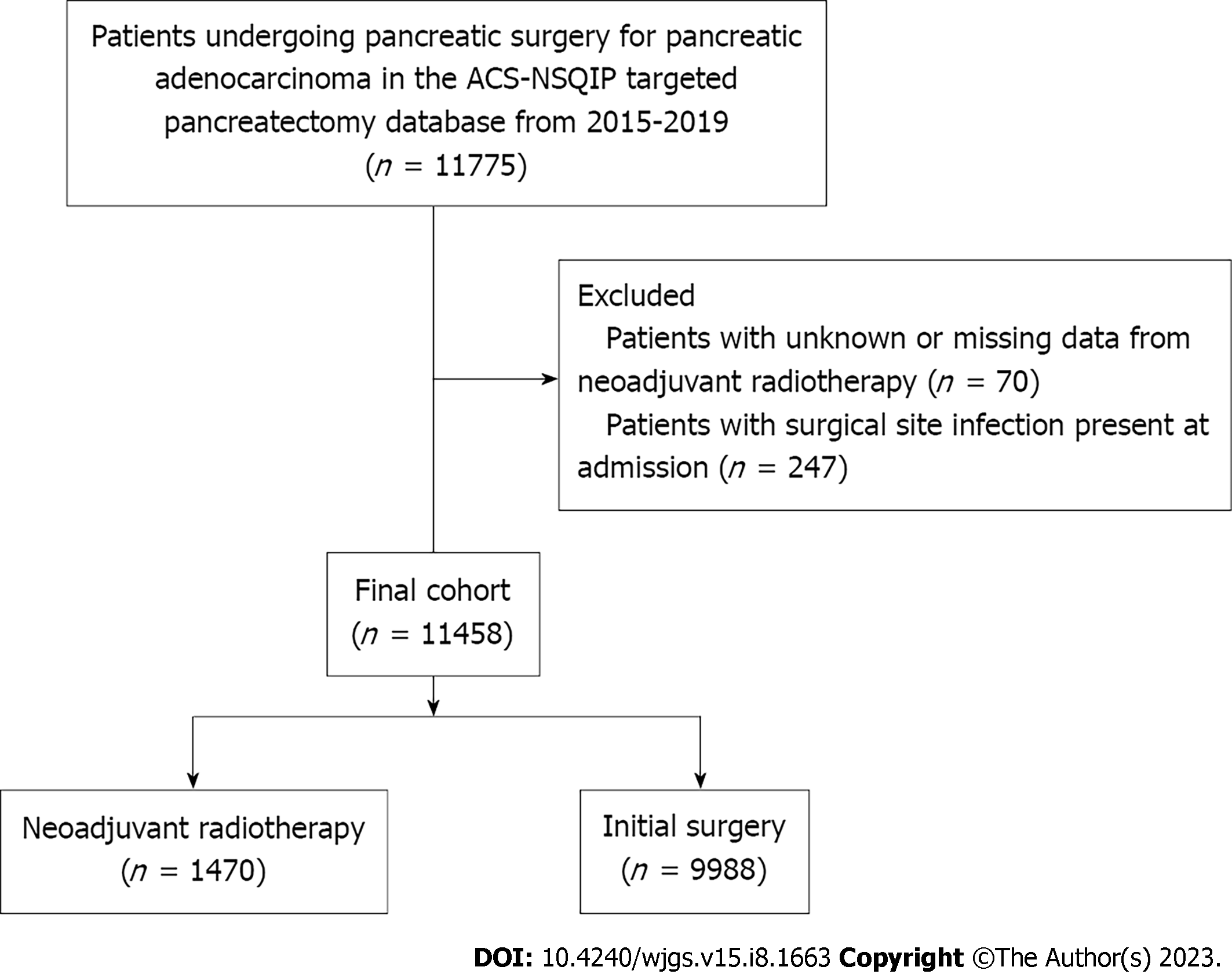
Query of the ACS-NSQIP database identified a total of 11775 patients who underwent surgery for pancreatic adenocarcinoma from 2015 to 2019. Patients who had data missing with regards to neoadjuvant radiation therapy status, and patients in whom SSIs were reported upon admission, were excluded from the analyses. This left a final cohort of 11458 patients. The cohort was then split into two study groups based off neoadjuvant radiotherapy exposure: 1470 patients (12.8%) received neoadjuvant radiation therapy, compared with 9988 (87.2%) patients who proceeded directly to surgery (Figure 1).
Patients undergoing neoadjuvant radiotherapy were more likely to be younger, female, non-Hispanic white, diabetic, and of normal body weight (all P < 0.04). Conversely, patients undergoing surgery without radiotherapy were more likely to be Hispanic, overweight/obese, and jaundiced (all P < 0.002). They were also more likely to have chronic obstructive pulmonary disease, dyspnea, and hypertension (all P < 0.05) (Table 1).
| Characteristic | Subcategory | Initial surgery | Neoadjuvant radiation | P value |
| n = 9988 | n = 1470 | |||
| Age in yr | Less than 50 | 511 (5.1) | 89 (6.1) | |
| 50-59 | 1638 (16.4) | 304 (20.7) | ||
| 60-69 | 3526 (35.3) | 585 (39.8) | < 0.001a | |
| 70-79 | 3287 (32.9) | 430 (29.3) | ||
| 80 and above | 1026 (10.3) | 62 (4.2) | ||
| Male | 5340 (53.5) | 743 (50.5) | 0.036a | |
| Race | White | 7392 (81.6) | 1203 (84.3) | |
| Black/African American | 748 (8.3) | 127 (8.9) | ||
| Hispanic | 462 (5.1) | 54 (3.8) | < 0.001a | |
| Asian | 427 (4.7) | 38 (2.7) | ||
| Other | 33 (0.4) | 5 (0.4) | ||
| Not reported | 926 | 43 | ||
| BMI | Normal | 3683 (36.9) | 618 (42.0) | |
| Underweight | 244 (2.4) | 37 (2.5) | ||
| Overweight | 3591 (36) | 505 (34.4) | 0.002a | |
| Obese | 1614 (16.2) | 203 (13.8) | ||
| Morbidly obese | 856 (8.6) | 107 (7.3) | ||
| Diabetes | 2859 (28.6) | 461 (31.4) | 0.031a | |
| Smoking | 1650 (16.5) | 238 (16.2) | 0.751 | |
| COPD | 394 (3.9) | 42 (2.9) | 0.042a | |
| Dyspnea | 507 (5.1) | 57 (3.9) | 0.047a | |
| HTN | 5418 (54.2) | 693 (47.1) | < 0.001a | |
| Preoperative steroid use | 239 (2.4) | 42 (2.9) | 0.283 | |
| Jaundice | 5759 (58.0) | 431 (29.7) | < 0.001a | |
| Biliary stent | 6457 (64.7) | 979 (66.6) | 0.235 | |
| Albumin, mean ± SD | 3.69 (0.60) | 3.85 (0.50) | < 0.001a |
With regard to tumor characteristics and operative approaches, patients receiving neoadjuvant radiotherapy were more likely to have a lower T-stage, lower N-stage, receive an elective surgery, have a higher wound class, and have a smaller pancreatic duct size (all P < 0.04). Such patients were also more likely to undergo an open surgical approach that involved resection of an artery or vein (all P < 0.001) (Table 2).
| Characteristic | Subcategory | Initial surgery | Neoadjuvant radiation | P value |
| n = 9988 | n = 1470 | |||
| Elective surgery | 8921 (89.4) | 1423 (96.8) | < 0.001a | |
| T-stage | T0/T1 | 2969 (29.7) | 867 (59.0) | |
| T2 | 6133 (61.4) | 544 (37.0) | < 0.001a | |
| T3/T4 | 666 (6.7) | 32 (2.2) | ||
| Tis/unknown | 128 (1.3) | 39 (2.7) | ||
| N-stage | N0 | 2969 (29.7) | 867 (59.0) | |
| N1 | 6133 (61.4) | 544 (37.0) | < 0.001a | |
| N2 | 666 (6.7) | 32 (2.18) | ||
| M-stage | M0 | 7260 (72.7) | 1109 (75.4) | |
| M1 | 172 (1.7) | 20 (1.4) | 0.073 | |
| Unknown | 2556 (25.6) | 341 (23.2) | ||
| Wound class | Clean | 242 (2.4) | 33 (2.2) | |
| Clean-contaminated | 7915 (79.3) | 1142 (77.7) | 0.034a | |
| Contaminated | 1547 (15.5) | 233 (15.9) | ||
| Dirty | 284 (2.8) | 62 (4.2) | ||
| Pancreatic duct size | < 3 mm | 1987 (24.9) | 304 (26.3) | |
| 3-6 mm | 4527 (56.8) | 685 (59.2) | ||
| > 6 mm | 1461 (18.3) | 168 (14.5) | 0.007a | |
| Unknown | 2013 | 315 | ||
| Resection of artery or vein | 2217 (22.4) | 554 (37.9) | < 0.001a | |
| Surgery approach | Open | 9112 (91.2) | 1386 (94.4) | < 0.001a |
| Robotic/laparoscopic | 875 (8.8) | 83 (5.7) |
Within the first 30 d following surgery, bi-variable statistical analyses revealed that patients receiving surgical treatment only were more likely to experience an MI, PE, pneumonia, organ space infection, delayed gastric emptying, and pancreatic fistula (all P < 0.035). Such patients also had a higher 30-d mortality rate, longer hospital stay, a drain that remained in place after 30 d, and a longer total operative time (all P < 0.041). Patients undergoing neoadjuvant radiation were more likely to receive a DVT and receive a perioperative transfusion (all P < 0.024). On multivariate analyses of the 30-d outcomes that occurred at a rate of greater than 5% in both study groups, neoadjuvant radiotherapy was associated with longer total operative times and the need for a perioperative transfusion. Patients undergoing neoadjuvant radiotherapy, however, were statistically less likely to acquire an organ space infection or a pancreatic fistula compared with patients who underwent surgery alone (Tables 3 and 4). While total hospital stay, presence of drain on post-operative day 30, and delayed gastric emptying occurred at significantly lower rates in the neoadjuvant therapy group upon univariate statistical analyses, this difference was not statistically significant on multivariable analyses.
| Complication | Initial surgery | Neoadjuvant radiation | P value |
| n = 9988 | n = 1470 | ||
| Wound dehiscence | 110 (1.1) | 16 (1.1) | 0.965 |
| Ventilator dependent > 48 h | 223 (2.2) | 38 (2.6) | 0.398 |
| Stroke | 29 (0.3) | 3 (0.2) | 0.791 |
| Myocardial infarction | 124 (1.2) | 9 (0.6) | 0.035a |
| DVT | 269 (2.7) | 55 (3.7) | 0.024a |
| Pulmonary embolism | 108 (1.1) | 5 (0.3) | 0.004a |
| Pneumonia | 305 (3.1) | 30 (2.0) | 0.031a |
| UTI | 247 (2.5) | 32 (2.2) | 0.491 |
| Septic shock | 207 (2.1) | 34 (2.3) | 0.545 |
| Complication | Neoadjuvant radiotherapy crude OR (95%CI) | Crude P value | Neoadjuvant radiotherapy adjusted OR/IRR (95%CI) | Adjusted P value |
| Total operative time | 0.15 (0.13, 0.16) | < 0.001a | 1.14 (1.11,1.16)1 | < 0.001a |
| Perioperative transfusion | 1.49 (1.32, 1.69) | < 0.001a | 1.58 (1.37, 1.82)2 | < 0.001a |
| Superficial SSI | 1.13 (0.93, 1.38) | 0.217 | NP | NP |
| Organ space SSI | 0.76 (0.63, 0.91) | 0.004a | 0.80 (0.66, 0.97)3 | 0.020a |
| 30-d reoperation | 1.03 (0.80, 1.32) | 0.832 | NP | NP |
| 30-d mortality rate | 0.40 (0.21, 0.75) | 0.005a | NP | NP |
| 30-d readmission rate | 1.14 (0.98, 1.32) | 0.090 | NP | NP |
| Renal failure | 0.74 (0.37, 1.47) | 0.382 | NP | NP |
| Total hospital stay | 0.09 (0.06, 0.12) | < 0.001a | 0.99 (0.96, 1.02)4 | 0.640 |
| Drain in place on POD 30 | 0.76 (0.61, 0.99) | 0.041a | 0.82 (0.64, 1.06)5 | 0.124 |
| Pancreatic fistula | 0.47 (0.38, 0.58) | < 0.001a | 0.50 (0.40, 0.63)5 | < 0.001a |
| Delayed gastric emptying | 0.81 (0.69, 0.95) | 0.010a | 0.86 (0.72, 1.01)5 | 0.073 |
In the past, numerous studies have implicated single and multi-agent neoadjuvant chemotherapy with both tumor downstaging and an increased rate of R0 resections[17,18]. In comparison with neoadjuvant chemotherapy, however, evidence demonstrating similar advantages with neoadjuvant radiation therapy have been somewhat sparce. In 2019, Jiang et al[19] used the NCDB database to show an increased R0 resection and overall survival rate among patients who utilized neoadjuvant stereotactic body radiation therapy in addition to neoadjuvant chemotherapy compared to just neoadjuvant chemotherapy alone[19]. A paper by Chung et al[20] showed that higher doses of radiation (i.e. intensity-modulate radiation therapy) corresponded to increased 1 year survival and progression-free survival with no significant increase in short or long-term side effects[20]. Upon initial analyses of our study data, we found that patients undergoing neoadjuvant radiotherapy prior to surgery had smaller tumors and less positive lymph nodes on pathologic staging compared with those undergoing surgery alone. This suggests that neoadjuvant therapy successfully worked to downstage the tumors prior to surgical re-section. Although this effect could be confounded by the substantial difference in baseline demographic data between the two study groups, these results are nearly identical to those found in similarly designed NSQIP studies comparing neoadjuvant chemoradiation to surgery alone[9].
When examining our two primary outcome variables, we found a statistically significant increase in total operative time and perioperative transfusion requirements among patients receiving neoadjuvant radiation therapy compared to just surgery alone. This is the first time that such associations have been reported using multivariable analyses with patients receiving only neoadjuvant radiotherapy (vs neoadjuvant radiotherapy and/or chemotherapy). An analysis of 2005-2010 NSQIP data from Cho et al[14] found similar results, but with bi-variable analysis only[14]. Similarly, a study using NSQIP data from 2014 to 2015 showed that the perioperative transfusion requirement rate among patients receiving neoadjuvant therapy (chemotherapy and/or radiotherapy) was significantly higher than the rate in patients who progressed directly to surgery[21]. In 2021, Krell et al[22] showed that (with propensity score matching) there were increased total operative times and more frequent perioperative blood transfusions among those receiving neoadjuvant therapy (chemotherapy and/or radiotherapy) prior to surgery compared to those undergoing surgery alone[22].
Previously, it has been suggested that these differences in perioperative blood transfusions and total operative time are secondary to an increase in the number of borderline resectable cancers in the neoadjuvant therapy groups[14]. By definition, these tumors involve major blood vessels and usually require more complex dissections when compared to lower stage tumors. While the increased number of vascular resections within our neoadjuvant therapy group supports this conclusion, an alternative explanation may be that neoadjuvant radiotherapy itself impacts the complexity of the eventual surgical resection. Histologic evaluation of pancreatic tumors before and after neoadjuvant chemoradiation has shown a significant increase in the ratio of fibrosis to neoplastic cells, indicating a change in the tissue character following neoadjuvant therapy[23]. While such fibrosis has been theorized to be protective against post-operative complications like pancreatic fistulae formation, its distortional effect on classic tissue planes may make the surgery itself more difficult[24]. It is important for the operating surgeon to keep this in mind, as it may impact both the procedure type (i.e. open vs minimally invasive) and the expected post-operative complications.
Overall, the 30-d post-operative complication and morbidity rates were similar between both study groups. Of the variables that were analyzed using multivariable regression, only organ space infections and pancreatic fistula rates were significantly different between the two study groups. Numerous studies have detailed the decreased rate of both variables in patients receiving neoadjuvant therapy, suggesting that neoadjuvant radiotherapy may have a small positive effect on short term post-operative morbidity[9,10,14,21,25].
Although the data provided in this study lend credence to the safety and efficacy of neoadjuvant radiotherapy, there are some limitations to keep in mind. Data regarding the specific details of neoadjuvant radiotherapy regimen used (duration, intensity, timing, prior to surgery) is not available within the NSQIP database, making it impossible to account for related confounding factors in our data analyses. Additionally, NSQIP only collects data on post-operative outcomes that occur within 30 d of the index operation, making the results of this study difficult to extrapolate over a longer-termed period. Facility and surgeon data are also not reported in NSQIP, which again may represent confounding factors that our analyses did not consider. Finally, it is impossible to determine whether a patient’s tumor is resectable or borderline resectable (per NCCN guidelines) based on the information reported in NSQIP. As surgeries may be more difficult in borderline resectable patients, this represents a further confounding factor that could not be completely controlled for.
The results of this study contribute to the notion that neoadjuvant radiotherapy is both safe and effective to use prior to a pancreaticoduodenectomy for pancreatic adenocarcinoma. The study does, however, suggest that adverse intraoperative outcomes like total operative time and perioperative transfusion requirements may be increased among patients receiving surgery for cancer resection after neoadjuvant radiotherapy. Surgeons are encouraged to keep in mind the potential positive and negative effects that neoadjuvant radiotherapy has on the complexity of the eventual surgery when it is performed.
Pancreatic adenocarcinoma is currently the fourth leading cause of cancer-related deaths in the United States. In addition to neoadjuvant chemotherapy, neoadjuvant radiotherapy may improve negative margin resection rates. This study seeks to investigate the safety and efficacy of neoadjuvant radiotherapy in patients with pancreatic adenocarcinoma.
By better clarifying the benefits and drawbacks that are associated with neoadjuvant radiotherapy administration in patients with pancreatic adenocarcinoma, practitioners can make informed decisions regarding its use.
The primary objective of the study was to investigate the effect that neoadjuvant radiotherapy has on both intra-operative and 30-d postoperative morbidities in patients with pancreatic adenocarcinoma.
Using 2015-2019 data from the National Surgery Quality Improvement Program data set, we divided pancreatic adenocarcinoma patients into two groups based on neoadjuvant radiotherapy status. Then we performed univariable and multivariable analyses to identify differences in baseline characteristics and outcomes between the two groups.
When compared to patients with pancreatic adenocarcinoma who underwent surgical resection alone, patients who underwent neoadjuvant radiotherapy were more likely to have longer surgeries and higher perioperative blood loss. The neoadjuvant radiotherapy patients were also less likely to have organ space infections and pancreatic fistulae formation.
Neoadjuvant radiotherapy has significant effects on intraoperative and 30-d postoperative morbidity in patients with pancreatic adenocarcinoma. It may make eventual surgical resection of the cancer more complex.
Future research should focus on finding new methods that work to minimize the negative side effects associated with neoadjuvant radiotherapy in patients with pancreatic adenocarcinoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Tan CL, China; Yi SQ, Japan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2113] [Article Influence: 150.9] [Reference Citation Analysis (3)] |
| 2. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1708] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 3. | Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, Hong TS, Kwak EL, Lauwers GY, Ryan DP, Wargo JA, Lillemoe KD, Ferrone CR. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? Ann Surg. 2013;257:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 4. | Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW, Kishiwada M, Kitagawa H, Michalski CW, Wolfgang CL. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 504] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 5. | National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 2.2021). [cited 10 January 2022]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. |
| 6. | Lambert A, Schwarz L, Borbath I, Henry A, Van Laethem JL, Malka D, Ducreux M, Conroy T. An update on treatment options for pancreatic adenocarcinoma. Ther Adv Med Oncol. 2019;11:1758835919875568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 7. | Russo S, Ammori J, Eads J, Dorth J. The role of neoadjuvant therapy in pancreatic cancer: a review. Future Oncol. 2016;12:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Kang CM, Chung YE, Park JY, Sung JS, Hwang HK, Choi HJ, Kim H, Song SY, Lee WJ. Potential contribution of preoperative neoadjuvant concurrent chemoradiation therapy on margin-negative resection in borderline resectable pancreatic cancer. J Gastrointest Surg. 2012;16:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Cools KS, Sanoff HK, Kim HJ, Yeh JJ, Stitzenberg KB. Impact of neoadjuvant therapy on postoperative outcomes after pancreaticoduodenectomy. J Surg Oncol. 2018;118:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Cooper AB, Parmar AD, Riall TS, Hall BL, Katz MH, Aloia TA, Pitt HA. Does the use of neoadjuvant therapy for pancreatic adenocarcinoma increase postoperative morbidity and mortality rates? J Gastrointest Surg. 2015;19:80-6; discussion 86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN, Blaszkowsky LS, Clark JW, Faris JE, Goyal L, Kwak EL, Murphy JE, Ting DT, Wo JY, Zhu AX, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 652] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 12. | Lof S, Korrel M, van Hilst J, Alseidi A, Balzano G, Boggi U, Butturini G, Casadei R, Dokmak S, Edwin B, Falconi M, Keck T, Malleo G, de Pastena M, Tomazic A, Wilmink H, Zerbi A, Besselink MG, Abu Hilal M; European Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS). Impact of Neoadjuvant Therapy in Resected Pancreatic Ductal Adenocarcinoma of the Pancreatic Body or Tail on Surgical and Oncological Outcome: A Propensity-Score Matched Multicenter Study. Ann Surg Oncol. 2020;27:1986-1996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Cloyd JM, Tsung A, Hays J, Wills CE, Bridges JF. Neoadjuvant therapy for resectable pancreatic ductal adenocarcinoma: The need for patient-centered research. World J Gastroenterol. 2020;26:375-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Cho SW, Tzeng CW, Johnston WC, Cassera MA, Newell PH, Hammill CW, Wolf RF, Aloia TA, Hansen PD. Neoadjuvant radiation therapy and its impact on complications after pancreaticoduodenectomy for pancreatic cancer: analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). HPB (Oxford). 2014;16:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VW, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15:2059-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2951] [Article Influence: 368.9] [Reference Citation Analysis (35)] |
| 17. | Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, Strobel O, Jäger D, Ulrich A, Büchler MW. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg. 2016;264:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 382] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 18. | Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, Yopp AC, Mansour JC, Choti MA, Polanco PM. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol. 2017;35:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 305] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 19. | Jiang W, Haque W, Verma V, Butler EB, Teh BS. Neoadjuvant stereotactic body radiation therapy for nonmetastatic pancreatic adenocarcinoma. Acta Oncol. 2019;58:1259-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Chung SY, Chang JS, Lee BM, Kim KH, Lee KJ, Seong J. Dose escalation in locally advanced pancreatic cancer patients receiving chemoradiotherapy. Radiother Oncol. 2017;123:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Czosnyka NM, Borgert AJ, Smith TJ. Pancreatic adenocarcinoma: effects of neoadjuvant therapy on post-pancreatectomy outcomes - an American College of Surgeons National Surgical Quality Improvement Program targeted variable review. HPB (Oxford). 2017;19:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Krell RW, McNeil LR, Yanala UR, Are C, Reames BN. Neoadjuvant Therapy for Pancreatic Ductal Adenocarcinoma: Propensity-Matched Analysis of Postoperative Complications Using ACS-NSQIP. Ann Surg Oncol. 2021;28:3810-3822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Sasson AR, Wetherington RW, Hoffman JP, Ross EA, Cooper H, Meropol NJ, Freedman G, Pingpank JF, Eisenberg BL. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: analysis of histopathology and outcome. Int J Gastrointest Cancer. 2003;34:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Ishikawa O, Ohigashi H, Imaoka S, Teshima T, Inoue T, Sasaki Y, Iwanaga T, Nakaizumi A. Concomitant benefit of preoperative irradiation in preventing pancreas fistula formation after pancreatoduodenectomy. Arch Surg. 1991;126:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Hank T, Sandini M, Ferrone CR, Rodrigues C, Weniger M, Qadan M, Warshaw AL, Lillemoe KD, Fernández-Del Castillo C. Association Between Pancreatic Fistula and Long-term Survival in the Era of Neoadjuvant Chemotherapy. JAMA Surg. 2019;154:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |