Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1549
Peer-review started: April 4, 2023
First decision: May 12, 2023
Revised: May 22, 2023
Accepted: May 24, 2023
Article in press: May 24, 2023
Published online: July 27, 2023
Processing time: 108 Days and 12.2 Hours
Lung cancer is the leading cause of cancer deaths worldwide. Although lung cancer can metastasize to various organs such as the liver, lymph nodes, adrenal gland, bone, and brain, metastases to the digestive organs, especially the colon, are rare.
An 83-year-old man diagnosed with lung cancer received radiation and chemoimmunotherapy, resulting in a complete clinical response. One year after the initial lung cancer diagnosis, the patient presented with obstructive ileus caused by a tumor in the descending colon. An elective left hemicolectomy was successfully performed after the endoscopic placement of a self-expandable metallic stent (SEMS). Pathologically, the tumor of the descending colon was diagnosed as lung cancer metastasis. The postoperative course was uneventful, and the patient is in good condition 13 mo after surgery, with no signs of recurrence. The previous 23 cases of surgical resection of colonic metastasis from lung cancer were reviewed using PubMed to characterize their clinicopathological features and outcomes.
SEMS is useful for obstructive colonic metastasis as a bridge to surgery to avoid emergency operations.
Core Tip: Gastrointestinal metastases of lung cancer, especially colorectal metastases, are rare. We report a colonic obstruction caused by lung cancer metastasis to the descending colon, which was successfully treated with left hemicolectomy after endoscopic decompression with a self-expandable metallic stent. Although chemotherapy is the mainstay of treatment for gastrointestinal metastases of lung cancer, successful resection of solitary colonic metastases with prolonged survival suggests that surgical intervention may be the treatment of choice for selected patients with colonic metastasis from lung cancer.
- Citation: Nakayama Y, Yamaguchi M, Inoue K, Hamaguchi S, Tajima Y. Successful resection of colonic metastasis of lung cancer after colonic stent placement: A case report and review of the literature. World J Gastrointest Surg 2023; 15(7): 1549-1558
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1549.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1549
Lung cancer is the leading cause of cancer deaths worldwide[1]. It is estimated that half of the patients with lung cancer have distant metastasis at initial diagnosis[2]. However, gastrointestinal (GI) metastasis of lung cancer is uncommon[3-7], and metastasis to the colon or rectum is even rarer[3,4,6,7]. In addition, the greater frequency of metastases reported in autopsy cases[3,4,7], compared to clinical cases[2,6,8,9], suggests that lung cancer metastases are generally asymptomatic. Colonic metastases of lung cancer are identified following the development of GI tract symptoms such as abdominal pain, obstruction, and bleeding, and are difficult to diagnose in the early stage of the disease[10-14]. Whether surgery is the optimal treatment for GI metastasis is controversial because lung cancer with GI metastasis is often associated with a poor prognosis[2,5,6,11]. Meanwhile, it has been claimed that surgical resection of GI metastases can alleviate GI symptoms, facilitate subsequent management, including chemotherapy, and improve the patient’s quality of life[12,15-17].
Here, we describe a case of a patient with obstructive ileus caused by descending colon metastasis from lung cancer, in whom the metastatic tumor was resected after endoscopic decompression with a self-expandable metallic stent (SEMS). To the best of our knowledge, this is the first report of a successful tumor resection following a metallic colonic stent placement as a bridge to surgery. Decompression of colonic obstruction with a stent avoided emergency surgery and allowed safe tumor resection after the patient’s general condition stabilized.
An 83-year-old man presented with epigastralgia and left abdominal pain for 1 mo.
The patient’s symptoms had started a month ago, accompanied by poor appetite. No abnormal bowel movements, including constipation, were noted.
The patient had a medical history of type II diabetes mellitus, hyperlipidemia, thoracic aortic aneurysm, and cerebral infarction, which was under control with medical treatment, including anticoagulants. A lung tumor was detected in the right pulmonary hilar region on a computed tomography (CT) scan performed 1 year ago, and he was diagnosed with lung cancer because the transbronchial lung biopsy revealed squamous cell carcinoma. The clinical diagnosis was cT4N2M0 cStageⅢB following a positron emission tomography (PET)-CT scan. There were no obvious findings in the upper and lower GI endoscopic examination. The patient was treated with radiotherapy (60 Gy) and chemotherapy (low dose carboplatin), along with immunotherapy with durvalumab (human monoclonal antibody against programmed cell death-ligand 1), resulting in a complete clinical response of the lung lesion. Thereafter, the patient continued to have an outpatient follow-up for lung cancer. Although prostate cancer was detected during the treatment for lung cancer, it was adequately controlled with hormone therapy.
The patient denied any family history of malignant tumors.
Physical examination revealed mild left abdominal tenderness; however, no obvious abdominal mass could be palpated. There were no abnormalities in the chest or any neurological findings.
Laboratory data revealed mild anemia with hemoglobin and hematocrit levels of 10.3 g/dL and 31.0%, respectively. Levels of tumor markers (carbohydrate antigen 19-9 and carcinoembryonic antigen) were not elevated. Liver and renal function tests and electrolytes were within the normal range.
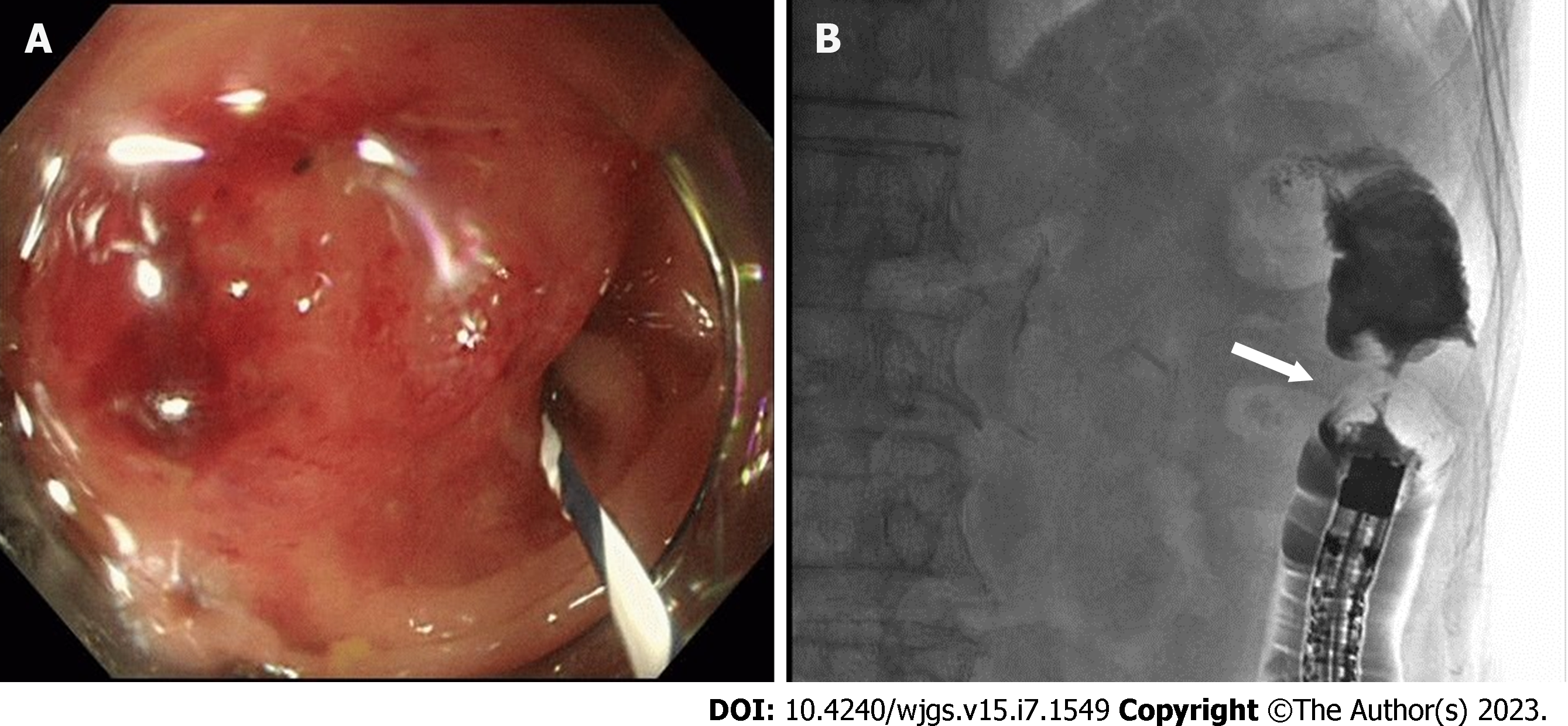
An upper GI endoscopy for upper abdominal pain detected an early gastric cancer (adenocarcinoma) in the anterior wall of the lower gastric body. In addition, an abdominal CT scan showed a thickening of the descending colon wall near the splenic flexure (Figure 1), suspecting primary colon cancer. A contrast-enhanced whole-body CT scan showed no metastatic lesions, swollen lymph nodes, or relapse of the primary lung cancer. Colonoscopy depicts a mass protruding into the lumen covered with smooth and reddish mucosa, the scope could not be advanced through the lesion (Figure 2A). Gastrografin enema shows an apple core sign with luminal narrowing (white arrow), approximately 3 cm in length, in the descending colon (Figure 2B). A circumferential protruding tumor in the descending colon with stent-induced mucosal changes (Figure 3A). Hematoxylin and eosin (HE) staining, the tumor shows the appearance of squamous cell carcinoma, mainly confined to the submucosal layer (Figure 3B). Immunohistochemical staining for CK5/6, p40, and CDX2. The tumor cells are positive for CK5/6 and p40, and negative for CDX2 (Figure 3C-E). HE staining of the lung biopsy specimen of the patient (Figure 3F). The patient was diagnosed with squamous cell lung cancer.
According to the pathological findings, the patient was definitively diagnosed with colonic metastasis from the primary lung cancer.
The postoperative course was uneventful, and the patient was discharged 16 d after surgery. He received a follow-up medical examination without adjuvant chemotherapy.
Eleven months after colonic surgery, he underwent endoscopic submucosal dissection for gastric cancer. Histologically, it was a primary gastric adenocarcinoma with successful tumor removal. The patient remained in good condition 13 mo after the colectomy, with no signs of metastasis or relapse of the primary lung cancer.
GI metastasis of primary lung cancer is a rare entity. Although a relatively high incidence of GI metastasis of lung cancer has been described in autopsy cases[3,4,7], ranging from 9.7%-14.0%, it is reported to be 0.19%-1.70%[2,9,11,17] in clinical cases, suggesting that the diagnosis of GI metastasis of lung cancer is difficult in clinical practice. Meanwhile, the incidence of GI metastasis shows an increasing trend[6,11], which may reflect the recent increase in the occurrence of lung cancer among women as well as men, more frequent endoscopic examinations performed in general hospitals, and the use of immunostaining study in the differential diagnosis of neoplasms showing an undifferentiated morphology[11]. Although the most frequently affected part of the GI tract by lung cancer is the esophagus[4], where direct invasion is possible, the most frequent site of GI metastasis is the small intestine[6,17]. The mechanism of GI metastasis of lung cancer is considered to be hematogenous and lymphogenous, and mass formation is usually observed in the submucosa of the intestinal wall in GI metastasis[9,12,18]. The small intestine with metastasis is prone to bleeding, perforation, and obstruction[2,3,6,11] because the submucosal layer has abundant blood and lymphatic vessels and a thin wall and small lumen. Colonic metastasis of lung cancer is rare, with an incidence of 2.1%-5.7% in autopsy cases[3-5,7]. Meanwhile, the incidence of colorectal metastases with clinical symptoms is reported to be 0.02%-0.30%[2,11,17], indicating that most patients with colorectal metastases are asymptomatic.
CT and PET-CT studies have been useful in diagnosing colorectal metastases from lung cancer[8,17,19]. Kim et al[8] reported that GI metastasis of lung cancer presents as an intraluminal polypoid mass or wall thickening with variable contrast enhancement patterns, predominantly isoattenuation; during colonoscopy, it may be observed as an elevated lesion without ulceration[20,21], a submucosal tumor with ulceration[9,18,22], or single or multiple nodules with or without ulceration[13,23,24]. Lung cancer tissue and metastatic colorectal cancer tissue may yield different results on a panel composed of cancer-related genes[25]; hence, a definitive diagnosis is made by biopsy and histopathological examination, especially confirmed by immunohistochemical staining. Cytokeratin7 (CK7), CK20, and thyroid transcription factor-1 (TTF-1) help distinguish between primary and metastatic colon adenocarcinoma[11,26,27]. In our patient, positive immunohistochemical staining for CK5/6 and p40, specific markers of lung squamous cell carcinoma, helped diagnose colonic metastasis of lung cancer. In GI metastasis of lung adenocarcinoma, a combination of CK7 and CK20 may differentiate them from primary GI adenocarcinoma, as primary GI cancers show a CK7(-)/CK20(+) pattern[10,11]. However, owing to the presence of CK7(+) and/or CK20(-) cases in some GI cancers, the addition of TTF-1, which is also a specific marker for lung cancer, can help differentiate primary GI cancers from metastasis of lung cancer[10] because most primary adenocarcinomas are TTF-1 positive. In contrast, metastatic adenocarcinomas of the lung are almost always TTF-1 negative[28].
The prognosis of lung cancer with GI metastasis is reported to be very poor[2,6,9], with an average time of fewer than 3 mo from the diagnosis of GI metastasis to death[2,5,6]. Although chemotherapy is the standard treatment for GI metastases, surgical intervention can be applied in patients with clinical symptoms such as bowel obstruction[17,29,30], intussusception[31,32], and perforation[33]. However, surgery for advanced lung cancer with multiple metastases is highly invasive and worsens the patient’s general condition, resulting in various perioperative complications[32-34] with mortality rates of 64%-100%[5,10,35]. Meanwhile, a relatively good prognosis can be achieved when GI metastases are curatively resected[6,10].
A total of 24 cases[12,16,17,22,25,29-34,36-46] of colorectal metastases of lung cancer treated with surgical resection, including our case, have been reported so far (Table 1); this includes 18 male (75%) and 6 female (25%) patients with a median age of 61.5 years (range 49-85 years). The most common symptom was abdominal pain, which presented in 14 patients (58.3%), followed by GI bleeding in 5 (20.8%). Three patients were asymptomatic and incidentally detected on follow-up CT examination for lung cancer. Colonic metastases were synchronous in 9 patients (37.5%) and metachronous in 15 patients (62.5%) with a median term of 11 mo (range 3-24 mo) from diagnosis of lung cancer to the confirmation of colonic metastasis. The number of colorectal metastasis was one in 22 patients and two in 2 patients. The tumors were located in the colon in 22 patients, the rectum in one, and the appendix in one. There was no specific distribution of colonic metastases. Seven patients (29.2%) had regional lymph node metastasis of the colorectum[14,28,34,41], and five patients (20.8%) had distant metastasis other than the colorectum such as the liver[46], stomach[47], small intestine[35,48], bone[49], and the bilateral lung[25]. Most colonic metastases showed the histological appearance of adenocarcinoma (11 patients); squamous cell carcinoma was observed in 7, pleomorphic carcinoma in 2, small cell carcinoma in 2, and adenosquamous cell carcinoma in 2 patients, which were naturally identical to the primary lung cancer. Non-small cell lung cancers, including adenocarcinoma[10,47], squamous cell carcinoma[47], and large cell adenocarcinoma[3,4], are more likely to metastasize to the GI system when compared to small cell carcinomas; a similar finding was observed in colorectal metastases. Colectomy with curative intent was performed in 11 patients and conservative surgery in 13. The median survival after colorectal surgery was 7 mo (range: A few days to 48 mo); 12 mo in patients receiving curative surgery and 3.1 mo in patients with conservative surgery. Patients who underwent curative resection of colorectal metastasis showed a better prognosis. Fujiwara et al[15] reported that postoperative survival for patients receiving radical surgery for GI metastases ranged from 40.0 mo to 93.6 mo, compared with 3.7 mo to 14.9 mo for those receiving conservative surgery. Nine patients with primary lung cancer and synchronous GI metastasis had a median survival time of 3.1 mo (range: A few days to 24 mo) after colectomy. On the other hand, 15 patients with metachronous GI metastasis showed a prolonged median survival time of 12 mo (range: 1-48 mo) after colectomy. Fujiwara et al[15] reported that the longer the time to metastatic recurrence in the GI tract after pneumonectomy for lung cancer, the longer the survival.
| No. | Ref. | Age | Sex | Initial stage of lung cancer | Treatment for lung cancer | Colorectal metastasis | Colorectal metastasis | Other than the colorectum | Colorectal metastasis | Lung cancer to colorectal metastasis | For colorectal metastasis | Treatment for colorectal metastasis | Colorectal metastasis | Month |
| 1 | Johnson et al[36], 1995 | 50 | M | NA | Lobectomy | Rectum | 1 | None | Sm | 12 mo | Rectal bleeding | Abdominoperineal resection of rectum | Dead | 19 mo |
| 2 | Carr et al[37], 1996 | 60 | F | NA | Pneumonectomy | T-colon | 1 | None | Sq | 12 mo | Rectal bleeding | Extended right hemicolectomy | Dead | 24 mo |
| 3 | Carr et al[37], 1996 | 52 | F | NA | Lobectomy | T-colon | 1 | None | ASC | 6 mo | Rectal bleeding | Extended left hemicolectomy | Alive | 24 mo |
| 4 | Carroll et al[38], 2001 | 68 | M | Stage IV | None | S-colin | 1 | None | Sq | Synchronous | Diarrhoea | Sigmoid colectomy | Dead | 6 mo |
| 5 | Uner et al[29], 2005 | 58 | M | Stage I B | Lobectomy | D-colon | 1 | None | Sq | 19 mo | Abdominal pain | Left hemicolectomy | Alive | 9 mo |
| 6 | Miyazaki et al[39], 2005 | 64 | M | Stage III A | Pneumonectomy | Appendix | 1 | None | Ad | 9 mo | Abdominal pain | Ileocecal resection | Dead | 12 mo |
| 7 | Kim et al[8], 2009 | 62 | M | Stage I B | NA | Colon | 1 | None | ASC | 169 d | Abdominal pain | Operation (unknown details) | Dead | 91 d |
| 8 | Ono et al[12], 2009 | 59 | M | Stage I A | Lobectomy | D-colon | 1 | Abdominal LN | Ad | 8 mo | Abdominal pain | Left hemicolectomy | Alive | 12 mo |
| 9 | Rashid et al[32], 2011 | 57 | M | Stage IV | None | Cecum | 1 | None | PC | Synchronous | Abdominal pain | Right hemicolectomy | Dead | 89 d |
| 10 | Sakai et al[16], 2012 | 60 | F | Stage IV | Chemoradiotherapy | S-colon | 1 | None | Sq | 6 mo | Abdominal pain | Sigmoid colectomy, partial transverse colectomy | Alive | 6 mo |
| 11 | Doussot et al[40], 2013 | 62 | M | Stage IV | Chemotherapy | A-colon | 1 | Bilateral lung metastases | Ad | Synchronous | Abdominal pain | Right hemicolectomy | Dead | 6 mo |
| 12 | Lin et al[31], 2014 | 78 | M | Stage IV | None | A-colon | 2 | 8 jejunal metastases | PC | Synchronous | Tarry stool | Segmental resection | Dead | 3 mo |
| 13 | Sifuentes et al[33], 2014 | 73 | M | Stage IV | None | S-colon | 1 | None | Ad | Synchronous | Abdominal pain | Hartmann’s procedure | Dead | 2 mo |
| 14 | Costa Almeida et al[41], 2015 | 49 | M | NA | Chemoradiotherapy | A-colon, S-colon | 2 | None | Sm | 24 mo | Abdominal pain | Right hemicolectomy, sigmoid loop colostomy | Dead | 3 mo |
| 15 | Vittorakis et al[22], 2018 | 49 | M | Stage I A | Bilobectomy | A-colon | 1 | Abdominal LN | Ad | 24 mo | Abdominal pain | Right colectomy | Alive | 12 mo |
| 16 | Choi et al[30], 2019 | 74 | M | Stage IV | None | A-colon | 1 | Abdominal LN | Ad | Synchronous | Abdominal pain | Right hemicolectomy | Dead | 41 d |
| 17 | Prabhakaran et al[42], 2020 | 85 | M | Stage IV | Immunotherapy | A-colon | 1 | None | Ad | Synchronous | Rectal bleeding | Right hemicolectomy | Alive | 24 mo |
| 18 | Wang et al[25], 2019 | 47 | F | Stage II B | Lobectomy, AC | S-colon | 1 | None | Ad | 3 mo | No | Sigmoid colectomy | Alive | 8 mo |
| 19 | Pararas et al[43], 2021 | 72 | F | Stage I B | Lobectomy, AC | D-colon | 1 | Liver, abdominal LN | Sq | 24 mo | No | Left hemicolectomy, segmentectomy of liver | Alive | 1 mo |
| 20 | Bhutta et al[34], 2021 | 61 | M | Stage IV | Chemotherapy | S-colon | 1 | None | Ad | Synchronous | Dyspnea | Sigmoidectomy | Dead | A few days |
| 21 | Catalano et al[44], 2022 | 78 | M | Stage II A | Lobectomy | T-colon | 1 | Stomach, abdominal LN | Ad | 5 mo | No | Partial colectomy | Alive | 48 mo |
| 22 | Cheng et al[45], 2023 | 74 | M | Stage IV | NA | T-colon | 1 | None | Sq | Synchronous | Abdominal pain | Partial transverse colon resection | NA | 19 mo |
| 23 | Luo et al[46], 2022 | 58 | F | Stage IV | Immunochemotherapy | A-colon | 1 | Ileum, bone, multiple LN | Ad | 11 mo | Vomiting | Right hemicolectomy, gastrojejunostomy | NA | NA |
| 24 | Present case | 83 | M | Stage III B | Radiochemoimmunotherapy | D-colon | 1 | Abdominal LN | Sq | 12 mo | Abdominal pain | Left hemicolectomy | Alive | 13 mo |
Although systemic chemotherapy is the standard treatment for lung cancer with GI metastasis, it cannot be used in cases associated with abdominal symptoms, such as abdominal pain, bleeding, and bowel obstruction, due to GI metastasis. Tumor resection[17,30], colostomy[14,48], and SEMS[49], followed by chemotherapy, have been reported in such patients. Although the usefulness of SEMS as a bridge to surgery for primary colorectal cancer is accumulating[50-54], little is known regarding the application of SEMS for metastatic colonic stricture. In our patient, the placement of SEMS for colonic obstruction was useful as a bridge to surgery, and prolonged survival was observed. The SEMS placement had no technical difficulty because the tumor had grown mainly in the submucosal layer without neoplastic changes in the mucosal surface.
On the other hand, the possibility of migration, perforation, and bleeding during a long-term placement of SEMS should be considered. The incidence of SEMS migration has been reported to be 5.6%-7.7% in cases of colonic stenosis caused by non-colonic malignancy with peritoneal carcinomatosis[55,56]. The incidence of perforation related to SEMS is reported to be 4.3%-8.3% in primary colorectal cancer[50,51,54,57]. In particular, bevacizumab-based chemotherapy is associated with a high risk of GI perforation[57]. It has also been claimed that perforation may result in further cancer recurrence and peritoneal dissemination[58,59]. In the present case, curative resection of colonic metastasis was achieved 18 d after SEMS implantation, in which the SEMS allowed careful evaluation of the tumor status and improved the patient’s general condition. In cases with solitary GI metastasis associated with GI symptoms, as in our case, SEMS may be useful as a bridge to surgery.
We report a rare case of colonic metastasis of lung cancer associated with bowel obstruction. Colonic metastasis was successfully resected following placement of SEMS, and it was suggested that SEMS could be used as a bridge to surgery to avoid emergency operation and allow a more adequate treatment planning in cases with obstructive colonic metastasis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gu GL, China; Lin Q, China S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1876] [Cited by in RCA: 1999] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 2. | Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang TH, Sheu CC, Tsai JR, Huang MS. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer. 2006;54:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Yoshimoto A, Kasahara K, Kawashima A. Gastrointestinal metastases from primary lung cancer. Eur J Cancer. 2006;42:3157-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Antler AS, Ough Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer. 1982;49:170-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | McNeill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the lung. Cancer. 1987;59:1486-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Hu Y, Feit N, Huang Y, Xu W, Zheng S, Li X. Gastrointestinal metastasis of primary lung cancer: An analysis of 366 cases. Oncol Lett. 2018;15:9766-9776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Ryo H, Sakai H, Ikeda T, Hibino S, Goto I, Yoneda S, Noguchi Y. [Gastrointestinal metastasis from lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1996;34:968-972. [PubMed] |
| 8. | Kim SY, Ha HK, Park SW, Kang J, Kim KW, Lee SS, Park SH, Kim AY. Gastrointestinal metastasis from primary lung cancer: CT findings and clinicopathologic features. AJR Am J Roentgenol. 2009;193:W197-W201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Taira N, Kawabata T, Gabe A, Furugen T, Ichi T, Kushi K, Yohena T, Kawasaki H, Higuchi D, Chibana K, Fujita K, Nakamoto A, Owan I, Kuba M, Ishikawa K. Analysis of gastrointestinal metastasis of primary lung cancer: Clinical characteristics and prognosis. Oncol Lett. 2017;14:2399-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Lee PC, Lo C, Lin MT, Liang JT, Lin BR. Role of surgical intervention in managing gastrointestinal metastases from lung cancer. World J Gastroenterol. 2011;17:4314-4320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Rossi G, Marchioni A, Romagnani E, Bertolini F, Longo L, Cavazza A, Barbieri F. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. J Thorac Oncol. 2007;2:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Ono H, Okabe M, Kimura T, Kawakami M, Nakamura K, Danjo Y, Takasugi H, Nishihara H. Colonic metastasis from primary carcinoma of the lung: report of a case and review of Japanese literature. Clin J Gastroenterol. 2009;2:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Gonzalez-Tallon AI, Vasquez-Guerrero J, Garcia-Mayor MA. Colonic Metastases From Lung Carcinoma: A Case Report and Review of the Literature. Gastroenterology Res. 2013;6:29-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Parker NA, McBride C, Forge J, Lalich D. Bowel obstruction caused by colonic metastasis of lung adenocarcinoma: a case report and literature review. World J Surg Oncol. 2019;17:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Fujiwara A, Okami J, Tokunaga T, Maeda J, Higashiyama M, Kodama K. Surgical treatment for gastrointestinal metastasis of non-small-cell lung cancer after pulmonary resection. Gen Thorac Cardiovasc Surg. 2011;59:748-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Sakai H, Egi H, Hinoi T, Tokunaga M, Kawaguchi Y, Shinomura M, Adachi T, Arihiro K, Ohdan H. Primary lung cancer presenting with metastasis to the colon: a case report. World J Surg Oncol. 2012;10:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Kim MS, Kook EH, Ahn SH, Jeon SY, Yoon JH, Han MS, Kim CH, Lee JC. Gastrointestinal metastasis of lung cancer with special emphasis on a long-term survivor after operation. J Cancer Res Clin Oncol. 2009;135:297-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Iwamuro M, Miyabe Y, Hanae K, Yoshinari K, Katsuyoshi T, Murakami T, Hirofumi M, Yamamoto K. Regression of metastatic colon tumour from primary adenocarcinoma of the lung due to fistulisation to the bowel lumen. Ecancermedicalscience. 2014;8:412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Xie X, Tu N, Wang Q, Cheng Z, Han X, Bu L. (18) F-FDG PET/CT imaging of small intestinal metastasis from pulmonary sarcomatoid carcinoma: Brief report and review of the literature. Thorac Cancer. 2020;11:2325-2330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Han SH, Lee JW, Hyun CL, Yoo SY, Lee JH, Kwon JM, Kim WK. Solitary rectal metastasis from primary small cell lung carcinoma. Thorac Cancer. 2012;3:284-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Khan AM, Khan S, Dave V, Bhurgri H. Small cell lung carcinoma presenting as a caecal polyp on surveillance colonoscopy. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Vittorakis S, Giannakopoulou G, Konstantinides K, Daskalaki A, Samitas K. Isolated colonic metastasis two years after resection of stage IA primary adenocarcinoma of the lung: A case report. Respir Med Case Rep. 2018;25:86-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Chen CH, Chen WM, Tung SY, Wu CS, Tong WL, Lee KF, Wei KL. Gastrointestinal metastasis from primary sarcomatoid carcinoma of the lung: a case report and review of the literature. World J Surg Oncol. 2015;13:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Wei SC, Su WC, Chang MC, Chang YT, Wang CY, Wong JM. Incidence, endoscopic morphology and distribution of metastatic lesions in the gastrointestinal tract. J Gastroenterol Hepatol. 2007;22:827-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Wang R, Liu K, Pan D, Ren F, Cui W, Jiang J, Wu S. Isolated sigmoid colon metastasis from lung micropapillary adenocarcinoma: a case report. Int J Clin Exp Pathol. 2019;12:3560-3564. [PubMed] |
| 26. | Rossi G, Pelosi G, Graziano P, Barbareschi M, Papotti M. A reevaluation of the clinical significance of histological subtyping of non--small-cell lung carcinoma: diagnostic algorithms in the era of personalized treatments. Int J Surg Pathol. 2009;17:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Chen ZM, Wang HL. Alteration of cytokeratin 7 and cytokeratin 20 expression profile is uniquely associated with tumorigenesis of primary adenocarcinoma of the small intestine. Am J Surg Pathol. 2004;28:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Jagirdar J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch Pathol Lab Med. 2008;132:384-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Uner A, Unsal D, Yilmaz E, Mentes BB, Bozkurt S, Ataoglu O. Colonic metastasis from squamous carcinoma of the lung: report of a case and review of the literature. Int J Clin Pract Suppl. 2005;92-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Choi SJ, Hong SK, Chae G, Ryu YJ, Park SB, Kim YH, Moon SB, Kim SY, Kim H. Solitary colonic metastasis from primary lung adenocarcinoma first presenting as intestinal obstruction: A case report. Medicine (Baltimore). 2019;98:e14063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 31. | Lin MW, Wu CT, Chang YL. Intussusception caused by intestinal metastasis from lung pleomorphic carcinoma. Ann Thorac Cardiovasc Surg. 2014;20 Suppl:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Rashid S, Rajan D, Jacob R, Dahl K, Prasad A, Singh J, Siddiqui G, Sasthakonar V, Freedman L, Gebre W, Takeshige U, Subramani K, Rizvon K, Mustacchia P. Colonic metastases from pleomorphic carcinoma of the lung presenting as an ileocecal intussusception. ISRN Gastroenterol. 2011;2011:137139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Sifuentes Giraldo WA, González García A, Chamorro Tojeiro S, Sánchez Sánchez O, Pian H, Vázquez Díaz M. Colonic perforation secondary to metastatic lung adenocarcinoma during anti-TNF treatment for ankylosing spondylitis. Acta Reumatol Port. 2014;39:72-76. [PubMed] |
| 34. | Bhutta SI, Ahmed Y, Zahid T, Rehman HU, Nur MM, Mahmood T, Calvert P. Colonic Metastasis of Primary Lung Cancer. Case Rep Oncol. 2021;14:901-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Leidich RB, Rudolf LE. Small bowel perforation secondary to metastatic lung carcinoma. Ann Surg. 1981;193:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Johnson AO, Allen MB. Rectal metastases from small cell lung cancer. Respir Med. 1995;89:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Carr CS, Boulos PB. Two cases of solitary metastases from carcinoma of the lung presenting as primary colonic tumours. Br J Surg. 1996;83:647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Carroll D, Rajesh PB. Colonic metastases from primary squamous cell carcinoma of the lung. Eur J Cardiothorac Surg. 2001;19:719-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Miyazaki K, Satoh H, Sekizawa K. Metastasis to appendix from lung adenocarcinoma. Int J Gastrointest Cancer. 2005;36:59-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Doussot A, Chalumeau C, Combier C, Cheynel N, Facy O. Infected colonic mass revealing a lung adenocarcinoma. Clin Res Hepatol Gastroenterol. 2013;37:e141-e142. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Costa Almeida CE, Dos Reis LS, Costa Almeida CM. Colonic metastases from small cell carcinoma of the lung presenting with an acute abdomen: A case report. Int J Surg Case Rep. 2015;9:75-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Prabhakaran S, Williams E, Kong JCH, Warrier SK, Farmer C. Unique case of lung cancer metastasis to a previous colonic anastomosis. ANZ J Surg. 2020;90:1186-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Pararas N, Kirkilessis G, Pikoulis A, Syrigos K, Pikoulis E. A Rare Case of a Metastatic Lung Squamous Cell Carcinoma to the Large Bowel and the Liver. Cureus. 2021;13:e13867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Catalano M, Marini A, Ferrari K, Voltolini L, Cianchi F, Comin CE, Castiglione F, Roviello G, Mini E. Gastric and colonic metastasis from NSCLC: A very unusual case report. Medicine (Baltimore). 2022;101:e28249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Cheng T, Fang J, Ren J, Wang S. Intestinal obstruction caused by colonic metastasis of lung squamous-cell carcinoma. Asian J Surg. 2023;46:1850-1851. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Luo Y, Mou K, Wang J, Luo J, Peng L, Ye H, Lin S. Colon metastasis from lung adenocarcinoma with BRAF V600E mutation: A case report. Front Immunol. 2022;13:970879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 47. | Garwood RA, Sawyer MD, Ledesma EJ, Foley E, Claridge JA. A case and review of bowel perforation secondary to metastatic lung cancer. Am Surg. 2005;71:110-116. [PubMed] |
| 48. | Weng MW, Wang HC, Chiou JC, Lin SL, Lai RS. Colonic metastasis from a primary adenocarcinoma of the lung presenting with acute abdominal pain: a case report. Kaohsiung J Med Sci. 2010;26:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Suzuki M, Okada K, Koyama N, Yamashita N, Yamagishi A, Yamada T, Yoshida H. Usefulness of a Colonic Stent for Colonic Obstruction Caused by Lung Cancer Metastasis. J Nippon Med Sch. 2021;88:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 50. | Tan CJ, Dasari BV, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg. 2012;99:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 51. | Kim EM, Park JH, Kim BC, Son IT, Kim JY, Kim JW. Self-expandable metallic stents as a bridge to surgery in obstructive right- and left-sided colorectal cancer: a multicenter cohort study. Sci Rep. 2023;13:438. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Axmarker T, Leffler M, Lepsenyi M, Thorlacius H, Syk I. Long-term survival after self-expanding metallic stent or stoma decompression as bridge to surgery in acute malignant large bowel obstruction. BJS Open. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Karoui M, Charachon A, Delbaldo C, Loriau J, Laurent A, Sobhani I, Tran Van Nhieu J, Delchier JC, Fagniez PL, Piedbois P, Cherqui D. Stents for palliation of obstructive metastatic colon cancer: impact on management and chemotherapy administration. Arch Surg. 2007;142:619-23; discussion 623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Alkhayal KA, Alshammari SA, Al-Mazrou AM, Almadi MA, Al-Obeed OA, Zubaidi AM, Traiki TAB, Alhassan NS. Short-term outcomes after self-expandable metal stent insertion for obstructing colon cancer: a retrospective cohort study. Ann Saudi Med. 2020;40:403-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 55. | Kim JH, Ku YS, Jeon TJ, Park JY, Chung JW, Kwon KA, Park DK, Kim YJ. The efficacy of self-expanding metal stents for malignant colorectal obstruction by noncolonic malignancy with peritoneal carcinomatosis. Dis Colon Rectum. 2013;56:1228-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Miłek T, Pasek K, Ciostek P, Alkalaya H, Timorek A, Sawicki W, Cendrowski K. Using our own developed stent in the palliative treatment of obstruction in the left half of the colon due to ovarian cancer. Ginekol Pol. 2017;88:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 57. | Kwon SJ, Yoon J, Oh EH, Kim J, Ham NS, Hwang SW, Park SH, Ye BD, Byeon JS, Myung SJ, Yang SK, Yang DH. Factors Associated with Clinical Outcomes of Palliative Stenting for Malignant Colonic Obstruction. Gut Liver. 2021;15:579-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 58. | Kim SJ, Kim HW, Park SB, Kang DH, Choi CW, Song BJ, Hong JB, Kim DJ, Park BS, Son GM. Colonic perforation either during or after stent insertion as a bridge to surgery for malignant colorectal obstruction increases the risk of peritoneal seeding. Surg Endosc. 2015;29:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Pattarajierapan S, Sukphol N, Junmitsakul K, Khomvilai S. Oncologic safety of colonic stenting as a bridge to surgery in left-sided malignant colonic obstruction: Current evidence and prospects. World J Clin Oncol. 2022;13:943-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |