Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1512
Peer-review started: January 26, 2023
First decision: February 7, 2023
Revised: February 22, 2023
Accepted: May 5, 2023
Article in press: May 5, 2023
Published online: July 27, 2023
Processing time: 175 Days and 16.8 Hours
Presence of liver metastatic disease in pancreatic ductal adenocarcinoma (PDAC), either synchronous or metachronous after pancreatic resection, is a terminal diagnosis that warrants management with palliative intent as per all international practice guidelines. However, there is an increasing interest on any potential value of surgical treatment of isolated oligometastatic disease in selected cases.
To present the published evidence on surgical management of PDAC liver metastases, synchronous and metachronous, and compare the outcomes of these treatments to the current standard of care.
A systematic review was performed in line with the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines to compare the outcomes of both synchronous and metachronous liver metastases resection to standard care.
356 studies were identified, 31 studies underwent full-text review and of these 10 were suitable for inclusion. When synchronous resection of liver metastases was compared to standard care, most studies did not demonstrate a survival benefit with the exception of one study that utilised neoadjuvant treatment. However, resection of metachronous disease appeared to confer a survival advantage when compared to treatment with chemotherapy alone.
A survival benefit may exist in resection of selected cases of metachronous liver oligometastatic PDAC disease, after disease biology has been tested with time and systemic treatment. Any survival benefit is less clear in synchronous cases; however an approach with neoadjuvant treatment and consideration of resection in some selected cases may confer some benefit. Future studies should focus on pathways for selection of cases that may benefit from an aggressive approach.
Core Tip: The focus of management for isolated liver oligometastatic disease in pancreatic ductal adenocarcinoma (PDAC) has typically been palliative. However, recently there is an increasing number of series reporting promising results from resection of oligometastatic disease limited in the liver. The findings of this systematic review, which summarises the current available literature, indicate that a survival benefit may exist in resection of selected cases of metachronous liver oligometastatic PDAC disease, after disease biology has been tested with time and systemic treatment. Any survival benefit is less clear in synchronous cases; however an approach with neoadjuvant treatment and consideration of resection in some selected cases may confer some benefit. Future studies should focus on pathways for selection of cases that may benefit from an aggressive approach.
- Citation: Halle-Smith JM, Powell-Brett S, Roberts K, Chatzizacharias NA. Resection of isolated liver oligometastatic disease in pancreatic ductal adenocarcinoma: Is there a survival benefit? A systematic review. World J Gastrointest Surg 2023; 15(7): 1512-1521
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1512.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1512
Pancreatic ductal adenocarcinoma (PDAC) remains a leading cause of cancer death globally, with less than 10% 5-year survival rates[1-3]. One of the reasons behind this poor prognosis is that a substantial number of patients have distant metastatic disease at presentation, with the majority been hepatic due to the portal drainage of the pancreas[4-6].
International practice guidelines consistently advise against resection of PDAC in the setting of liver metastases, instead favouring palliative systemic treatments or best supportive care[7-11], with a typical survival of less than 12 mo[12]. This is in contrast to other malignancies with more favourable biology, such as colorectal or breast, for which resection of liver metastases has been shown to confer a survival advantage in selected cases with an acceptable safety profile[13-16]. Nonetheless, there is an increasing body of evidence in the form of small case series and reports that present promising oncological outcomes following resection of metachronous and even synchronous isolated liver metastases from PDAC primary. Any potential oncological benefits from such an aggressive approach need to be considered, especially as up to 75% of patients who undergo surgical resection and adjuvant therapy for primary PDAC will experience disease recurrence within 2 years[1,17,18] and two thirds of those will have metastatic disease[19,20].
The aim of this systematic review is to present the published evidence on the surgical management of PDAC isolated liver metastases, synchronous and metachronous; and compare the outcomes of these treatments to the current standard of care.
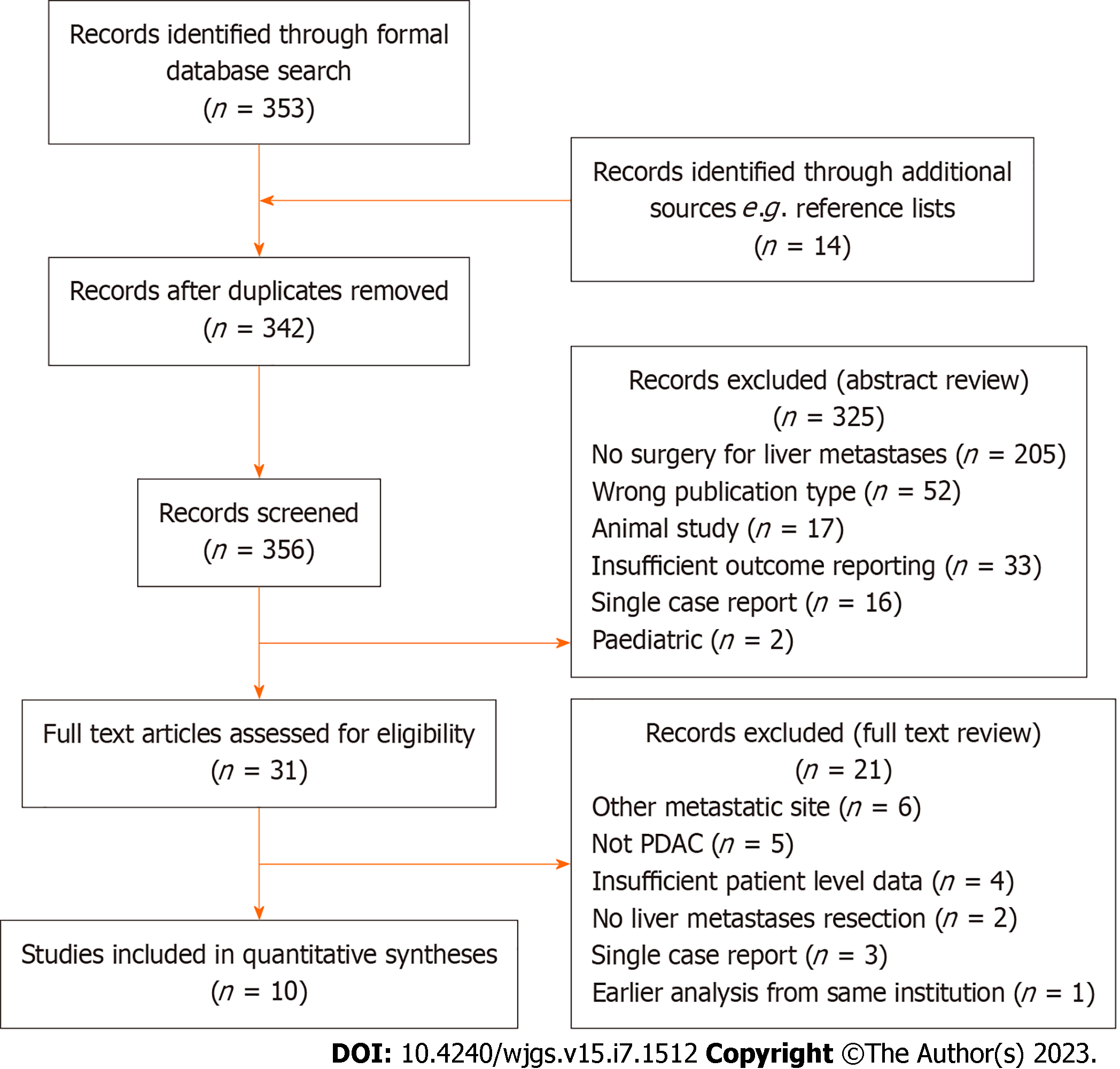
A systematic literature search was performed for studies reporting outcomes of resection of isolated liver metastases in patients with PDAC, in either a synchronous or metachronous setting, according to the Preferred Reporting Items for Systematic Review and Meta-Analyses[21]. Synchronous lesions were defined those appearing within 6 mo of the primary diagnosis, whilst metachronous those diagnosed after 6 mo[22]. Medical subject headings terms, keywords and synonyms for ‘pancreatic cancer’, ‘liver metastases’ and ‘surgical resection’ were combined to search Medline, Embase, Cochrane, PubMed and Reference Citation Analysis (https://www.referencecitationanalysis.com/) databases. Database searches included all results from inception of each database to October 30, 2022. The search term was applied to titles and abstracts and was limited to English language articles. Conference abstracts and letters to the Editor were excluded. Following the removal of duplicates two independent reviewers (Sarah Powell-Brett and James Halle-Smith) screened by title and abstract and then by full text review. Articles were considered appropriate if they reported outcomes following resection of either synchronous or metachronous liver metastases in PDAC patients. Any disputes were resolved by a third, independent, reviewer, the senior author (NC) (Figure 1). The full search term is available in Supplementary Figure 1.
The primary endpoint for this systematic review was median overall survival (OS) in PDAC patients with synchronous or metachronous isolated liver metastases treated with either surgical resection or an alternative treatment modality, for example chemotherapy. Secondary outcomes included disease free survival (DFS), peri-operative morbidity and mortality. As such, for each study survival data were collected in addition to relevant demographic, primary tumour and perioperative details. Data extraction elements were defined in advance and two reviewers independently extracted data using a pre-piloted extraction template (Sarah Powell-Brett and James Halle-Smith).
The literature search identified a total of 356 studies, of which 31 full-text articles were screened and of these 10 articles were suitable for inclusion with a total of 449 patients[23-32] (Figure 1 and Table 1). Of these, 9 reported outcomes of surgical resection for synchronous isolated liver metastases[23-25,27-32] and 4 reported outcomes for resection of metachronous metastases[23,26,30,31]. There were 3 studies that reported outcomes for both synchronous and metachronous metastases[23,30,31] (Table 1). There was a mix of single- and multicentre studies included and all resections were performed in specialist hepato-pancreato-biliary centres. There were no criteria mentioned for the diagnosis or operability of PDAC liver metastases.
| Ref. | Country and Units | Single or multicentre | Metastasectomy timing | Control group? | Total patients included | Total no controls | Gender (% male) | Pancreas resection types included |
| Dünschede et al[23], 2010 | Germany | Single | Metachronous and synchronous | Yes | 13 | 10 | 54 | PD, DP, TP |
| Hackert et al[31], 2017 | Germany | Single | Metachronous and synchronous | No | 85 | - | 55 | PD, DP, TP |
| Hamad et al[24], 2022 | United States | Single | Synchronous only | Yes | 137 | - | - | PD only |
| Klein et al[25], 2012 | Germany | Single | Synchronous only | Yes | 22 | 22 | 64 | PD, DP, TP |
| Shi et al[32], 2016 | China | Single | Synchronous only | Yes | 30 | 39 | 67 | PD, DP, TP |
| Schwarz et al[26], 2020 | Germany, Austria and United States | Multicentre | Metachronous only | Yes | 25 | 8 | 42 | NR |
| Tachezy et al[27], 2016 | Greece, France, Italy and Germany | Multicentre | Synchronous only | Yes | 69 | 69 | 63 | PD, DP, TP |
| Takeda et al[28], 2023 | Japan | Single | Synchronous only | Yes | 5 | - | 80 | NR |
| Yang et al[29], 2020 | China | Single | Synchronous only | Yes | 48 | 10 | 58 | PD, DP |
| Zanini et al[30], 2015 | Italy | Single | Metachronous and synchronous | No | 15 | - | 53 | PD, DP, TP |
In the 9 studies that reported outcomes of synchronous liver metastases resection, there were a total of 393 patients. The median number of metastases resected ranged 2-3 and the diameter 1–9 cm when reported. Non-anatomical resections (NAR) were the most common type of liver resection (133/152, 88%), with segmentectomies (15/152 10%) and hemihepatectomies (4/152, 3%) also been performed (Table 2). The postoperative morbidity rates ranged between 15% and 68% and mortality between 0 and 4.1%. DFS was reported in 2 studies with a range of 5.2 to 20.9 mo. OS was reported in all studies and ranged from 7.8 to 33 mo. Two of the studies also reported 5-year survival results at 3.3% and 5.8% (Table 2). The extent of liver resection and its relationship to survival was reported in 3 studies but was not found to be significant[26,30,31].
| Ref. | n of patients | n of metastases (median, IQR) | Mean diameter of LM, cm | Type of liver resection | Primary resection R0 rate | Post op morbidity | Post op mortality | DFS (months) | OS (months) | 5-yr survival |
| Dünschede et al[23], 2010 | 9 | 3 (1-5) | 3.5 (1-9) | NAR (6), Seg (1), HH (2) | 100% | 33% | 0% | - | 8 | 0% |
| Hackert et al[31], 2017 | 62 | - | - | NAR (59), Seg (2), HH (1) | - | 47% | 1.60% | - | 10.6 | - |
| Hamad et al[24], 2022 | 137 | - | - | - | - | - | - | - | 15.6 | - |
| Klein et al[25], 2012 | 22 | - | - | NAR (15), Seg (7) | 32% | 27% | 0% | - | 7.4 | 0% |
| Shi et al[32], 2016 | 30 | - | - | - | - | - | - | - | 15.7 | 3.3% |
| Tachezy et al[27], 2016 | 69 | 2 (1-2) | - | - | 58% | 68% | 1% | - | 14.5 | 5.8% |
| Takeda et al[28], 2023 | 5 | 2 (2-2) | - | - | - | 23% CD3+ | 0% | 20.9 | 33 | - |
| Yang et al[29], 2020 | 48 | - | - | NAR (43), Seg (4), HH (1) | 100% | 15% | 4.1% | - | 7.8 | 0% |
| Zanini et al[30], 2015 | 11 | 2 (1-2) | 2.4 (1.75-2.5) | NAR (1), Seg (10) | - | 27% CD3+; 54% overall | 0% | 5.2 | 9.1 | 0% |
There were 5 studies that compared operative and non-operative treatment of patients with synchronous isolated liver metastases. The control groups varied between the studies, with some comparing the resection group to PDAC patients without liver metastases, whilst others compared to patients receiving chemotherapy only or surgical palliative bypass only. A formal meta-analysis was therefore not appropriate owing to the heterogenous nature of the control groups. Instead, median OS is reported in Table 3. Two studies reported reduced OS amongst the resection group[23,25], two similar OS[29,32] and one superior OS in the synchronous liver metastases resection group[27].
| Ref. | Treatment modality | n of patients | Median age | n of mets | Max met diameter | Adjuvant chemotherapy | OS | P value | |
| Dünschede et al[23], 2010 | Control | Gemcitabine, no surgical resection | 5 | 63 | 1 | 3.5 (1-9) | NR | Median 11 mo | NR |
| Intervention | Synchronous resection of pancreatic primary and liver metastases | 9 | 55 | 3 | Mean 3.5 cm | NR | Median 8 mo | ||
| Klein et al[25], 2012 | Control | Pancreatic primary resection only. No liver metastases | 22 | Mean 57.5 | - | - | 22 (100) | Median 14.6 mo | 0.015 |
| Intervention | Synchronous resection of pancreatic primary and liver metastases | 22 | Mean 57.5 | - | - | 22 (100) | Median 7.6 mo | ||
| Shi et al[32], 2016 | Control | Palliative bypass and gemcitabine | 39 | Mean 63 | - | - | NR | Median 16.9 mo | 0.085 |
| Intervention | Synchronous resection of pancreatic primary and liver metastases | 30 | Mean 62.2 | - | - | NR | Median 15.7 mo | ||
| Tachezy et al[27], 2016 | Control | No resection, palliative bypass/exploration and chemotherapy (FOLFIRINOX or Gemcitabine) | 69 | Median 62 | 2 (1-8) | - | 58 (84) | Median 7.5 mo | < 0.001 |
| Intervention | Synchronous resection of pancreatic primary and liver metastases | 69 | Median 65 | 2 (1-11) | - | 58 (84) | Median 14.5 mo | ||
| Yang et al[29], 2020 | Control | Systemic gemcitabine-based chemotherapy, no surgery | 31 | Mean 61.1 | - | - | NR | Median 7.6 mo | 0.37 |
| Intervention | Synchronous resection of pancreatic primary and liver metastases | 48 | Mean 61.1 | - | - | NR | Median 7.8 mo |
In the 4 studies that reported outcomes of metachronous isolated liver metastases resection, a total of 56 patients were included. The time from primary resection to detection of liver metastases was given in two studies and ranged 8-13 mo. The remaining two studies reported the time from primary resection to liver metastasectomy and the median values were 17.8 and 18.4 mo. The number of lesions resected ranged 1-2 and the diameter of these lesions was 1.0-2.5 cm. NAR was the most common (28/56, 50%) type of liver resection reported, followed by segmentectomy (19/56, 34%) and hemihepatectomy (9/56, 16%). The postoperative morbidity ranged 32%-75% and mortality 0%-4.3%. OS from metastasectomy was reported in 3 of the 4 studies, with one reporting OS from time of detection of liver metastases, and ranged from 14.8-31 mo. DFS was reported in 2 studies and ranged from 8 to 14.9 mo (Table 4). One study reported patients surviving longer than 5 years after resection of metachronous liver metastases[31].
| Ref. | n of patients | Age (median IQR) | n of Mets (median, IQR) | Mean diameter of LM, cm | Type of liver resection | Time to metastases detection or resection | Postop morbidity | Postop mortality | OS1 (median, months) | 5-yr survival |
| Dünschede et al[23], 2010 | 4 | 42 (41–81) | 1.75 (1–2) | 2.2 (1–3) | NAR (3), Seg (1), HH (6) | 92 | 0 | 0 | 31 | 0% |
| Hackert et al[31], 2017 | 23 | 60.4 (mean, for SM and MM) | NAR (14), Seg (3), HH (6) | 18.43 | 34% | 4.30% | 14.8 | - | ||
| Schwarz et al[26], 2020 | 25 | 63.8 | 1 (1,2) | - | NAR (8), Seg (15), HH (2) | 17.83 | 32% CD1; 12% CD3 | 0% | 36.8 | 0% |
| Zanini et al[30], 2015 | 4 | 48 (40.5-55.25) | 1 (1-1) | 2.2 (2-2.5) | NAR (3), HH (1) | 8.02 | 25% CD3+; 75% overall | 0% | 11.4 | 0% |
There were 2 studies that compared operative and non-operative treatment of patients presenting with metachronous liver metastases after PDAC resection. Meta-analysis was not possible due to the format in which OS was reported. Both studies reported a survival benefit with resection, with a median OS of 31 mo from LM detection in the resection group compared to 11 mo in one study[23] and 36.8 mo from LM resection compared to 9.2 mo in the other one[26] (Table 5).
| Ref. | Treatment modality | n of patients | Median age | n of mets | Max met diameter | Overall survival1 (median, months) | P value | |
| Dünschede et al[23], 2010 | Control | Systemic chemotherapy (gemcitabine), no surgery | 5 | 42 | 1.75 | 2.2 cm (average) | 11 (8-19) | < 0.05 |
| Intervention | Resection of liver metastases | 4 | 42 | 1.75 | 2.2 cm (average) | 31 (20-51) | ||
| Schwarz et al[26], 2020 | Control | Systemic chemotherapy (gemcitabine), no surgery | 8 | Median 69.4 | 2 | - | 9.2 | 0.0007 |
| Intervention | Resection of liver metastases | 25 | Median 63.8 | 1 | - | 36.8 |
Diagnosis of metastatic disease in the liver from primary PDAC is a palliative diagnosis. Nonetheless, with the advances in systemic treatment and surgical techniques, a question that is recently becoming more and more relevant is whether a more aggressive approach with surgical resection of PDAC oligometastatic disease could confer survival benefit in selected cases. This question becomes more prominent in cases of synchronous isolated and limited liver metastases that are identified intra-operatively despite full pre-operative staging investigations or in cases of metachronous liver oligometastatic disease where tumour biology has been assessed with monitoring and systemic treatment. This systematic review analyses the published evidence on the surgical management of PDAC isolated liver metastases, synchronous and metachronous, and how these compare to the current standard of care.
The identification of metastatic disease during the laparotomy in patients with PDAC and no evidence of metastases in pre-operative staging investigations upgrades the disease stage and changes the management intent from potentially curative to palliative as per all the international guidelines. Despite utilising all modern imaging staging modalities, this can still occur in around 15%[33,34] of the cases. Nonetheless, a number of series reported outcomes with a more aggressive approach, resecting isolated oligometastatic liver disease at the same time as the pancreatic resection. The reported OS was generally poor and ranged between 7.8 and 15.7 mo, with only one study reporting OS of 33 mo. Of note, long-term survival following synchronous resection was reported in some of the included studies[27,32]. It is unsurprising that OS was reduced in patients undergoing synchronous liver metastases resection when compared to PDAC patients without metastatic disease[25], given the more advanced stage of the disease. Interestingly though when resection of liver metastases was compared to palliative treatment (surgical bypass and chemotherapy)[23,32], survival after bypass and gemcitabine was superior to that of the groups treated with simultaneous resection of the primary and liver metastases in both of these studies. This may be explained by the likely longer time interval from surgery to chemotherapy in patients that had synchronous resection owing to the more complex post-operative course with higher risk for complications and prolonged recovery. Furthermore, the current suggested standard chemotherapy regimen is FOLFIRINOX[12,35], which also has a more cytotoxic profile and therefore even less likely to be tolerated well early in the post-operative period after major resection. Only one study reported superior survival amongst patients undergoing synchronous resection compared to palliative treatment[27]. However, in this study the majority of patients received neoadjuvant chemotherapy so only those with a favourable response and therefore less aggressive tumour biology proceeded to synchronous resection. On the contrary, patients who did not respond to neoadjuvant therapy and therefore likely had more aggressive disease biology did not proceed to resection and were included in the non-resection group. Therefore, any survival advantage reported is likely mainly attributed to the benefits of the systemic treatment, as well as the patient and disease biology selection by the neoadjuvant approach, rather than the surgical treatment. Indeed, the prognostic significance of response to preoperative chemotherapy has recently also been shown in another study reporting outcomes of chemotherapy and resection in metastatic PDAC patients with liver, lung and peritoneal spread[36].
The survival outcomes reported after resection of metachronous liver metastases were more promising and ranged between 11.4 and 31 mo. Most of the patients included in these studies have received adjuvant chemotherapy (mainly gemcitabine based) after the pancreatic primary resection and had liver metastasectomy about 1.5 years later[26,31]. Therefore, the likely more favourable disease biology, with stability on and after systemic treatment can once again possibly explain the recorded survival. Whilst it was not possible to perform a formal meta-analysis comparing the outcomes after resection of metachronous liver metastases to the control group (palliative chemotherapy), a survival benefit of over one year was observed in both studies included. Of note, both studies used gemcitabine based chemotherapy regimens, known to have inferior results to the current standards of care (FOLFIRINOX)[12,35]. More importantly one study also reported a 8.1% 5-year survival rate with metastasectomy[31]. Even though the study included both synchronous and metachronous liver metastases resections and the 5-year survival rates were not reported separately for the two groups, it is more likely that the metachronous group had longer OS.
The limitations of this review include its narrative descriptive nature, as well as the retrospective nature of the studies included with limited number of patients introducing patient and treatment selection bias. It is also focused on surgical resection as opposed to ablation, radiotherapy or other forms of liver directed therapies. The type, timing and duration of systemic treatment were not standardised or consistent among the studies. Younger or fit patients with less aggressive disease were therefore more likely to have received a more aggressive treatment approach including surgical resection. A meta-analysis was not appropriate, due to heterogeneity between the 5 studies in the synchronous group and the lack of reported OS confidence intervals in the one of the two studies in the metachronous group. Finally, most patients in the included studies received gemcitabine based chemotherapy regimens, which confer inferior outcomes to FOLFIRINOX which is the current standard of care[12]. Nonetheless, this review presents and analyses the best current available evidence on the resection of PDAC isolated liver oligometastatic disease.
In conclusion, the evidence on surgical management of PDAC isolated liver metastases is scarce and inconclusive. A survival benefit may exist in selected metachronous cases when disease biology has been tested with time and systemic treatment. Survival benefit is less clear in synchronous cases; however an approach with neoadjuvant treatment and consideration of resection in some selected cases may confer some benefit. Future studies should focus on pathways for selection of cases that may benefit from an aggressive approach, including patient selection, tumour genetic testing and individualised systemic treatment, as well as novel markers for treatment response.
Pancreatic ductal adenocarcinoma (PDAC) remains a leading cause of cancer death globally, with a substantial number of patients presenting with metastatic disease and typical survival of less than 12 mo. Furthermore, up to 75% of patients who undergo surgical resection and adjuvant therapy for primary PDAC will experience disease recurrence within 2 years and two thirds of those will have metastatic disease. International practice guidelines consistently suggest palliative treatment pathways for these patients. Nonetheless, there is an increasing body of evidence in the form of small case series and reports that present promising oncological outcomes following resection of metachronous and even synchronous isolated liver metastases from PDAC primary.
A number of patients with oligometastatic disease may benefit from an aggressive approach which includes surgical resection.
The aim of this systematic review is to present the published evidence on the surgical management of PDAC isolated liver metastases, synchronous and metachronous; and compare the outcomes to the current standard of care (palliative treatment).
A systematic literature search was performed for studies reporting outcomes of resection of isolated liver metastases in patients with PDAC, in either a synchronous or metachronous setting, according to the Preferred Reporting Items for Systematic Review and Meta-Analyses. Synchronous lesions were defined those appearing within 6 mo of the primary diagnosis, whilst metachronous those diagnosed after 6 mo. The primary endpoint for this systematic review was median overall survival in PDAC patients with synchronous or metachronous isolated liver metastases treated with either surgical resection or an alternative treatment modality, for example chemotherapy. Secondary outcomes included disease free survival, peri-operative morbidity and mortality.
The literature search identified a total of 356 studies, of which 31 full-text articles were screened and of these 10 articles were suitable for inclusion with a total of 449 patients. Nine studies reported outcomes of surgical resection for synchronous isolated liver metastases and 4 reported outcomes for resection of metachronous metastases (3 studies reported outcomes for both).
In conclusion, the evidence on surgical management of PDAC isolated liver metastases is scarce and inconclusive. A survival benefit may exist in selected metachronous cases when disease biology has been tested with time and systemic treatment. Survival benefit is less clear in synchronous cases; however an approach with neoadjuvant treatment and consideration of resection in some selected cases may confer some benefit.
Future studies should focus on pathways for selection of cases that may benefit from an aggressive approach, including patient selection, tumour genetic testing and individualised systemic treatment, as well as novel markers for treatment response.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li S, China; Rossi RE, Italy; Yu CZ, China S-Editor: Fan JR L-Editor: A P-Editor: Xu ZH
| 1. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1361] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 2. | Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1327] [Cited by in RCA: 1394] [Article Influence: 174.3] [Reference Citation Analysis (0)] |
| 3. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1346] [Article Influence: 149.6] [Reference Citation Analysis (2)] |
| 4. | Matsuda Y, Hagio M, Naito Z, Ishiwata T. Clinicopathological features of 30 autopsy cases of pancreatic carcinoma. J Nippon Med Sch. 2012;79:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Mierke F, Hempel S, Distler M, Aust DE, Saeger HD, Weitz J, Welsch T. Impact of Portal Vein Involvement from Pancreatic Cancer on Metastatic Pattern After Surgical Resection. Ann Surg Oncol. 2016;23:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Bissolati M, Sandri MT, Burtulo G, Zorzino L, Balzano G, Braga M. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol. 2015;36:991-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Conroy T, Galais MP, Raoul JL, Bouché O, Gourgou-Bourgade S, Douillard JY, Etienne PL, Boige V, Martel-Lafay I, Michel P, Llacer-Moscardo C, François E, Créhange G, Abdelghani MB, Juzyna B, Bedenne L, Adenis A; Fédération Francophone de Cancérologie Digestive and UNICANCER-GI Group. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 8. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4890] [Article Influence: 407.5] [Reference Citation Analysis (0)] |
| 9. | Tempero MA, Malafa MP, Behrman SW, Benson AB 3rd, Casper ES, Chiorean EG, Chung V, Cohen SJ, Czito B, Engebretson A, Feng M, Hawkins WG, Herman J, Hoffman JP, Ko A, Komanduri S, Koong A, Lowy AM, Ma WW, Merchant NB, Mulvihill SJ, Muscarella P 2nd, Nakakura EK, Obando J, Pitman MB, Reddy S, Sasson AR, Thayer SP, Weekes CD, Wolff RA, Wolpin BM, Burns JL, Freedman-Cass DA. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2014;12:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 10. | Bellon E, Gebauer F, Tachezy M, Izbicki JR, Bockhorn M. Pancreatic cancer and liver metastases: state of the art. Updates Surg. 2016;68:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Seufferlein T, Bachet JB, Van Cutsem E, Rougier P; ESMO Guidelines Working Group. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii33-vii40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 12. | Conroy T, Castan F, Lopez A, Turpin A, Ben Abdelghani M, Wei AC, Mitry E, Biagi JJ, Evesque L, Artru P, Lecomte T, Assenat E, Bauguion L, Ychou M, Bouché O, Monard L, Lambert A, Hammel P; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol. 2022;8:1571-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 183] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 13. | Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, Costa A, Winer EP, Cardoso F; ESO-MBC Task Force. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Lillemoe HA, Vauthey JN. Surgical approach to synchronous colorectal liver metastases: staged, combined, or reverse strategy. Hepatobiliary Surg Nutr. 2020;9:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Cetin B, Bilgetekin I, Cengiz M, Ozet A. Managing Synchronous Liver Metastases in Colorectal Cancer. Indian J Surg Oncol. 2018;9:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Teh C, Tejpar S, Van Cutsem E, Vauthey JN, Påhlman L; of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (2)] |
| 17. | Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dörken B, Oettle H, Riess H. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;35:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 18. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 19. | Groot VP, Daamen LA, Hagendoorn J, Borel Rinkes IHM, Busch OR, van Santvoort HC, Besselink MG, Molenaar IQ; Dutch Pancreatic Cancer Group. Current Strategies for Detection and Treatment of Recurrence of Pancreatic Ductal Adenocarcinoma After Resection: A Nationwide Survey. Pancreas. 2017;46:e73-e75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Ratnayake B, Savastyuk AY, Nayar M, Wilson CH, Windsor JA, Roberts K, French JJ, Pandanaboyana S. Recurrence Patterns for Pancreatic Ductal Adenocarcinoma after Upfront Resection Versus Resection Following Neoadjuvant Therapy: A Comprehensive Meta-Analysis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15892] [Article Influence: 1589.2] [Reference Citation Analysis (1)] |
| 22. | Moertel C. Introduction and Presentation of Data. In Multiple Primary Malignant Neoplasms. Cancer. 1966;1-21. |
| 23. | Dünschede F, Will L, von Langsdorf C, Möhler M, Galle PR, Otto G, Vahl CF, Junginger T. Treatment of metachronous and simultaneous liver metastases of pancreatic cancer. Eur Surg Res. 2010;44:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 24. | Hamad A, Underhill J, Ansari A, Thayaparan V, Cloyd JM, Li Y, Pawlik TM, Tsung A, Abushahin L, Ejaz A. Surgical treatment of hepatic oligometastatic pancreatic ductal adenocarcinoma: An analysis of the National Cancer Database. Surgery. 2022;171:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Klein F, Puhl G, Guckelberger O, Pelzer U, Pullankavumkal JR, Guel S, Neuhaus P, Bahra M. The impact of simultaneous liver resection for occult liver metastases of pancreatic adenocarcinoma. Gastroenterol Res Pract. 2012;2012:939350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Schwarz C, Fitschek F, Primavesi F, Stättner S, Margonis GA, Weiss MA, Stavrou GA, Oldhafer KJ, Kornprat P, Wundsam H, Fischer I, Längle F, Függer R, Hauer A, Klug R, Kieler M, Prager G, Schindl M, Stremitzer S, Bodingbauer M, Sahora K, Kaczirek K. Metachronous hepatic resection for liver only pancreatic metastases. Surg Oncol. 2020;35:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Tachezy M, Gebauer F, Janot M, Uhl W, Zerbi A, Montorsi M, Perinel J, Adham M, Dervenis C, Agalianos C, Malleo G, Maggino L, Stein A, Izbicki JR, Bockhorn M. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery. 2016;160:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 28. | Takeda T, Sasaki T, Okamoto T, Kasuga A, Matsuyama M, Ozaka M, Inoue Y, Takahashi Y, Saiura A, Sasahira N. Outcomes of pancreatic cancer with liver oligometastasis. J Hepatobiliary Pancreat Sci. 2023;30:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 29. | Yang J, Zhang J, Lui W, Huo Y, Fu X, Yang M, Hua R, Wang L, Sun Y. Patients with hepatic oligometastatic pancreatic body/tail ductal adenocarcinoma may benefit from synchronous resection. HPB (Oxford). 2020;22:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Zanini N, Lombardi R, Masetti M, Giordano M, Landolfo G, Jovine E. Surgery for isolated liver metastases from pancreatic cancer. Updates Surg. 2015;67:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Hackert T, Niesen W, Hinz U, Tjaden C, Strobel O, Ulrich A, Michalski CW, Büchler MW. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 32. | Shi HJ, Jin C, Fu DL. Preoperative evaluation of pancreatic ductal adenocarcinoma with synchronous liver metastasis: Diagnosis and assessment of unresectability. World J Gastroenterol. 2016;22:10024-10037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB Jr. MDCT in Pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol. 2004;182:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | De Rosa A, Cameron IC, Gomez D. Indications for staging laparoscopy in pancreatic cancer. HPB (Oxford). 2016;18:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Primrose PJ. Pancreatic cancer in adults: diagnosis and management NICE guideline. (e-pub ahead of print 2018). United Kingdom: ELSEVIER, 2020: e3. |
| 36. | Hank T, Klaiber U, Hinz U, Schütte D, Leonhardt CS, Bergmann F, Hackert T, Jäger D, Büchler MW, Strobel O. Oncological Outcome of Conversion Surgery After Preoperative Chemotherapy for Metastatic Pancreatic Cancer. Ann Surg. 2022;277:e1089-e1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |