Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1465
Peer-review started: February 1, 2023
First decision: April 3, 2023
Revised: April 24, 2023
Accepted: May 25, 2023
Article in press: May 25, 2023
Published online: July 27, 2023
Processing time: 170 Days and 10.1 Hours
Total mesorectal excision along the “holy plane” is the only radical surgery for rectal cancer, regardless of tumor size, localization or even tumor stage. However, according to the concept of membrane anatomy, multiple fascial spaces around the rectum could be used as the surgical plane to achieve radical resection.
To propose a new membrane anatomical and staging-oriented classification system for tailoring the radicality during rectal cancer surgery.
A three-dimensional template of the member anatomy of the pelvis was esta
The fascia propria of the rectum, urogenital fascia, vesicohypogastric fascia and parietal fascia lie side by side around the rectum and form three spaces (medial, middle and lateral), and blood vessels and nerves are precisely positioned in the fascia or space. Three types of radical surgery for rectal cancer are described, as are a few subtypes that consider nerve preservation. The surgical planes of the proposed radical surgeries (types A, B and C) correspond exactly to the medial, middle, and lateral spaces, respectively.
Three types of radical surgery can be precisely defined based on membrane anatomy, including nerve-sparing procedures. Our classification system may offer an optimal tool for tailoring rectal cancer surgery.
Core Tip: Total mesorectal excision (TME) is the only surgical option for rectal cancer. It is necessary to establish a variety of surgical procedures apart from Heald’s TME to tailor radical surgery for rectal cancer patients. In this study, we clarified the three-dimensional membrane anatomy of the pelvis and proposed a new anatomical and staging-oriented classification system comprising three types of radical rectal cancer surgery (types A to C). This classification may provide a useful tool for uniting terminology and tailoring rectal cancer surgery.
- Citation: Jiang HH, Ni ZZ, Chang Y, Li AJ, Wang WC, Lv L, Peng J, Pan ZH, Liu HL, Lin MB. New classification system for radical rectal cancer surgery based on membrane anatomy. World J Gastrointest Surg 2023; 15(7): 1465-1473
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1465.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1465
Total mesorectal excision (TME), first described by Heald et al[1] in 1982, has been the gold standard surgical procedure for rectal cancer. TME has completely converted the traditional concepts of radical resection from an organ-centered to mesentery-centered model[2]. Radical surgery emphasizes the importance of dissecting the rectum and its mesentery along the embryological space formed by fasciae[3] and is referred to as mesenteric-based surgery[4] or membrane anatomy-guided surgery in China[5,6]. The “membrane” may refer to the fascia, peritoneum, mesentery or ligament. Currently, membrane anatomy-guided surgery has been widely used in the clinic and thus has different names, such as the fascial anatomy[7], mesenteric anatomy[4], space anatomy[8] or fascia space priority-guided approach[9].
In fact, the concept of membrane anatomy has long existed in gynecology and can be traced back to 1919 and the radical hysterectomy approach of Wilhelm Latzko[10]. Since then, a variety of tailored radical hysterectomy procedures have been developed apart from standard radical hysterectomy[11]. However, TME is currently the only surgical option for rectal cancer, regardless of tumor size, localization or even tumor stage. Furthermore, pelvic lymph node dissection is integrated into the Querleu-Morrow (QM) classification system of radical surgery for cervical cancer and is an integral part of type C surgery (i.e., classical radical hysterectomy)[11]. But for rectal cancer, lateral lymph node dissection (LLND) and TME seem to be two distinct surgeries. A routine LLND is not recommended unless lateral lymph node enlargement is clinically suspicious for metastasis after preoperative chemoradiotherapy[12]. Therefore, it is necessary to establish a variety of surgical procedures apart from Heald’s TME for tailoring rectal cancer surgery.
Notably, the QM classification system was established completely based on anatomy and does not include any discussion on the role of oncological outcome. This classification uses stable anatomical landmarks to describe surgical resection margins. For example, the lateral resection margins for types A to D surgeries are the level between the cervix and ureter, the ureteral tunnel, the medial aspect of the internal iliac artery and the pelvic sidewall, respectively[13] (Figure 1A). The artificial division of the dissection margin may raise questions regarding the rationality of this classification. In fact, the ureter, internal iliac vessels and pelvic wall muscles are covered with the urogenital fascia, vesicohypogastric fascia and parietal fascia, respectively, in the view of membrane anatomy[14]. Therefore, the QM classification is actually based on fascial anatomy. Just because the scientificity of the QM classification can be explained by membrane anatomy theory, this article aims to establish a classification for radical rectal cancer surgery on the basis of clarifying the three-dimensional membrane anatomy of the pelvic cavity.
The anatomical study was based on cadaveric dissection and laparoscopic surgical observation. Cadaveric dissection was performed on 25 formalin-preserved cadavers and 1 fresh-frozen cadaver (13 males and 13 females) at the Anatomy Department. Surgical observations of 212 rectal cancer patients who underwent TME and 20 patients with suspected lateral lymph node metastasis who underwent LLND at our hospital were carried out. Details regarding the dissection procedures or surgical procedures have been described previously[14,15]. This study was conducted in accordance with the Helsinki Declaration[16] and was approved by our local ethics committee (LL-2020-SCI-001), and the work has been reported based on the STROBE guidelines.
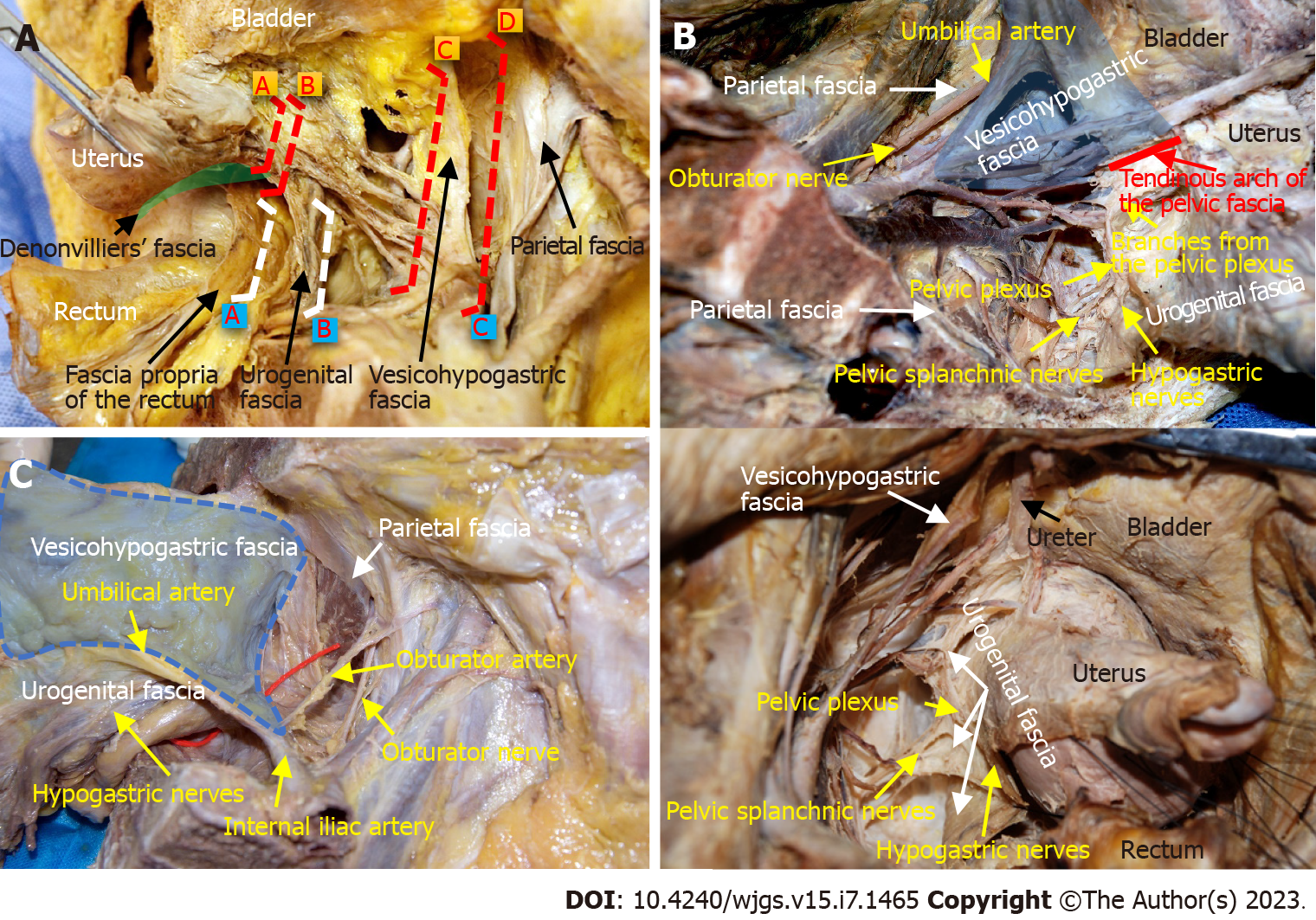
The pelvic membrane anatomy emphasizes the importance of integrity and continuity to describe the fasciae, corresponding interfaces, blood vessels and nerves[17]. Generally, the fascia propria of the rectum, urogenital fascia, vesicohypogastric fascia and parietal fascia lie side by side around the rectum and form three spaces (medial, middle and lateral), and blood vessels and nerves are precisely positioned in the fascia or space (Figures 1A and 2).
Fasciae related to rectal cancer surgery: The fascia propria of the rectum presents as a thin layer of fascia surrounding the mesorectum. The fascia propria of the rectum and visceral fascia are two different layers of fascia (Figure 2A).
The urogenital fascia originates from the perirenal fascia. The anterior and posterior layers of the renal fascia contact intimately below the inferior pole of the kidney to form a sandwich-like fascia sheath. This fascia sheath serves as a corridor carrying the hypogastric nerves (HGNs), ureters and genital vessels. The urogenital fascia surrounds the rectum posterolaterally and extends anterolaterally to its termination in the pelvis. Superiorly, it joins the lateral surface of the uterus/vagina (female) or the prostate (male). Inferiorly, it attaches to the tendinous arch of the pelvic fascia (Figure 1B). Actually, the urogenital fascia[18], urogenital sheath[19], visceral fascia[20], mesoureter[13] ureterohypogastric nerve fascia[21], prehypogastric nerve fascia[22], hypogastric nerve sheath[22], urogenital-hypogastric sheath[23], uterosacral ligament[13] and the deep layer of vesicouterine ligament[24] are anatomical terms used to describe the different parts of the same structure in the literature.
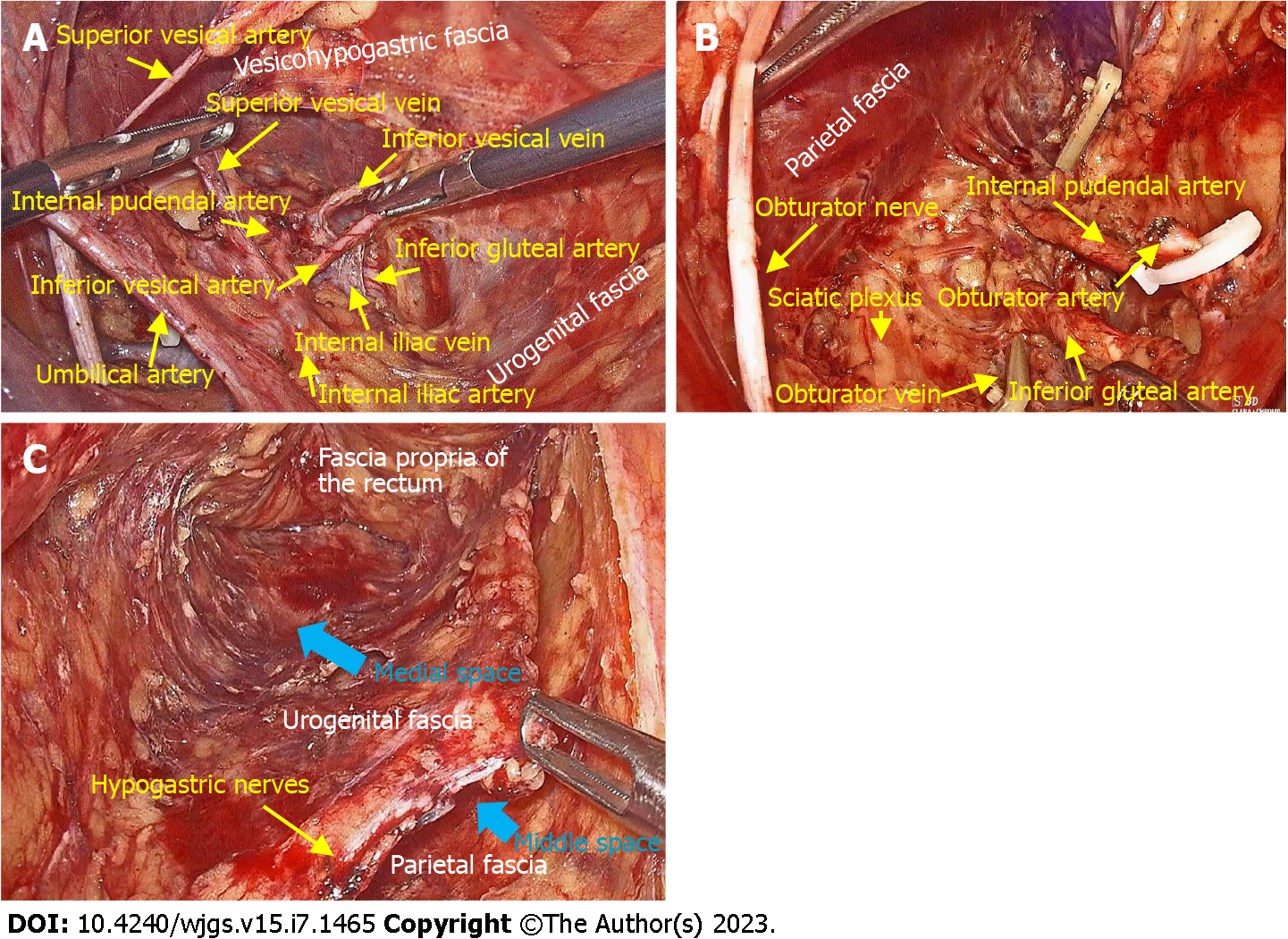
The vesicohypogastric fascia is a triangle-shaped fascia bordered by the umbilical artery, the lateral wall of the bladder and the tendinous arch of the pelvic fascia. Contrary to the traditional view, we found that the bottom boundary of this triangular fascia is not the inferior vesical artery but the tendinous arch of the pelvic fascia[14]. Moreover, the vesicohypogastric fascia is not a continuation of the urogenital fascia but a single-layered fascia covering the lateral surface of the anterior trunks of the internal iliac vessels and their branches. These branches are all located medial to the vesicohypogastric fascia except the obturator vessels, which extend laterally to the obturator canal (Figures 1B and 1C).
The parietal fascia covers the pelvic muscles and bones. Although the definition of the presacral fascia has previously been controversial, it is now recognized as part of the parietal fascia in classic anatomical textbooks, including Gray’s Anatomy[25]. Therefore, the parietal fascia consists of four parts: The obturator fascia, piriformis fascia, superior fascia of the pelvic diaphragm and presacral fascia.
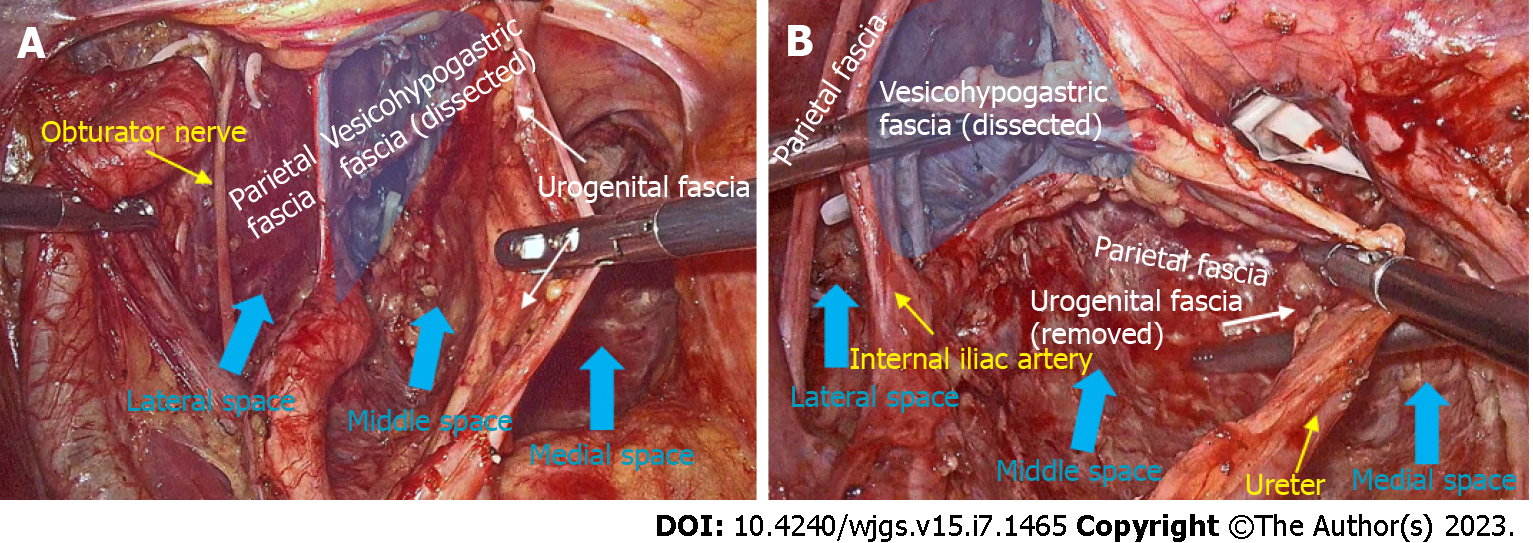
Fascial spaces related to rectal cancer surgery: The medial space is located between the fascia propria of the rectum and the urogenital fascia and includes the following spaces from classical surgical anatomy: Okabayashi’s pararectal space[26], Yabuki space[27], Okabayashi’s paravaginal space[28] or Fujii space[28]. The HGNs and pelvic plexus are sandwiched in the urogenital fascia (Figure 2A).
The middle space lies between the urogenital fascia and the vesicohypogastric fascia, and corresponds to Heald’s “holy plane”[3], Latzko’s pararectal space[26] or medial paravesical space[29] in traditional anatomy. This space contains mostly blood vessels but also the pelvic splanchnic nerves. The anterior trunks of the internal iliac vessels and most of their branches run in this space, including the uterine artery, middle rectal artery, and superficial and deep uterine veins (Figure 3A).
The lateral space is the space between the vesicohypogastric fascia and the parietal fascia, and includes the following spaces from classical surgical anatomy: Prevesical space, perivesical space or presacral space[29]. The contents of this space are mainly nerves, including the lumbosacral trunk, sciatic plexus, sciatic nerve and obturator nerve. The obturator vessels also run in this space (Figure 3B).
Nerve anatomy related to rectal cancer surgery: The HGNs run downward in the urogenital fascia and join the pelvic splanchnic nerves penetrating from the parietal fascia, thereby forming the pelvic plexus. The pelvic plexus is located external to the junction of the urogenital fascia and Denonvilliers’ fascia, and gives off several nerve branches, including the rectal branch, uterine branch and vesical branch (Figure 1B).
For simplification, this classification system is based only on the lateral extent of resection. Radical rectal cancer surgeries are classified into three types (A, B and C), and each type can be precisely defined on a three-dimensional model of membrane anatomy (Figures 1A and 2).
The dissection plane for type A surgery is located in the medial space, and the “fascia tube” formed by the fascia propria of the rectum is resected while the urogenital fascia is preserved (Figures 2A and 3C). According to the European Society for Medical Oncology (ESMO) clinical practice guidelines[30], type A surgery is adapted to rectal cancer classified as very early stage (cT1N0) with adverse histopathological features or early stage (cT1-2N0).
The dissection plane for type B surgery is located in the middle space, and the “mesorectum” formed by the urogenital fascia and Denonvilliers’ fascia is resected, and thus type B surgery corresponds to the so-called classical TME (Figure 3C). ESMO guidelines recommend neoadjuvant therapy followed by TME for patients with intermediate-stage rectal cancer, including cT3a/b with clear mesorectal fascia in the lower rectum and cT3a/b, N0-1 without tumor deposit or extramural vascular invasion (EMVI) in the mid- or high rectum. However, the guidelines also suggest that TME alone is a standard only if high-quality mesorectal resection is assured[30]. As the gold standard surgical treatment for rectal cancer, classical TME can meet the requirements of radical excision.
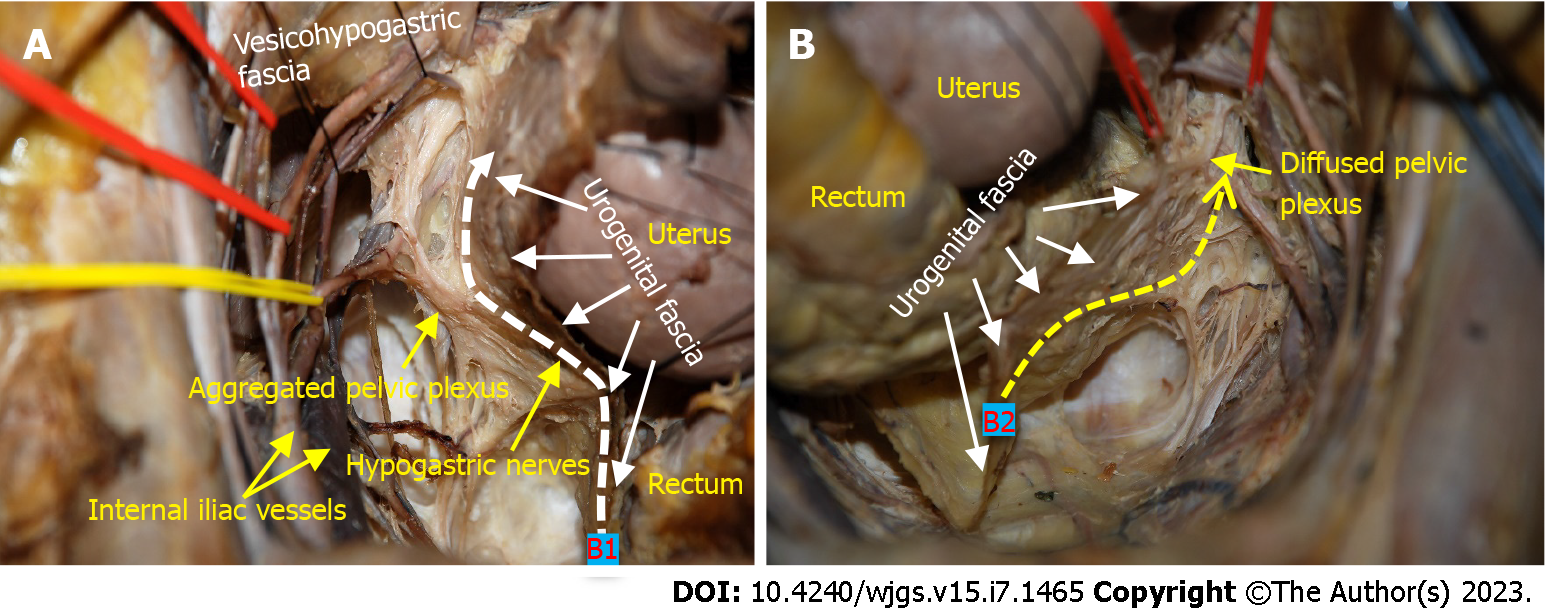
Type B surgery can be further divided into two subcategories: B1 with nerve preservation and B2 without preservation of autonomic nerves. From a purely anatomical point of view, the possibility of preserving autonomic nerves largely depends on the morphology of the pelvic plexus. According to its configuration and its relation to the urogenital fascia, we divide the pelvic plexus into two categories: Aggregated and diffuse shapes[31]. The former has been described in the classical anatomy textbooks as a roughly quadrilateral plaque, with areolar tissue between the pelvic plexus and the urogenital fascia[31]. Therefore, the pelvic plexus can be preserved during resection of the urogenital fascia, which corresponds to the type B1 procedure (Figure 4A). A diffuse pelvic plexus presents as a flattened neurofascial network without a fixed form and is in tight contact with the urogenital fascia; they seem to be inseparable from each other by sharp dissection. Therefore, the pelvic plexus has to be resected together with the urogenital fascia, which corresponds to the type B2 procedure (Figure 4B).
The dissection plane for type C surgery is located in the lateral space. The aim of type C surgery is to completely dissect all three spaces. Therefore, type C surgery is equivalent to TME plus LLND. The ESMO guidelines declare that neoadjuvant therapy is a preferred option for patients with locally advanced disease (>cT3b or EMVI+), and LLND is only performed when the lateral lymph nodes are still enlarged after chemoradiotherapy[30].
Two subcategories are defined for the type C procedure: With (C1) (Figure 5A) and without (C2) preservation of autonomic nerves. Type C2 is indicated for patients with tumors invading the autonomic nerves. The involved nerves must be resected with the urogenital fascia and sometimes with the branches of the internal iliac vessels to achieve oncological radicality (Figure 5B).
TME principles are based on the knowledge that the mesorectal envelope confers protection against tumor dissemination until the terminal stages, and sharp dissection along the “holy plane” allows an en-bloc resection of the lesions[20]. In the traditional anatomy, the space between the visceral and parietal fasciae was the only option for radical rectal cancer surgery[3]. However, there is an onion-like multilayered fascial structure around the rectum, with multiple spaces formed[32]. Thus theoretically, we can take different spaces as the surgical plane to achieve radical resection according to local invasion of the tumor. In this paper, we proposed a new anatomical and staging-oriented classification system for tailoring the radicality during rectal cancer surgery.
We developed a classification comprising three types of radical rectal cancer surgery (A-C) using the theory of “four fasciae and three spaces”, first proposed by our research team[14]. Type A surgery is performed in the medial space between the fascia propria of the rectum and urogenital fascia. Traditionally, the fascia propria of the rectum is considered part of the visceral fascia[3]. However, we have demonstrated that the visceral fascia is actually the urogenital fascia, and the fascia propria of the rectum and visceral fascia are two independent layers of fascia[15]. Given that the fascia propria is defined as an intact envelope around the mesorectum, we named this surgery urogenital fascia-preserving TME[15]. In fact, most of the robotic and laparoscopic TME reported thus far are actually type A surgeries, as the urogenital fascia containing the HGNs is preserved rather than resected, which can be confirmed with photos or videos of these surgical procedures[33,34]. In addition, Denonvilliers’ fascia is located anterior to the “fascial tube” composed of the fascia propria of the rectum. Thus, type A surgery does not require dissection of Denonvilliers’ fascia, which may provide an anatomical basis for the Denonvilliers’ fascia-preserving TME proposed in recent years[35].
Type B surgery corresponds to the classical TME. The visceral fascia together with Denonvilliers’ fascia constitutes the morphology of the “mesorectum”, which has been described in classical anatomy texts. Anterolateral dissection is conducted in the middle space between the urogenital fascia (i.e., visceral fascia) and vesicohypogastric fascia, and therefore requires dissection of the urogenital fascia related to the rectum. It is important to note that the HGNs and pelvic plexus are sandwiched in the urogenital fascia and constitute the so called “nerve plane” described by Fujii[36]. The possibility of preservation of autonomic nerves depends on proper dissection between the urogenital fascia and pelvic plexus. The aggregated pelvic plexus, presenting as a roughly quadrilateral plaque, can be separated from the urogenital fascia by delicate dissection to complete a nerve-sparing type B1 procedure. In contrast, the diffuse pelvic plexus is inextricably intertwined with the urogenital fascia. This inevitably damages the pelvic plexus during dissection of the urogenital fascia, which is called the type B2 procedure.
Type C surgery is equivalent to TME plus LLND and aims for complete resection of all three spaces. The medial space corresponds to the resection area of TME, while the middle space and the lateral space constitute the surgical field for LLND. Routine LLND is controversial[12], but a recent meta-analysis demonstrated that LLND reduces local recurrence of locally advanced lower rectal cancer in the absence of preoperative neoadjuvant chemoradiotherapy[37]. According to the Japanese Classification of Colorectal Cancer (Version 9)[38], lateral lymph nodes include internal iliac lymph nodes (#263), obturator lymph nodes (#283), external iliac lymph nodes (#293), common iliac lymph nodes (#273), lateral sacral lymph nodes (#260), median sacral lymph nodes (#270), and abdominal aortic bifurcation lymph nodes (#280). According to the principle of membrane anatomy, we propose a fascia-to-space surgical approach to perform LLND[14]. Specifically, we first dissect the urogenital fascia, vesicohypogastric fascia and parietal fascia to develop fascial spaces, and this process allows the removal of #293, #273 and #280. Then, we perform dissection of spaces to remove #263 and #270 in the middle space and #283 and #260 in the lateral space (Figure 3C). Type C surgery also has two subcategories, and the possibility of preservation of autonomic nerves depends on tumor invasion and the type of pelvic plexus.
We established a new classification system for radical rectal cancer surgery based on membrane anatomy, which may provide a useful tool for unifying terminology and tailoring radical surgery for rectal cancer. Based on the same membrane anatomical system, we also elucidated the anatomical basis of the QM classification for cervical cancer. Therefore, an in-depth study of the membrane anatomy may contribute to establishing a universal and standardized radical surgery classification system for pelvic cancers. However, our new classification depends on a clearly described anatomical and surgical concept, and requires further investigation and verification through randomized control studies.
The concept of membrane anatomy has been widely used in clinical practice, especially in rectal surgery and gynecology, but there are many differences between them. The hysterectomy includes a variety of procedures, and the radicality of hysterectomy was classified according to Querleu-Morrow classification. However, total mesorectal excision (TME) is currently the only surgical option for rectal cancer, regardless of tumor size, localization or even tumor stage. Therefore, it is necessary to establish a variety of surgical procedures apart from Heald’s TME for tailoring rectal cancer surgery.
Previous work has shown that there is an onion-like multilayered fascial structure around the rectum, with multiple spaces formed. According to the principle of membrane anatomy, we can take different spaces as the surgical plane to achieve radical resection according to local invasion of the tumor.
This study aims to establish a new classification for radical rectal cancer surgery on the basis of clarifying the three-dimensional membrane anatomy of the pelvic cavity.
Detailed pelvic dissections were performed on 26 cadavers, and surgical observations were conducted in 212 rectal patients undergoing laparoscopic TME with or without lateral lymph node dissection (LLND). A three-dimensional model of member anatomy of the pelvis was established, and the related anatomical nomenclatures were clearly clarified. Then, we proposed a membrane anatomical and staging-oriented classification for radical rectal cancer surgery.
Both cadaveric dissection and laparoscopic observation show that, the fascia propria of the rectum, urogenital fascia, vesicohypogastric fascia and parietal fascia lie side by side around the rectum and form three spaces (medial, middle and lateral). Thus, a new classification system for radical rectal cancer surgery was proposed based only on the lateral extent of resection. We described three types of radical surgery for rectal cancer, which can be precisely defined on a three-dimensional anatomical template, including a few subtypes that consider nerve preservation. The surgical planes of the proposed radical surgeries (types A to C) were located in the medial, middle, and lateral spaces, respectively. Types A surgery is a urogenital fascia-preserving procedure, type B surgery corresponds to the classical TME, and type C surgery is equivalent to TME plus LLND.
In this study, a new anatomical and staging-oriented classification system for rectal cancer surgery was established, and may serve as a valuable tool for unifying terminology and tailoring the radicality during surgery for rectal cancer.
We proposed a new and promising classification for radical rectal cancer surgery. However, this classification is established on the basis of anatomical and surgical concept and lacks the support of clinical outcome data, and therefore further clinical investigations are warranted to confirm its role.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dimofte GM, Romania; Gad EH, Egypt S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1937] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 2. | Coffey JC. Surgical anatomy and anatomic surgery - Clinical and scientific mutualism. Surgeon. 2013;11:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med. 1988;81:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 492] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Coffey JC, Dillon M, Sehgal R, Dockery P, Quondamatteo F, Walsh D, Walsh L. Mesenteric-Based Surgery Exploits Gastrointestinal, Peritoneal, Mesenteric and Fascial Continuity from Duodenojejunal Flexure to the Anorectal Junction--A Review. Dig Surg. 2015;32:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Xie D, Gao C, Lu A, Liu L, Yu C, Hu J, Gong J. Proximal segmentation of the dorsal mesogastrium reveals new anatomical implications for laparoscopic surgery. Sci Rep. 2015;5:16287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Shen J, Dong X, Liu Z, Wang G, Yang J, Zhou F, Lu M, Ma X, Li Y, Tang C, Luo X, Zhao Q, Zhang J. Modularized laparoscopic regional en bloc mesogastrium excision (rEME) based on membrane anatomy for distal gastric cancer. Surg Endosc. 2018;32:4698-4705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Mike M, Kano N. Laparoscopic surgery for colon cancer: a review of the fascial composition of the abdominal cavity. Surg Today. 2015;45:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Yang X, Wang J, Wang Y, Fan Q, Li Y. Surgical Technique Based on Space Anatomy for Laparoscopic Radical Trachelectomy with Uterine Artery Preservation. J Laparoendosc Adv Surg Tech A. 2021;31:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Sun Y, Yang HJ, Zhang ZC, Zhou YD, Li P, Zeng QS, Zhang XP. Fascial space priority approach for laparoscopic complete mesocolic excision (CME) plus central vascular ligation or extended lymphadenectomy (CVL/D3) in right-sided colon cancer (with video). Tech Coloproctol. 2022;26:311-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Latzko W. Klinisches und anatomisches zur radikaloperation des gebarmutterkrebses. Zbl Gynak. 1919;43:715-722. |
| 11. | Querleu D, Cibula D, Abu-Rustum NR. 2017 Update on the Querleu-Morrow Classification of Radical Hysterectomy. Ann Surg Oncol. 2017;24:3406-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 12. | Takemasa I. Advances and controversies in treatment for locally advanced rectal cancer over the past decades: West meets East. Ann Gastroenterol Surg. 2020;4:314-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Cibula D, Abu-Rustum NR, Benedetti-Panici P, Köhler C, Raspagliesi F, Querleu D, Morrow CP. New classification system of radical hysterectomy: emphasis on a three-dimensional anatomic template for parametrial resection. Gynecol Oncol. 2011;122:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Jiang HH, Liu HL, Li AJ, Wang WC, Lv L, Peng J, Pan ZH, Chang Y, Lin MB. Laparoscopic lateral lymph node dissection in two fascial spaces for locally advanced lower rectal cancer. World J Gastroenterol. 2021;27:3654-3667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 15. | Liu H, Chang Y, Li A, Wang W, Lv L, Peng J, Pan Z, Jiang H, Lin M. Laparoscopic total mesorectal excision with urogenital fascia preservation for mid-low rectal cancer: Anatomical basis and clinical effect - Experimental research. Int J Surg. 2022;99:106263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14-18. [PubMed] |
| 17. | Liu HL, Chang Y, Lin MB. [Scientific interpretation of membrane anatomy theory and standardized application of membrane anatomy terms]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:634-642. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Diarra B, Stoppa R, Verhaeghe P, Mertl P. About prolongations of the urogenital fascia into the pelvis: an anatomic study and general remarks on the interparietal-peritoneal fasciae. Hernia. 1997;1:191-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Uhlenhuth E, Day EC. The visceral endopelvic fascia and the hypogastric sheath. Surg Gynecol Obstet. 1948;86:9-28. [PubMed] |
| 20. | Heald RJ, Moran BJ. Embryology and anatomy of the rectum. Semin Surg Oncol. 1998;15:66-71. [PubMed] [DOI] [Full Text] |
| 21. | Matsumoto A, Arita K. A technique of laparoscopic lateral pelvic lymph node dissection based on vesicohypogastric fascia and ureterohypogastric nerve fascia for advanced low rectal cancer. Surg Endosc. 2017;31:945-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Kinugasa Y, Murakami G, Suzuki D, Sugihara K. Histological identification of fascial structures posterolateral to the rectum. Br J Surg. 2007;94:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Yang XF, Luo GH, Ding ZH, Li GX, Chen XW, Zhong SZ. The urogenital-hypogastric sheath: an anatomical observation on the relationship between the inferomedial extension of renal fascia and the hypogastric nerves. Int J Colorectal Dis. 2014;29:1417-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Fujii S, Takakura K, Matsumura N, Higuchi T, Yura S, Mandai M, Baba T. Precise anatomy of the vesico-uterine ligament for radical hysterectomy. Gynecol Oncol. 2007;104:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Standring S. Gray’s anatomy e-book: the anatomical basis of clinical practice. Netherlands: Elsevier, 2021. |
| 26. | Yabuki Y. Twenty-first century radical hysterectomy - Journey from descriptive to practical anatomy. Gynecol Oncol Rep. 2020;34:100623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Yabuki Y, Asamoto A, Hoshiba T, Nishimoto H, Nishikawa Y, Nakajima T. Radical hysterectomy: An anatomic evaluation of parametrial dissection. Gynecol Oncol. 2000;77:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Muallem MZ, Diab Y, Sehouli J, Fujii S. Nerve-sparing radical hysterectomy: steps to standardize surgical technique. Int J Gynecol Cancer. 2019;29:1203-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Cosma S, Ferraioli D, Mitidieri M, Ceccaroni M, Zola P, Micheletti L, Benedetto C. A simplified fascial model of pelvic anatomical surgery: going beyond parametrium-centered surgical anatomy. Anat Sci Int. 2021;96:20-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1199] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 31. | Lin M, Chen W, Huang L, Ni J, Yin L. The anatomy of lateral ligament of the rectum and its role in total mesorectal excision. World J Surg. 2010;34:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Sato T, Hashimoto M. Morphological analysis of the fascial lamination of the trunk. Bull Tokyo Med Dent Univ. 1984;31:21-32. [PubMed] |
| 33. | Zhou H, Ruan C, Sun Y, Zhang J, Wang Z, Hu Z. Nerve-guided laparoscopic total mesorectal excision for distal rectal cancer. Ann Surg Oncol. 2015;22:550-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Baca B, Benlice C, Ozben V, Hamzaoglu I, Karahasanoglu T. Totally Robotic Autonomic Nerve-Preserving Total Mesorectal Excisions: Step-by-Step Technical Tips and Tricks. Dis Colon Rectum. 2020;63:562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Wei B, Zheng Z, Fang J, Xiao J, Han F, Huang M, Xu Q, Wang X, Hong C, Wang G, Ju Y, Su G, Deng H, Zhang J, Li J, Chen T, Huang Y, Huang J, Liu J, Yang X, Wei H; Chinese Postoperative Urogenital Function (PUF) Research Collaboration Group. Effect of Denonvilliers' Fascia Preservation Versus Resection During Laparoscopic Total Mesorectal Excision on Postoperative Urogenital Function of Male Rectal Cancer Patients: Initial Results of Chinese PUF-01 Randomized Clinical Trial. Ann Surg. 2021;274:e473-e480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Fujii S. Anatomic identification of nerve-sparing radical hysterectomy: a step-by-step procedure. Gynecol Oncol. 2008;111:S33-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Gao X, Wang C, Yu Y, Singh D, Yang L, Zhou Z. Lateral lymph node dissection reduces local recurrence of locally advanced lower rectal cancer in the absence of preoperative neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. World J Surg Oncol. 2020;18:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication]. J Anus Rectum Colon. 2019;3:175-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 436] [Article Influence: 72.7] [Reference Citation Analysis (0)] |