Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1397
Peer-review started: March 13, 2023
First decision: March 28, 2023
Revised: April 21, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: July 27, 2023
Processing time: 130 Days and 11.6 Hours
For cases of middle and low biliary obstruction with left and right hepatic duct dilatation, the type of approach and whether different approaches affect the difficulty of puncture operation and intraoperative and postoperative complications have not been discussed in detail.
To compare the efficacy of different percutaneous transhepatic biliary stent placements and catheter drainage in treating middle and low biliary obstruction.
A retrospective analysis was performed on the medical records of 424 patients with middle and low biliary obstruction who underwent percutaneous liver puncture biliary stent placement and catheter drainage at the Department of Interventional Radiology, Shaanxi Provincial People’s Hospital between March 2016 and March 2022. Based on the puncture path, patients were categorized into two groups: Subxiphoid left hepatic lobe approach group (Group A, 224 cases) and right intercostal, right hepatic lobe approach group (Group B, 200 cases). Liver function improvement, postoperative biliary bleeding incidence, post
All 424 surgeries were successful without adverse events. Group A comprised 224 cases, and Group B had 200 cases. There was no statistically significant difference in basic data between Group A and Group B (P > 0.05). No significant difference in postoperative biliary bleeding incidence was observed between the groups (P > 0.05). The decreased rates for total bilirubin (Group A: 69.23 ± 4.50, Group B: 63.79 ± 5.65), direct bilirubin (Group A: 79.30 ± 11.19, Group B: 63.62 ± 5.64), and alkaline phosphatase (Group A: 60.51 ± 12.23, Group B: 42.68 ± 23.56) in the 1st wk after surgery were significantly faster in Group A than in Group B. The decreased rate of gamma-glutamyl transpeptidase was also significantly faster in Group A at both 3 d (Group A: 40.56 ± 10.32, Group B: 32.22 ± 5.12) and 1 wk (Group A: 73.19 ± 7.05, Group B: 58.81 ± 18.98) after surgery (P < 0.05). Group A experienced significantly less peritoneal effusion leakage around the drainage tube than Group B (P < 0.05). The patient survival rate was higher in Group A compared to Group B (P < 0.05).
In treating jaundice patients with middle and low biliary obstruction, a percutaneous left liver puncture demonstrated better clinical efficacy than a percutaneous right liver puncture.
Core Tip: For patients with unresectable middle to low level malignant biliary obstruction, percutaneous transhepatic biliary drainage has been widely used in clinical practice. The selection of the approach and whether different approaches will affect the difficulty of puncture surgery, as well as intraoperative and postoperative complications, have not been discussed in detail. This study compared the clinical efficacy of two different puncture pathways in the treatment of middle and low level biliary obstruction. The authors found that the clinical efficacy of patients undergoing percutaneous left liver puncture was superior to that of patients undergoing percutaneous right liver puncture.
- Citation: Yang YB, Yan ZY, Jiao Y, Yang WH, Cui Q, Chen SP. Different percutaneous transhepatic biliary stent placements and catheter drainage in the treatment of middle and low malignant biliary obstruction. World J Gastrointest Surg 2023; 15(7): 1397-1404
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1397.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1397
In patients with unresectable middle and low malignant biliary obstruction, the primary cause is often extrahepatic biliary obstruction and obstruction below the cystic duct opening. Common symptoms include yellowing of the skin and sclera, high fever, abdominal pain, and decreased appetite. In cases of complete obstruction, the stool color may become pale or even clay-like[1]. Percutaneous transhepatic cholangial drainage (PTCD), a widely used palliative care method[2,3], offers advantages such as a straightforward procedure, effective jaundice relief, and convenient bile drainage, making it a popular choice in clinical settings.
For cases of middle and low biliary obstruction with left and right hepatic duct dilatation, the type of approach and whether different approaches affect the difficulty of puncture operation and intraoperative and postoperative complications have not been discussed in detail. This study compared the clinical efficacy of two different puncture paths in treating middle and low biliary obstruction.
Between March 2016 and March 2022, 424 medical records of patients with inoperable middle and low malignant biliary obstruction were analyzed. Patients were divided into two groups based on the puncture approach: Group A, consisting of 224 cases using the subxiphoid left hepatic lobe approach; and Group B, consisting of 200 cases using the right intercostal, right hepatic lobe approach. Inclusion criteria[4,5] were as follows: (1) Clinical presentation, magnetic resonance cholangiopancreatography, computed tomography, ultrasound, and other imaging and laboratory exami
Traditional disinfection and local puncture anesthesia were employed. The puncture site was typically situated at the lower xiphoid process on the right abdominal wall (left bile duct approach) or between the 8th and 9th intercostal spaces of the right seasonal rib (right bile duct approach). The puncture direction and angle were designed for digital subtraction angiography. A Cook 22 G/15 cm biliary puncture needle was used to access the bile duct of the liver, and the needle core was removed. The successful puncture was confirmed by observing the outflow of pale-yellow bile, and a compatible 0.018-inch short guidewire was introduced through the puncture needle. After removing the puncture needle, a matching coaxial introducer sheath (4 F) was inserted along the guidewire. A contrast medium was administered through the introducer sheath to assess the location, extent, and severity of the obstruction of the bile duct. A 0.035-inch ultra-smooth guidewire was inserted through the introducer sheath, and the obstruction site was adjusted.
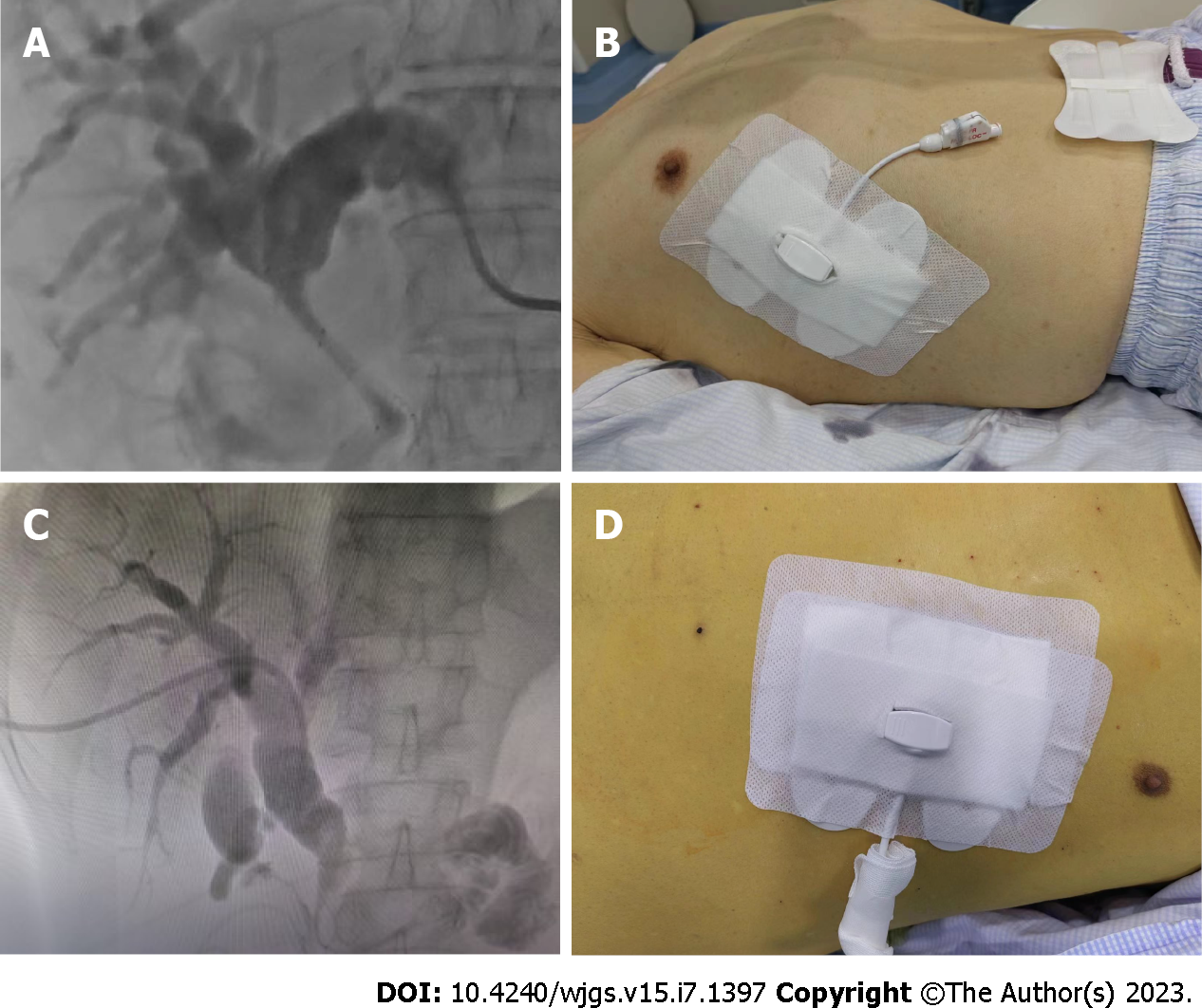
A memory alloy stent was introduced into the biliary system using a pusher along the guidewire, ensuring that the upper end of the stent was above the obstruction, and its lower end was within the obstructed bile duct and duodenum. Both ends needed to extend approximately 1 cm beyond the narrowed section. After the stent was deployed, the metal stent could open smoothly. An 8.5 F external biliary drainage tube was placed through the guidewire, and contrast medium was injected again to verify the resolution of the bile duct obstruction, adequate bile drainage, absence of clotting in the biliary tract, firm fixation of the drainage tube, and proper connection with the drainage bag. Figure 1 displays the imaging data for percutaneous stent placement and catheter drainage of the left/right hepatic ducts. Prior to the procedure, all patients received an intramuscular injection of 10 mg diazepam, 70 mg pethidine hydrochloride, and 10 mg racemolamine hydrochloride.
SPSS23.0 statistical software was used for data analysis. Measurement data were expressed as mean ± SD, and count data were expressed as numbers (percentage). An independent sample t-test was used for comparison between the two groups. The χ2 test was used for comparison between groups. The survival rate was analyzed with the Kaplan-Meier survival curve, and P < 0.05 was considered statistically significant.
A total of 424 patients participated in the study, with no severe adverse events (such as death and critical cardiac or cerebrovascular incidents) observed during the follow-up period. The cohort included 227 males (53.5%) and 197 females (46.5%), with an average age of 68.92 ± 0.36 years. Among the patients, 46 cases (10.8%) had diabetes, 79 cases (18.6%) were smokers, 101 cases (23.8%) had coronary heart disease, and 45 cases (10.6%) experienced hypertension. Additionally, 33 cases (7.8%) presented with hyperlipidemia. No significant differences in the baseline characteristics were identified between the two groups (P > 0.05) (Table 1).
| Clinical features | Group A, n = 224 | Group B, n = 200 | t value | P value |
| Male sex | 124 | 103 | 0.632 | 0.427 |
| Age in yr | 69.76 ± 12.24 | 68.46 ± 14.45 | 0.816 | 0.675 |
| Diabetes | 21 | 25 | 1.060 | 0.302 |
| Smoking | 45 | 34 | 0.665 | 0.415 |
| Coronary heart disease | 53 | 48 | 0.070 | 0.935 |
| Hypertension | 23 | 22 | 0.060 | 0.870 |
| Hyperlipidemia | 14 | 19 | 1.555 | 0.212 |
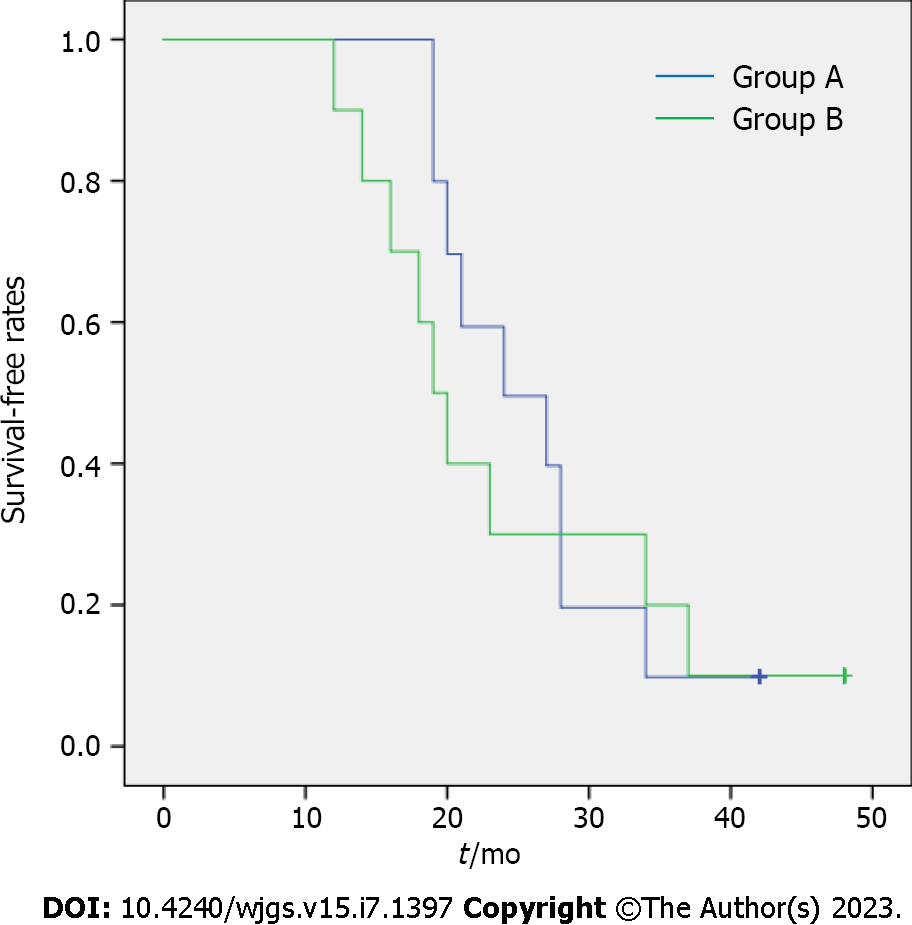
No significant differences were observed between the two groups regarding the incidence of postoperative biliary bleeding and the duration of postoperative pain (P > 0.05). A comparison of postoperative liver function indices revealed that the total bilirubin reduction rate, direct bilirubin reduction rate, and alkaline phosphatase reduction rate were significantly higher in Group A during the 1st wk after surgery. Moreover, the gamma-glutamyl transpeptidase reduction rate in Group A was considerably faster than in Group B at both 3 d and 1 wk post-surgery (P < 0.05). Group A also exhibited significantly lower leakage of peritoneal effusion around the drainage tube compared to Group B (P < 0.05). The survival rate for patients in Group A surpassed that of Group B (P < 0.05) (Table 2 and Figure 2).
| Group A, n = 224 | Group B, n = 200 | t value | P value | |
| Biliary tract bleeding | 2 | 1 | 0.232 | 0.630 |
| Duration of postoperative pain in h | 2.10 ± 0.99 | 2.40 ± 1.89 | -0.443 | 0.663 |
| Peritoneal fluid leaking from around the drainage tube | 13 | 31 | 10.681 | 0.001 |
| Survival time in mo | 26.2 ± 7.39 | 16.1 ± 2.72 | 4.054 | 0.001 |
| Total bilirubin in µmol/L | ||||
| Preoperative | 208.23 ± 81.81 | 233.52 ± 92.41 | -0.648 | 0.525 |
| After 3 d | 152.11 ± 86.21 | 141.15 ± 46.55 | 0.354 | 0.728 |
| After 1 wk | 64.09 ± 26.89 | 88.29 ± 45.40 | -1.451 | 0.164 |
| Rate of decline 3 d after surgery | 30.40 ± 11.84 | 37.59 ± 13.65 | -1.259 | 0.224 |
| Rate of decline 1 wk after surgery | 69.23 ± 4.50 | 63.79 ± 5.65 | 2.379 | 0.029 |
| Direct bilirubin in µmol/L | ||||
| Preoperative | 170.73 ± 69.82 | 194.38 ± 72.66 | -0.742 | 0.468 |
| After 3 d | 106.62 ± 61.87 | 114.37 ± 38.77 | -0.336 | 0.741 |
| After 1 wk | 34.26 ± 23.37 | 72.83 ± 33.03 | -3.014 | 0.007 |
| Rate of decline 3 d after surgery | 39.96 ± 12.05 | 40.05 ± 12.78 | -0.015 | 0.988 |
| Rate of decline 1 wk after surgery | 79.30 ± 11.19 | 63.62 ± 5.64 | 3.956 | 0.001 |
| Alanine aminotransferase (U/L) | ||||
| Preoperative | 127.20 ± 95.72 | 170.10 ± 109.80 | -0.931 | 0.364 |
| After 3 d | 64.60 ± 24.35 | 102.20 ± 51.81 | -2.077 | 0.052 |
| After 1 wk | 31.60 ± 14.72 | 43.00 ± 13.40 | -10.810 | 0.087 |
| Rate of decline 3 d after surgery | 35.99 ± 33.13 | 34.46 ± 9.89 | 0.140 | 0.890 |
| Rate of decline 1 wk after surgery | 67.84 ± 22.83 | 60.38 ± 25.41 | 0.691 | 0.498 |
| Aspartate aminotransferase (U/L) | ||||
| Preoperative | 254.00 ± 192.84 | 144.90 ± 42.79 | 1.746 | 0.098 |
| After 3 d | 51.60 ± 13.14 | 71.40 ± 12.17 | -3.495 | 0.003 |
| After 1 wk | 36.60 ± 15.12 | 38.60 ± 16.04 | -0.287 | 0.778 |
| Rate of decline 3 d after surgery | 72.31 ± 15.13 | 46.81 ± 18.41 | 3.383 | 0.003 |
| Rate of decline 1 wk after surgery | 80.45 ± 13.15 | 68.51 ± 18.28 | 1.676 | 0.111 |
| Alkaline phosphatase in IU/L | ||||
| Preoperative | 995.00 ± 398.24 | 587.70 ± 199.03 | 2.893 | 0.010 |
| After 3 d | 798.00 ± 161.08 | 467.00 ± 155.36 | 4.677 | 0.001 |
| After 1 wk | 371.60 ± 129.25 | 305.80 ± 115.78 | 1.199 | 0.246 |
| Rate of decline 3 d after surgery | 14.24 ± 17.92 | 19.27 ± 15.73 | -0.666 | 0.514 |
| Rate of decline 1 wk after surgery | 60.51 ± 12.23 | 42.68 ± 23.56 | 2.125 | 0.048 |
| Gamma-glutamyl transpeptidase in IU/L | ||||
| Preoperative | 704.30 ± 364.56 | 434.80 ± 111.44 | 2.236 | 0.038 |
| After 3 d | 417.00 ± 202.68 | 293.80 ± 71.79 | 1.812 | 0.087 |
| After 1 wk | 181.40 ± 78.62 | 161.70 ± 52.93 | 0.657 | 0.519 |
| Rate of decline 3 d after surgery | 40.56 ± 10.32 | 32.22 ± 5.12 | 2.286 | 0.035 |
| Rate of decline 1 wk after surgery | 73.1 9 ± 7.05 | 58.81 ± 18.98 | 2.246 | 0.038 |
For patients with jaundice undergoing treatment for middle and low level malignant biliary obstruction, those receiving percutaneous left hepatic puncture demonstrated significantly better liver function improvement, reduced peritoneal effusion leakage around the drainage tube, and enhanced survival compared to patients undergoing percutaneous right hepatic puncture.
Pancreatic head cancer and periampullary cancer (including ampullary cancer, lower common bile duct cancer, and duodenal papilla cancer) are frequent causes of middle and low biliary obstruction as well as a group of gastrointestinal malignancies characterized by insidious onset, rapid progression, and poor treatment outcomes and prognosis[6-10]. Many patients are diagnosed at advanced stages, missing the opportunity for curative surgery. Hence, interventions to alleviate biliary obstruction are necessary to extend survival and improve patient quality of life[11,12]. Kubo et al[13] demonstrated that interventional surgery for malignant obstructive jaundice has broad applicability, minimal invasiveness, high success rates, and rapid postoperative recovery, with PTCD being a common procedure[14-16].
Currently, there is some debate surrounding the advantages and disadvantages of percutaneous left hepatic biliary stent placement and catheter drainage vs percutaneous right hepatic biliary stent placement and catheter drainage for treating low malignant obstructive jaundice. Dumonceau et al[17] investigated the benefits of PTCD and endoscopic retrograde cholangiopancreatography in treating biliary obstruction, concluding that PTCD is a simpler procedure with shorter transhepatic access and broader drainage coverage, making it more effective than endoscopic retrograde cholangiopancreatography for patients with biliary obstruction. However, Inoue et al[18] reported that improper puncture during percutaneous liver biliary drainage and stent implantation might result in vascular damage and biliary bleeding, with percutaneous right hepatic puncture causing more liver parenchyma damage and liver injury. Additionally, patients carrying a drainage bag on their right side experience a significant reduction in their quality of life. Due to the limited selection of percutaneous left hepatic puncture routes and scarce literature, many believe this approach is challenging and exposes patients to high radiation levels. Thus, few studies in China have investigated the treatment of low malignant obstructive jaundice with biliary stent placement and catheter drainage via percutaneous left hepatic puncture.
In line with a humanistic care approach and aiming to enhance long-term survival, our hospital predominantly opted for percutaneous left hepatic biliary stent placement and catheter drainage in treating middle-low malignant obstructive jaundice. We compared the clinical efficacy of two distinct puncture paths in addressing low biliary obstruction. Our study revealed that the reduction rates of total bilirubin, direct bilirubin, aspartate aminotransferase, and gamma-glutamyl transpeptidase in Group A were significantly faster than those in Group B presurgery and post-surgery (P < 0.05). Liver function recovery was notably superior in comparison to percutaneous right hepatic puncture. Factors contributing to these results include the slender left hepatic duct, the small left hepatic lobe, and minimal liver damage. Furthermore, stent implantation ensures an unobstructed common bile duct.
Owing to the intact bile duct in the right hepatic lobe, increased bile duct tension accelerates bile excretion. After the right hepatic puncture, the bile duct was exposed to the external environment, leading to a decrease in intrahepatic bile duct tension and a slower bile excretion rate. Consequently, patients experienced a gradual decrease in bilirubin levels and a prolonged recovery period from liver damage in the short term. We observed and analyzed the significantly lower peritoneal effusion leakage around the drainage tube in Group A compared to Group B, considering the diminished liver function and protein synthesis in patients with advanced tumor stages, which contributed to ascites development. The puncture location for patients who underwent percutaneous right hepatic puncture resulted in a relatively low puncture point in both supine and lateral positions. Excessive abdominal pressure frequently accompanies abdominal fluid leakage from the puncture site, substantially diminishing quality of life. In contrast, the elevated puncture point in patients receiving percutaneous left hepatic puncture led to minimal fluid exudation at the puncture site, whether standing, lying laterally, or supine, resulting in a negligible impact on the quality of life. Group A displayed a marginally higher survival rate, which we attributed to the reduced effect on quality of life, improved mood and comfort, and consequently better mental state, dietary intake, and nutrition, ultimately leading to a longer patient survival time.
Both domestic and international findings combined with our study revealed that percutaneous left hepatic biliary stenting and catheter drainage for treating low biliary obstruction substantially enhanced liver function, quality of life, postoperative pain duration, leakage of peritoneal effusion around the drainage tube, and overall patient survival.
This research was carried out retrospectively and reflected the experiences of a single institution. Additionally, our study acknowledges the constraint posed by the limited sample size, which may result in variations among some of the documented findings.
In managing low biliary obstruction, percutaneous left hepatic biliary stent placement and catheter drainage demonstrated superior therapeutic efficacy compared to percutaneous right hepatic stent placement and catheter drainage. Consequently, for patients experiencing low biliary obstruction, prioritizing percutaneous left hepatic biliary stenting and catheter drainage is recommended.
For cases of middle and low biliary obstruction with left and right hepatic duct dilatation, the type of approach and whether different approaches affect the difficulty of puncture operation and intraoperative and postoperative complications have not been discussed in detail.
This study compared the clinical efficacy of two different puncture paths in treating middle and low biliary obstruction. The study prioritized the optimal puncture pathway.
This study compared the efficacy of different pathways to find the best improvement in patient quality of life and survival rate.
A retrospective analysis was performed on the medical records of 424 patients with middle and low biliary obstruction who underwent percutaneous liver puncture biliary stent placement and catheter drainage between March 2016 and March 2022. Based on the puncture path, patients were categorized into two groups: subxiphoid left hepatic lobe approach group (Group A) and right intercostal, right hepatic lobe approach group (Group B). Liver function improvement, postoperative biliary bleeding incidence, postoperative pain duration, and abdominal effusion leakage around the drainage tube were compared between the two groups at 3 d and 1 wk after the operation.
The decreased rates for total bilirubin, direct bilirubin, and alkaline phosphatase 1 wk after surgery were significantly faster in Group A than in Group B. The decreased rate of gamma-glutamyl transpeptidase was also significantly faster in Group A at both 3 d and 1 wk after surgery. Group A experienced significantly less peritoneal effusion leakage around the drainage tube than Group B. The patient survival rate was higher in Group A compared to Group B.
The study proposed a humanistic care perspective, improving patient survival treatment in the later stage and prioritized the optimal puncture pathway.
This research was carried out retrospectively and reflected the experiences of a single institution. More studies should be performed in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Praktiknjo M, Germany; Reddy KR, United States S-Editor: Wang JL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Kang MJ, Yun EH, Jung KW, Park SJ. Incidence, mortality and survival of gallbladder, extrahepatic bile duct, and pancreatic cancer using Korea central cancer registry database: 1999-2019. Ann Hepatobiliary Pancreat Surg. 2022;26:220-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 2. | Jung JH, Yoon SJ, Lee OJ, Shin SH, Han IW, Heo JS. Surgical outcomes and prognostic factors of distal common bile duct adenocarcinoma: chronological analysis in a single high-volume institutional experience. BMC Surg. 2022;22:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Zhang H, Li F, Huang J, Huo C. Fishing line assisted endoscopic placement of multiple plastic biliary stents for unresectable malignant hilar biliary obstruction: a retrospective study. BMC Gastroenterol. 2021;21:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Yamashita Y, Tachikawa A, Shimokawa T, Yamazaki H, Itonaga M, Sakai Y, Sugiyama H, Nakai Y, Tanaka K, Isayama H, Kitano M. Covered versus uncovered metal stent for endoscopic drainage of a malignant distal biliary obstruction: Meta-analysis. Dig Endosc. 2022;34:938-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Camacho JC, Brody LA, Covey AM. Treatment of Malignant Bile Duct Obstruction: What the Interventional Radiologist Needs to Know. Semin Intervent Radiol. 2021;38:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Kurita A, Uza N, Asada M, Yoshimura K, Takemura T, Yazumi S, Kodama Y, Seno H. Stent placement above the sphincter of Oddi is a useful option for patients with inoperable malignant hilar biliary obstruction. Surg Endosc. 2022;36:2869-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Gao DJ, Yang JF, Ma SR, Wu J, Wang TT, Jin HB, Xia MX, Zhang YC, Shen HZ, Ye X, Zhang XF, Hu B. Endoscopic radiofrequency ablation plus plastic stent placement versus stent placement alone for unresectable extrahepatic biliary cancer: a multicenter randomized controlled trial. Gastrointest Endosc. 2021;94:91-100.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Bernon MM, Shaw J, Burmeister S, Chinnery G, Hofmeyr S, Kloppers JC, Jonas E, Krige JE. Distal malignant biliary obstruction: a prospective randomized trial comparing plastic and uncovered self-expanding metal stents in the palliation of symptomatic jaundice. S Afr J Surg. 2018;56:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Coronel M, Lee JH, Coronel E. Endoscopic Ultrasound for the Diagnosis and Staging of Biliary Malignancy. Clin Liver Dis. 2022;26:115-125. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Jin H, Pang Q, Liu H, Li Z, Wang Y, Lu Y, Zhou L, Pan H, Huang W. Prognostic value of inflammation-based markers in patients with recurrent malignant obstructive jaundice treated by reimplantation of biliary metal stents: A retrospective observational study. Medicine (Baltimore). 2017;96:e5895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Felici C, Mannavola F, Stucci LS, Duda L, Cafforio P, Porta C, Tucci M. Circulating tumor cells from melanoma patients show phenotypic plasticity and metastatic potential in xenograft NOD.CB17 mice. BMC Cancer. 2022;22:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Sakai Y, Iwai T, Shimura K, Gon K, Koizumi K, Ijima M, Chiba K, Nakatani S, Sugiyama H, Tsuyuguchi T, Kamisawa T, Maetani I, Kida M. Safety and efficacy of metallic stent for unresectable distal malignant biliary obstruction in elderly patients. World J Gastroenterol. 2018;24:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kubo N, Harimoto N, Shibuya K, Ishii N, Tsukagoshi M, Igarashi T, Watanabe A, Araki K, Miyazaki M, Kuwano H, Shirabe K. Successful treatment of isolated bile leakage after hepatectomy combination therapy with percutaneous transhepatic portal embolization and bile duct ablation with ethanol: a case report. Surg Case Rep. 2018;4:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Hyun H, Choi SY, Kim KA, Ko SB. Safety and Efficacy of Percutaneous Biliary Covered Stent Placement in Patients with Malignant Biliary Hilar Obstruction; Correlation with Liver Function. Cardiovasc Intervent Radiol. 2016;39:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Melenhorst MC, Scheffer HJ, Vroomen LG, Kazemier G, van den Tol MP, Meijerink MR. Percutaneous Irreversible Electroporation of Unresectable Hilar Cholangiocarcinoma (Klatskin Tumor): A Case Report. Cardiovasc Intervent Radiol. 2016;39:117-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Park JK, Woo YS, Noh DH, Yang JI, Bae SY, Yun HS, Lee JK, Lee KT, Lee KH. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc. 2018;88:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 17. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline – Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 484] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 18. | Inoue T, Naitoh I, Okumura F, Ozeki T, Anbe K, Iwasaki H, Nishie H, Mizushima T, Sano H, Nakazawa T, Yoneda M, Joh T. Reintervention for stent occlusion after bilateral self-expandable metallic stent placement for malignant hilar biliary obstruction. Dig Endosc. 2016;28:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |