Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1375
Peer-review started: January 5, 2023
First decision: April 3, 2023
Revised: April 20, 2023
Accepted: May 26, 2023
Article in press: May 26, 2023
Published online: July 27, 2023
Processing time: 197 Days and 7.9 Hours
Preoperative anemia is associated with increased postoperative morbidity and mortality and increased perioperative transfusion risk. For surgical patients, this affects physical and cognitive ability and quality of life, but it is an important and modifiable risk factor.
To determine the effect of preoperative anemia on the prognosis of gastric cancer (GC) patients and generate a prognostic nomogram to predict the postoperative overall survival (OS) of GC patients with preoperative anemia.
Clinicopathological and follow-up data of GC patients treated at Zhejiang Provincial People's Hospital (China) from 2010 to 2015 were collected. Inde
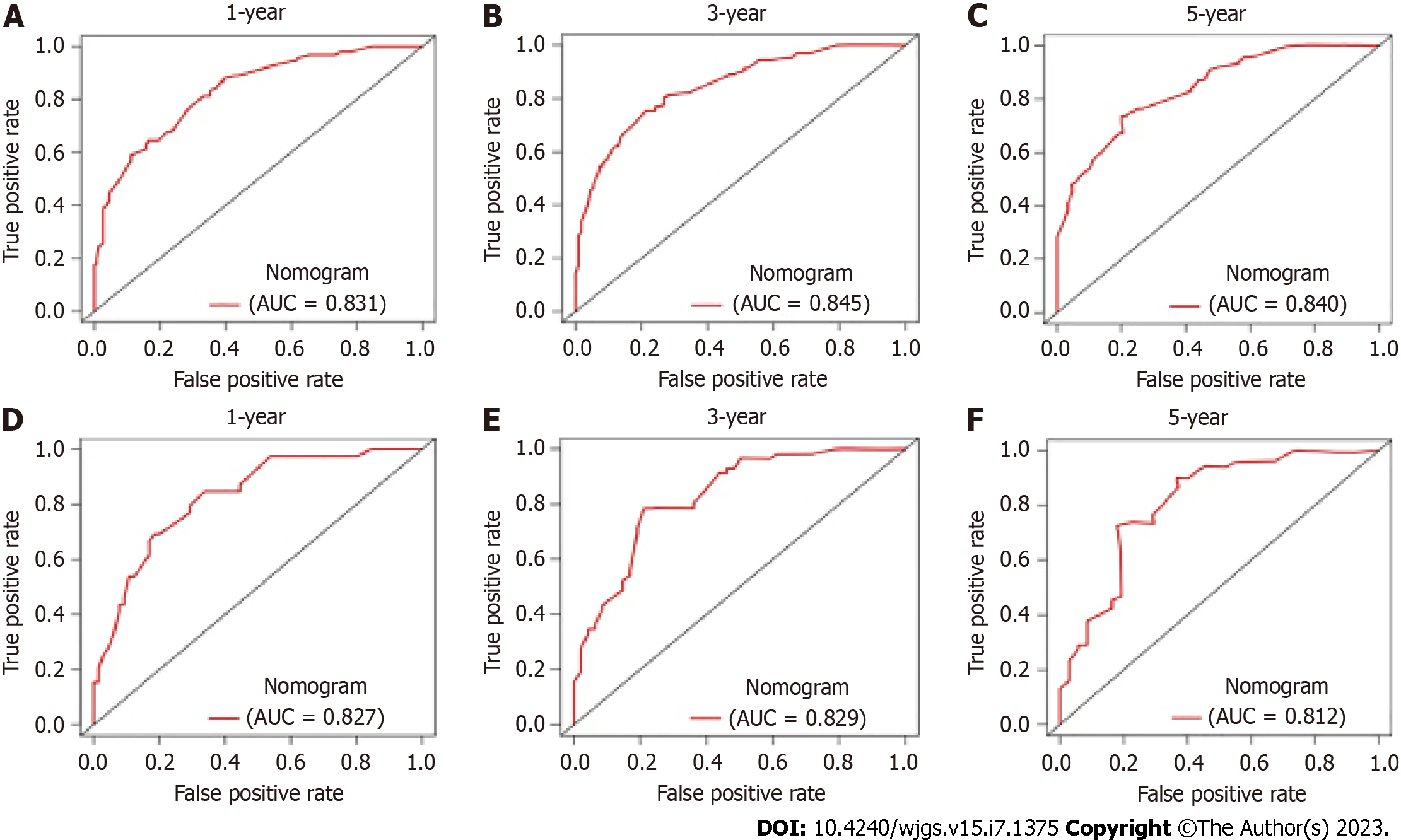
Nine hundred and sixty GC patients were divided into two groups (preoperatively anemic and nonanemic), and postoperative survival analysis was performed on both groups, yielding a shorter postoperative survival for preoperatively anemic patients than for nonanemic patients. A total of 347 GC patients with preoperative anemia were included. Age, preoperative alpha-fetoprotein level, monocyte count, lymphocyte count, clinicopathological stage, liver metastasis, and GC type were identified as independent prognostic factors for OS. The area under the ROC curve (AUC) of the nomogram for predicting 1-, 3-, and 5-year OS was 0.831, 0.845, and 0.840, respectively, for the training cohort, and the corresponding AUC values in the validation cohort were 0.827, 0.829, and 0.812, respectively. Calibration curves and DCA indicated good performance of the nomogram.
In all, we have successfully produced and verified a useful nomogram for predicting OS in GC patients with preoperative anemia. This nomogram based on a variety of clinicopathological indices can provide an effective prognostic assessment and help clinicians choose an appropriate treatment strategy for GC patients with preoperative anemia.
Core Tip: In this work, we evaluated a large amount of clinical information of gastric cancer patients that were collected and then screened for independent prognostic factors by univariate and multivariate Cox regression analyses. These independent prognostic factors were then used to construct a nomogram to predict 1-, 3-, and 5-year overall survival (OS) in gastric cancer patients with preoperative anemia, and the nomogram was evaluated by calibration curves, receiver operating characteristic curves, and decision curve analysis. Finally, we successfully developed and validated a valuable nomogram to predict OS in gastric cancer patients with preoperative anemia.
- Citation: Long Y, Zhou XL, Zhang CL, Wang YN, Pan WS. Nomogram based on clinical characteristics for predicting overall survival in gastric cancer patients with preoperative anemia. World J Gastrointest Surg 2023; 15(7): 1375-1387
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1375.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1375
Gastric cancer (GC) is the fourth most frequently diagnosed cancer in the world and is responsible for approximately 951600 new cases each year[1]. Operative resection plus adjacent treatment is the main therapy for GC. However, despite advances in the diagnosis and treatment of GC, the prognosis remains poor and GC remains the third leading cause of cancer-related deaths with approximately 723100 deaths[1-4]. Prevention and individualized treatment are considered the optimal options to reduce deaths[5-7], and preoperative anemia diagnosis might help to adjust individualized treatment. Absolute lymphocyte and monocyte counts can predict survival in patients with metastatic cancer, the overall survival rate of patients with reduced lymphocyte counts is low, and there is an apparent correlation between monocyte counts and survival[8]. Patients with an absolute monocyte count of 300 to 899 monocytes per cubic millimeter had a significantly better prognosis than those with higher or lower counts[8].
Patients with advanced GC have a high prevalence of anemia, but with high variability, ranging from 10% to 30%[9,10]. Cancer-associated anemia (CRA) is linked to various pathological and clinical factors, such as bleeding, lack of nutrition, and bone-marrow depression[11]. Myelosuppression can be due to invasion of malignant cells and chemotherapy[12,13]. Anemia is a hematological abnormality present in most patients with cancer, and its prevalence varies according to the type of cancer and stage of disease. It has been hypothesized that 30% to 90% of cancer patients present with anemia at the time of diagnosis[14,15]. To assess anemia status within 2 wk prior to surgery, anemia is defined as a hemoglobin level of < 120 g/L for men and < 110 g/L for women, and mild anemia is defined as a hemoglobin level > 90 g/L but below normal, according to the criteria suggested by the National Cancer Institute, and clinical practice guide published by the Chinese Society of Clinical Oncology (CSCO)[16]. According to previous reports, tumor-associated blood loss, bone marrow involvement, cytokine-mediated disease, and iron or folic acid nutritional deficiency play a key role in the development and maintenance of CRA[14]. Pretreatment anemia is seen frequently in cancer patients and can adversely affect their quality of life (QOL) and survival[17,18]. Iron metabolism disorders, tumor-related bleeding, catabolic abnormalities, and nutritional inadequacies in cancer patients all play a key role in anemia pathogenesis[17,18]. Further research is needed to clarify the underlying mechanism of the relationship between anemia and negative prognosis in GC. In most studies, pretreatment anemia is related to a poorer prognosis in cancer patients[19-22]. Anemia is associated with the nutritional status of patients. Although the prognosis of patients with preoperative anemia is worse than that of patients without, there are differences in prognosis among anemic patients, with some having a relatively good prognosis. At present, no one has proposed a predictive model for postoperative overall survival (OS) in GC patients with preoperative anemia.
Nomograms are a convenient prediction tool that provides accurate prediction of individual outcomes and have been utilized to estimate the prognosis of cancer patients[23]. Preoperative anemia predicts poor GC prognosis, including OS and disease-free survival. Hence, preoperative anemia is a conveniently and cost-effectively available blood-borne biomarker to predict GC prognosis. Therefore, this study used a nomogram to predict postoperative survival at 1, 3, and 5 years in GC patients with preoperative anemia.
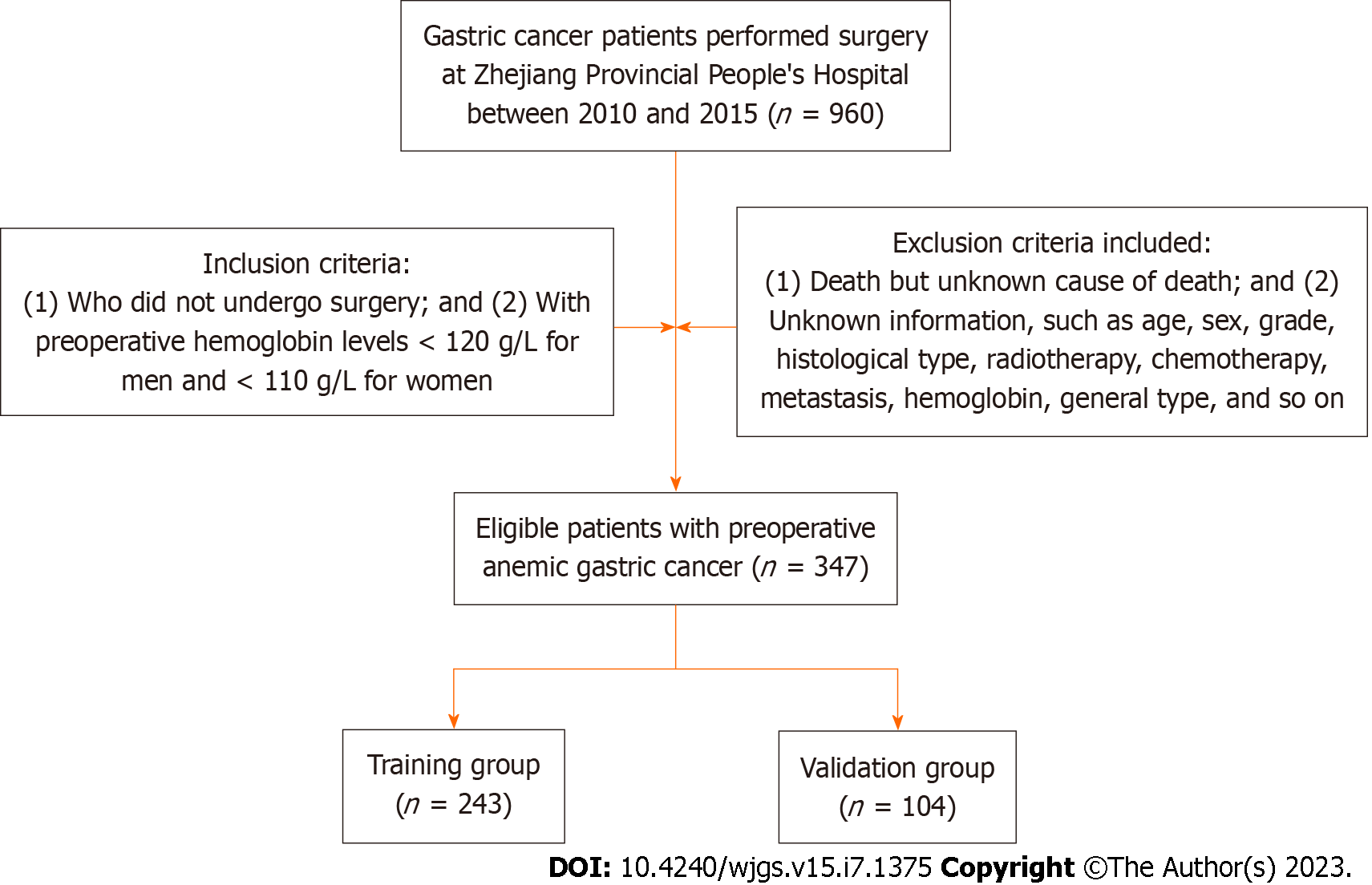
Data of patients who were diagnosed with GC and underwent surgery at Zhejiang Provincial People's Hospital (China) between 2010 and 2015 were included in this study, with the last follow-up date being January 2018. GC patients with preoperative anemia were screened according to the criteria suggested by the National Cancer Institute, and the clinical practice guide published by the CSCO. Clinical information for patients was collected, including age, sex, histological differentiation, clinicopathological stage, tumor size, tumor number, monocytes, lymphocytes, hemoglobin, preoperative alpha-fetoprotein (AFP) level, preoperative CA125 Level, preoperative CA199 Level, and follow-up status. The inclusion criteria were as follows: (1) GC patients who did not undergo surgery; and (2) GC patients with a preoperative hemoglobin level < 120 g/L for men and < 110 g/L for women. The exclusion criteria included: (1) Unknown cause of death; and (2) Unknown information, such as age, sex, grade, histological type, radiotherapy, chemotherapy, metastasis, hemoglobin, and GC type. In this study, data for both the training and validation sets were obtained from Zhejiang Provincial People's Hospital, and the research was based on the Declaration of Helsinki (as revised in 2013). This study was authorized by the Ethics Committee of Zhejiang Provincial People's Hospital, No. 2019KY017. This study was eligible for waiver of informed consent.
The variables included in this study were demographics, cancer characteristics, laboratory data, and metastasis. Demographic variables included age and sex. Cancer features included tumor size, histological differentiation, clinicopathological stage, and GC type. Laboratory data included tumor markers and blood work. Metastatic data included peritoneal, lymphatic, liver, and distant metastases. X-tile software (Yale University, New Haven, CT, United States) was utilized to validate the optimal cutoff values for age, tumor size, preoperative AFP level, lymphocytes, monocytes, red cell distribution width (RDW), red blood cell specific volume (HCT), mean corpuscular hemoglobin (MCH), and mean corpuscular volume (MCV)[24]. For OS, 73 years was the optimal cutoff for age, 3.5 cm for tumor size, 2.60 ng/mL for AFP, 1.2 × 109/L for lymphocytes, 0.47 × 109/L for monocytes, 18.9% for RDW, 0.34 L/L for HCT, 30.7 pg for MCH, and 87.30 fL for MCV.
All statistical analyses were conducted using SPSS 24.0 (IBM) and R software (version 3.6.1). Statistically significant cutoff values needed to meet a P value < 0.05 (two sided). Univariate and multivariate Cox regression analyses were used to identify independent prognostic factors. Receiver operating characteristic (ROC) curves for the prognostic nomogram were created[25]. The area under the ROC curve (AUC) was employed to assess the performance of the nomogram. Calibration curves were generated to compare the projected and real results. The range of threshold probability and size of benefits were defined by decision curve analysis (DCA)[26].
A total of 347 patients met our inclusion criteria, and after randomization, 243 were included in the training set and 104 in the validation set (Figure 1). The clinical features of the patients in both study groups are summarized in Table 1. In the training set, 183 (75.30%) and 60 (24.70%) of the patients were male and female, respectively. Their median age at diagnosis was 68 years (range 28-87 years), and the median follow-up time was 29 mo. In the validation set, 75 (72.12%) and 29 (27.88%) patients were males and females, respectively. The median age at diagnosis was 66 years (range 32-89 years), and the median follow-up time was 28.5 mo. The percentage of patients with a tumor size < 3.5 cm was 26.34% and 27.88% in the training and validation sets, respectively. In addition, there were no significant differences in sex, age, or type of surgery between the groups, although there were significant differences in pathological stage, histological differentiation, depth of gastric wall infiltration, type of metastasis, lymphocyte count, monocyte count, and preoperative AFP level (P < 0.05).
| Characteristic | Total cohort, n = 347 | Training cohort, n = 243 | Validation cohort, n = 104 |
| Age, yr | |||
| < 73 | 233 (67.1%) | 75 (72.1%) | 158 (65.0%) |
| ≥ 73 | 114 (32.9%) | 85 (35.0%) | 29 (27.9%) |
| Sex | |||
| Female | 89 (25.6%) | 60 (24.7%) | 29 (27.9%) |
| Male | 258 (74.4%) | 183 (75.3%) | 75 (72.1%) |
| Tumor size, cm | |||
| < 3.5 | 93 (26.8%) | 64 (26.3%) | 29 (27.9%) |
| ≥ 3.5 | 254 (73.2%) | 179 (73.7%) | 75 (72.1%) |
| Stage | |||
| I | 61 (17.6%) | 42 (17.3%) | 19 (18.3%) |
| II | 83 (23.9%) | 61 (25.1%) | 22 (21.1%) |
| III | 181 (52.1%) | 125 (51.4%) | 56 (53.8%) |
| IV | 24 (6.3%) | 15 (6.2%) | 7 (6.7%) |
| Liver metastasis | |||
| No | 326 (94.0%) | 227 (93.4%) | 99 (95.2%) |
| Yes | 21 (6.0%) | 16 (6.6%) | 5 (4.8%) |
| Lymphocyte count, × 109/L | |||
| < 1.2 | 134 (38.6%) | 101 (41.6%) | 33 (31.7%) |
| ≥ 1.2 | 213 (61.4%) | 142 (58.4%) | 71 (68.3%) |
| AFP, ng/mL | |||
| < 2.6 | 205 (59.1%) | 143 (58.8%) | 62 (59.6%) |
| ≥ 2.6 | 142 (40.9%) | 100 (41.2%) | 42 (43.1%) |
| Type of surgery | |||
| Partial excision | 191 (55.0%) | 130 (53.4%) | 61 (58.7%) |
| Total gastrectomy | 156 (45.0%) | 113 (46.6%) | 43 (41.3%) |
| GC type | |||
| Ulcer type | 289 (83.3%) | 207 (85.1%) | 82 (78.8%) |
| Polyp type | 26 (7.5%) | 16 (6.6%) | 10 (9.6%) |
| Diffuse type | 11 (3.2%) | 8 (3.3%) | 3 (2.9%) |
| Others | 21 (6.0%) | 12 (5.0%) | 9 (8.7%) |
| Peritoneal metastasis | |||
| No | 322 (92.8%) | 226 (93.1%) | 96 (92.3%) |
| Yes | 25 (7.2%) | 17 (6.9%) | 8 (7.7%) |
| Lymphatic metastasis | |||
| No | 95 (27.4%) | 64 (26.6%) | 31 (29.8%) |
| Yes | 252 (72.6%) | 179 (73.4%) | 73 (70.2%) |
| Remote metastasis | |||
| No | 323 (93.1%) | 228 (93.8%) | 95 (91.3%) |
| Yes | 24 (6.9%) | 15 (6.2%) | 9 (8.9%) |
| Vascular invasion | |||
| No | 195 (56.2%) | 136 (66.0%) | 59 (56.7%) |
| Yes | 152 (43.8%) | 107 (44.0%) | 45 (43.3%) |
| Histological differentiation | |||
| Highly or moderately differentiated | 98 (28.2%) | 70 (28.85) | 28 (26.9%) |
| Lowly or undifferentiated | 222 (64.0%) | 155 (63.8%) | 67 (64.4%) |
| Indolent cell or mucinous adenocarcinoma | |||
| Monocyte count, × 109/L | 27 (7.8%) | 18 (7.4%) | 9 (8.7%) |
| < 0.47 | |||
| ≥ 0.47 | 172 (49.6%) | 101 (41.6%) | 71 (68.3%) |
| Red cell distribution width, % | 175 (50.4%) | 142 (58.4%) | 33 (31.7%) |
| < 18.9 | 307 (88.5%) | 216 (88.9%) | 91 (87.5%) |
| ≥ 18.9 | 40 (11.5%) | 27 (11.1%) | 13 (12.5%) |
| Red blood cell specific volume, L/L | |||
| < 0.34 | 266 (76.7%) | 192 (79.0%) | 74 (71.2%) |
| ≥ 0.34 | 81 (23.3%) | 51 (21.0%) | 30 (28.8%) |
| Mean corpuscular hemoglobin, pg | |||
| < 30.70 | 297 (85.6%) | 211 (86.8%) | 86 (82.7%) |
| ≥ 30.70 | 50 (14.4%) | 32 (13.2%) | 18 (17.3%) |
| Mean corpuscular volume, fL | |||
| < 87.30 | 187 (53.9%) | 134 (55.1%) | 53 (51.0%) |
| ≥ 87.30 | 160 (46.1%) | 109 (44.9%) | 51 (49.0%) |
Finally, 347 GC patients were screened as having preoperative anemia and divided randomly into a training cohort and a validation cohort. Prognostic factors affecting survival independently were investigated in the training cohort, and a prognostic nomogram was developed. Then, the nomogram was verified in the validation group. The detailed process of patient selection is shown in Figure 1.
The 960 GC patients were divided into two groups (preoperatively anemic and nonanemic) according to patient follow-up data. Postoperative survival analysis was performed for both cohorts, yielding a shorter postoperative survival for preoperatively anemic patients than for nonanemic patients and a statistically significant difference in postoperative survival between the two cohorts (Figure 2).
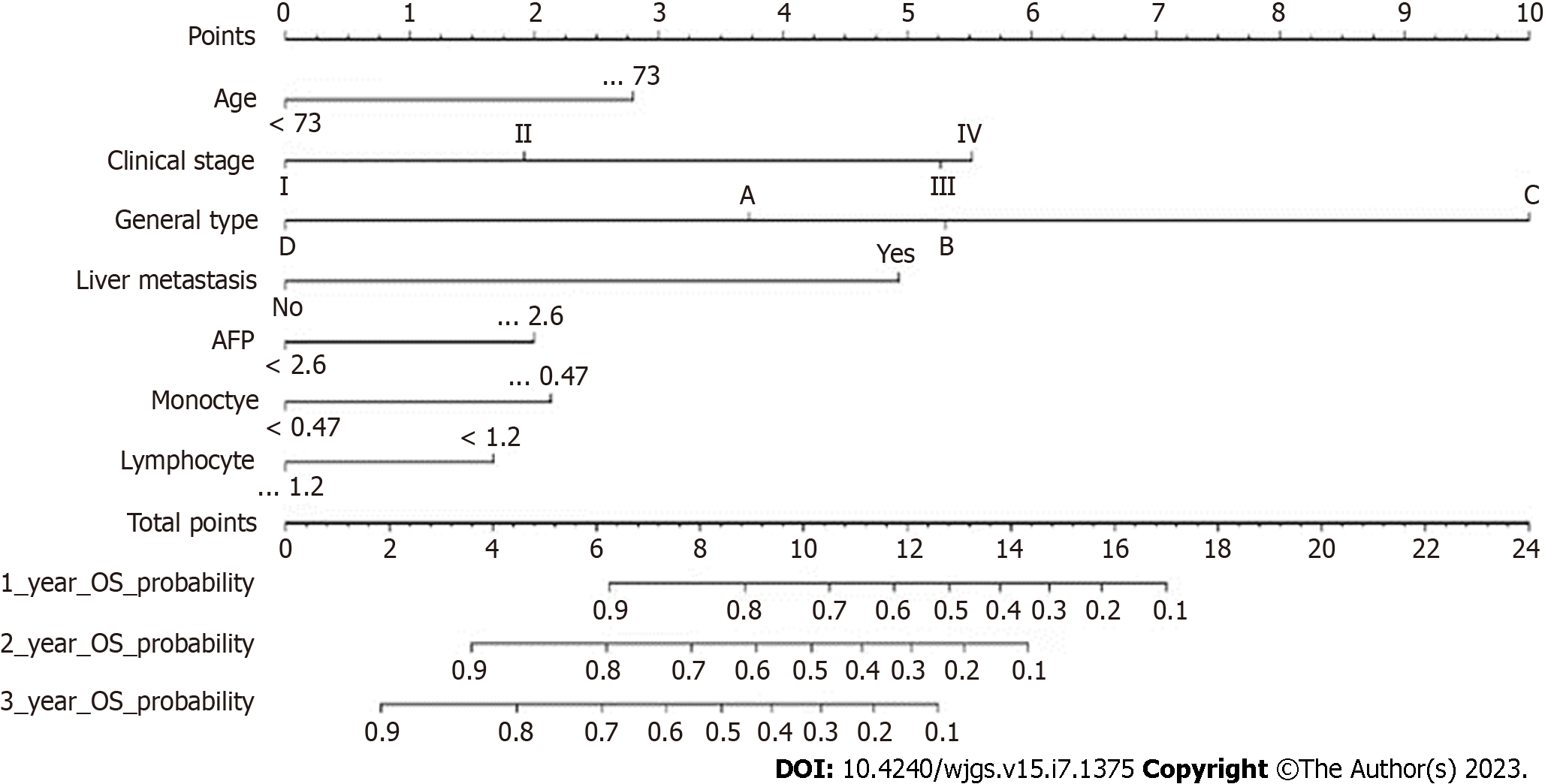
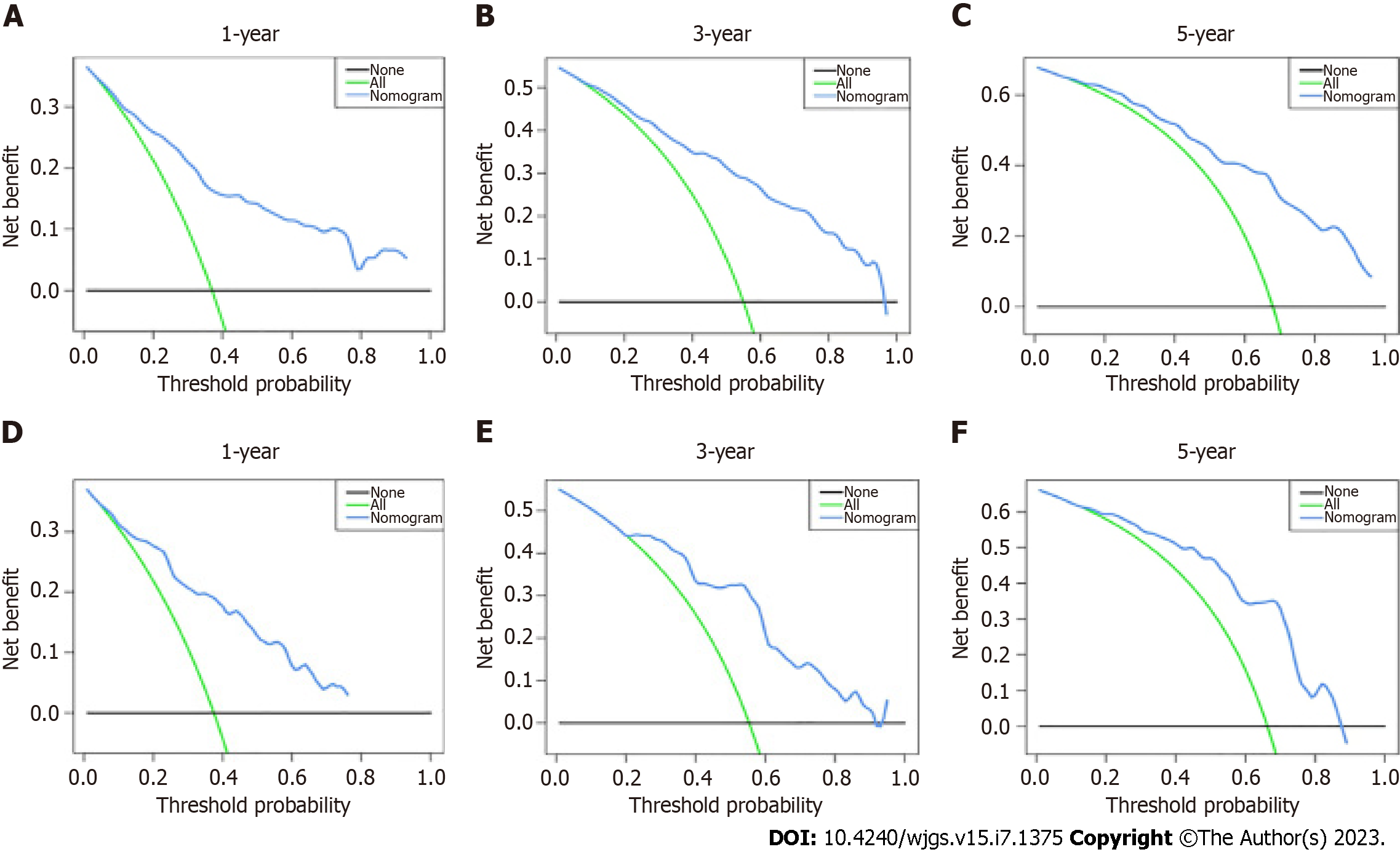
The results of using univariate Cox proportional hazards regression to screen prognostic factors showed that age, tumor size, GC type, clinical stage, liver metastasis, monocytes, lymphocytes, preoperative AFP level, peritoneal metastasis, lymphatic metastasis, vascular invasion, histological differentiation, and RDW were factors associated with OS (Table 2). Then, all factors associated with OS were included in the multivariate Cox analysis, and age, preoperative AFP level, monocytes, lymphocytes, clinicopathological stage, liver metastasis, and GC type were determined to be independent OS-related factors (Table 2). An OS prognostic nomogram was established by combining the corresponding independent prognostic factors (Figure 3). In summary, the nomogram predicted 1-, 3-, and 5-year OS for each patient by summarizing the scores shown on the bottom scale. The AUCs of the nomogram were 0.831, 0.845, and 0.840 for predicting 1-, 3-, and 5-year OS in the training group, separately, and the corresponding AUCs were 0.827, 0.829, and 0.812 in the validation group, respectively (Figure 4). Furthermore, the calibration curves for the 1-, 3- and 5-year OS in both the training and validation cohorts showed close agreement between the real results and the projected results by the column line plots (Figure 5). The DCA showed good predictive efficiency of column line graphs for OS in preoperatively anemic GC patients (Figure 6).
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age, yr | ||||
| < 73 | ||||
| ≥ 73 | 1.886 (1.367-2.602) | < 0.001 | 2.137 (1.532-2.981) | < 0.001 |
| Sex | ||||
| Female | ||||
| Male | 1.365 (0.924-2.016) | 0.118 | ||
| Tumor size, cm | ||||
| < 3.5 | ||||
| ≥ 3.5 | 2.399 (1.561-3.685) | < 0.001 | ||
| Stage | ||||
| I | ||||
| II | 2.274 (1.142-4.526) | 0.019 | 1.726 (0.846-3.521) | 0.133 |
| III | 5.296 (2.827-9.919) | < 0.001 | 4.231 (2.192-8.167) | < 0.001 |
| IV | 14.598 (6.501-32.780) | < 0.001 | 4.908 (1.426-16.897) | < 0.001 |
| Liver metastasis | ||||
| No | ||||
| Yes | 5.046 (2.943-8.653) | 0.001 | 3.573 (1.302-9.804) | 0.013 |
| Monocyte count, × 109/L | ||||
| < 0.47 | ||||
| ≥ 0.47 | 2.006 (1.363-2.953) | 0.019 | 1.819 (1.225-2.700) | 0.003 |
| Lymphocyte count, × 109/L | ||||
| < 1.2 | ||||
| ≥ 1.2 | 0.683 (0.498-0.939) | < 0.001 | 0.645 (0.463-0.898) | 0.009 |
| AFP, ng/mL | ||||
| < 2.6 | ||||
| ≥ 2.6 | 1.983 (1.443-2.725) | < 0.001 | 1.720 (1.238-2.390) | 0.001 |
| Type of surgery | ||||
| Partial excision | ||||
| Total Gastrectomy | 1.292 (0.940-1.775) | 0.114 | ||
| GC type | ||||
| Ulcer type | ||||
| Polyp type | 0.97 (0.475-1.979) | 0.933 | 1.527 (0.736-3.167) | 0.225 |
| Diffuse type | 2.715 (1.256-5.869) | 0.011 | 5.131 (2.266-11.621) | < 0.01 |
| Others | 0.17 (0.042-0.687) | 0.013 | 0.353 (0.082-1.517) | 0.162 |
| Peritoneal metastasis | ||||
| No | ||||
| Yes | 3.531 (2.086-5.595) | < 0.001 | ||
| Lymphatic metastasis | ||||
| No | ||||
| Yes | 2.879 (1.857-4.465) | < 0.001 | ||
| Vascular invasion | ||||
| No | ||||
| Yes | 1.831 (1.332-2.519) | < 0.001 | ||
| Histological differentiation | ||||
| Highly or moderately differentiated | ||||
| Lowly or | ||||
| undifferentiated | 1.848 (1.255-2.271) | 0.002 | ||
| Indolent cell or mucinous adenocarcinoma | 1.363 (0.690-2.691) | 0.372 | ||
| Red cell distribution width, % | ||||
| < 18.9 | ||||
| ≥ 18.9 | 1.88 (1.203-2.938) | 0.006 | ||
| Red blood cell specific volume, L/L | ||||
| < 0.34 | ||||
| ≥ 0.34 | 1.505 (0.98-2.31) | 0.062 | ||
| Mean corpuscular hemoglobin, pg | ||||
| < 30.7 | ||||
| ≥ 30.7 | 0.838 (0.674-1.042) | 0.112 | ||
| Mean corpuscular volume, fL | ||||
| < 87.30 | ||||
| ≥ 87.30 | 1.114 (0.948-1.309) | 0.189 | ||
The prevalence of anemia was 25.2%, and pretreatment anemia was an independent prognostic factor for lymph node metastasis-free survival, recurrence-free survival, and OS[27]. In our study, we created a nomogram to predict postoperative OS in GC patients with preoperative anemia, and by getting data for several easily obtainable variables on the nomogram for each GC patient, a total score could be calculated. Therefore, the postoperative OS of GC patients with preoperative anemia can be easily calculated from the nomogram, providing guidance for further clinical management.
Shen et al[28] found that preoperative anemia was significantly associated with tumor size, depth of infiltration, lymph node metastasis, and advanced tumor stage[28]. Liu et al[29] found that preoperative anemia was associated with tumor size[29]. In addition, several clinical studies have reported that preoperative anemia is an important risk factor for postoperative complications in GC and is negatively correlated with physical and nutritional status[16,30-32]. GC patients without anemia might tolerate surgery and adjuvant therapy better, whereas anemic patients need to be treated before surgery with adjuvant therapy and followed closely[33]. Absolute counts of lymphocytes and monocytes predicted survival in patients with metastatic cancer; overall survival was lower in patients with reduced lymphocyte counts, and patients with absolute monocyte counts of 300 to 899 monocytes per cubic millimeter had a significantly better prognosis than those with higher or lower counts[8]. Patients with liver metastases more often showed high expression of AFP, and histopathological type and tumor location did not affect the status of tumor markers[34]. AFP positivity is associated with liver metastases from gastric cancer, and liver metastases from gastric cancer results in a poorer prognosis[35-37]. AFP-producing gastric cancer was associated with venous invasion, deeper invasion of the gastric wall, and higher liver metastasis rate, with poorer overall survival in the AFP-positive group than in the AFP-negative group[36]. AFP-producing gastric cancers with liver metastases had deeper gastric wall infiltration and more pronounced lymphatic and venous invasion[36]. Saito et al[38] observed that a large tumor size was an independent prognostic factor for a worse prognosis. Large tumor size stimulates angiogenesis, which increases tumor cell proliferation[38]. However, to date, no predictive models have been developed, which means that postoperative OS in GC patients cannot be predicted by combining all independent preoperative anemia-related predictors. In our study, the results showed that age, preoperative AFP level, monocyte count, lymphocyte count, clinicopathological stage, liver metastasis, and GC were significant predictors of postoperative OS in preoperatively anemic GC patients. After two sets of data from the training and validation sets were compared to improve the accuracy and reliability of the study, we used ROC, calibration, and DCA curves to assess the accuracy of the model. We found that this prognostic model has good accuracy. Individualized GC treatment is a multidisciplinary collaborative and complementary approach aimed at enhancing the outcomes of cancer treatment and is currently the focus of many medical studies. Nomograms integrate more potential independent prognostic risk factors to personalize the prediction of patient survival and thus develop better treatment options. The calibration plots show good agreement between projected probabilities and practical observations, thus affirming their reliability and reproducibility. The accuracy of the nomogram is higher than that of any individual predictor, which also indicates the importance of the integrated prediction model.
Although the nomogram has excellent accuracy, it is inevitable that our work has some limitations. First, although the nomogram was validated externally and the results were consistent, all patients in the study were from China, and the data had geographical limitations. Second, in China, there is no major public GC database available for analysis and this study is a single-center data study with a limited sample size, so the information might be incomplete. In addition, treatment bias may have occurred. In assessing the correlation between hemoglobin levels and efficacy, the effect of chemotherapy could not be excluded. In addition, this study did not include chemotherapy or radiotherapy because of there was some incomplete data.
In conclusion, GC patients with preoperative anemia have a shorter survival than those without, and we used general clinical data to generate and verify a nomogram for predicting the 1-, 3-, and 5-year survival in such patients. The prognostic nomogram had greater discriminatory power and clinical applicability than the prognostic factors alone, and we used ROC, calibration, and DCA curves to assess the precision of the model and revealed that the prognostic model had high precision.
There are differences in prognosis among anemic patients, with some having a relatively good prognosis, but no one has proposed a predictive model for postoperative overall survival (OS) in gastric cancer (GC) patients with preoperative anemia.
To predict postoperative OS in GC patients with preoperative anemia using a nomogram.
The purpose of this study was to determine the effect of preoperative anemia on the prognosis of GC patients and generate a prognostic nomogram to predict the postoperative OS of GC patients with preoperative anemia.
Clinicopathological and follow-up data of GC patients treated at Zhejiang Provincial People's Hospital (China) from 2010 to 2015 were collected. Independent prognostic factors were screened by univariate and multivariate Cox regression analyses. Then, these factors were used to construct a nomogram.
The area under the operating characteristic (ROC) curve (AUC) of the nomogram for predicting the 1-, 3-, and 5-year OS were 0.831, 0.845, and 0.840, respectively, for the training cohort, and the corresponding AUC values in the validation cohort were 0.827, 0.829, and 0.812, respectively. Calibration curves and decision curve analysis indicated good performance of the nomogram.
We have successfully produced and verified a useful nomogram for predicting OS in preoperatively anemic GC patients.
Our study provides a tool for predicting OS by known clinicopathological and follow-up data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghaffari K, Iran; Win TT, Malaysia S-Editor: Li L L-Editor: Wang TQ P-Editor: Xu ZH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21373] [Article Influence: 2137.3] [Reference Citation Analysis (3)] |
| 2. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2646] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 3. | Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000;26:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Sun W, Yan L. Gastric cancer: current and evolving treatment landscape. Chin J Cancer. 2016;35:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 795] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 6. | Roukos DH, Kappas AM. Perspectives in the treatment of gastric cancer. Nat Clin Pract Oncol. 2005;2:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Shiozaki H, Shimodaira Y, Elimova E, Wadhwa R, Sudo K, Harada K, Estrella JS, Das P, Badgwell B, Ajani JA. Evolution of gastric surgery techniques and outcomes. Chin J Cancer. 2016;35:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Bruckner HW, Lavin PT, Plaxe SC, Storch JA, Livstone EM. Absolute granulocyte, lymphocyte, and moncyte counts. Useful determinants of prognosis for patients with metastatic cancer of the stomach. JAMA. 1982;247:1004-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T, Esaki T, Nagase M, Fujitani K, Yamaguchi K, Ura T, Hamamoto Y, Morita S, Okamoto I, Boku N, Hyodo I. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438-4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 379] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 10. | Hironaka S, Sugimoto N, Yamaguchi K, Moriwaki T, Komatsu Y, Nishina T, Tsuji A, Nakajima TE, Gotoh M, Machida N, Bando H, Esaki T, Emi Y, Sekikawa T, Matsumoto S, Takeuchi M, Boku N, Baba H, Hyodo I. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Pham CM, Syed AA, Siddiqui HA, Keller RA, Kowalewski C. Case of metastatic basal cell carcinoma to bone marrow, resulting in myelophthisic anemia. Am J Dermatopathol. 2013;35:e34-e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kamei A, Gao G, Neale G, Loh LN, Vogel P, Thomas PG, Tuomanen EI, Murray PJ. Exogenous remodeling of lung resident macrophages protects against infectious consequences of bone marrow-suppressive chemotherapy. Proc Natl Acad Sci U S A. 2016;113:E6153-E6161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:11S-26S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 369] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 15. | Tas F, Eralp Y, Basaran M, Sakar B, Alici S, Argon A, Bulutlar G, Camlica H, Aydiner A, Topuz E. Anemia in oncology practice: relation to diseases and their therapies. Am J Clin Oncol. 2002;25:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Experts Committee on Cancer -Related Anemia; Chinese Society of Clinical Oncology (CSCO). Clinical practice guidelines on cancer-related anemia (2012-2013 Edition). Chin Clin Oncol. 2012;1:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Mercadante S, Gebbia V, Marrazzo A, Filosto S. Anaemia in cancer: pathophysiology and treatment. Cancer Treat Rev. 2000;26:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 143] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Aapro M, Österborg A, Gascón P, Ludwig H, Beguin Y. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol. 2012;23:1954-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Hu K, Harrison LB. Impact of anemia in patients with head and neck cancer treated with radiation therapy. Curr Treat Options Oncol. 2005;6:31-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Choi YS, Yi CM, Sin JI, Ye GW, Shin IH, Lee TS. Impact of hemoglobin on survival of cervical carcinoma patients treated with concurrent chemoradiotherapy is dependent on lymph node metastasis findings by magnetic resonance imaging. Int J Gynecol Cancer. 2006;16:1846-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Grogan M, Thomas GM, Melamed I, Wong FL, Pearcey RG, Joseph PK, Portelance L, Crook J, Jones KD. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999;86:1528-1536. [PubMed] [DOI] [Full Text] |
| 22. | Dunphy EP, Petersen IA, Cox RS, Bagshaw MA. The influence of initial hemoglobin and blood pressure levels on results of radiation therapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1989;16:1173-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2397] [Article Influence: 239.7] [Reference Citation Analysis (0)] |
| 24. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 2941] [Article Influence: 147.1] [Reference Citation Analysis (0)] |
| 25. | Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1735] [Cited by in RCA: 2031] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 26. | Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3515] [Cited by in RCA: 3483] [Article Influence: 183.3] [Reference Citation Analysis (1)] |
| 27. | Zhang Y, Chen Y, Chen D, Jiang Y, Huang W, Ouyang H, Xing W, Zeng M, Xie X, Zeng W. Impact of preoperative anemia on relapse and survival in breast cancer patients. BMC Cancer. 2014;14:844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Shen JG, Cheong JH, Hyung WJ, Kim J, Choi SH, Noh SH. Pretreatment anemia is associated with poorer survival in patients with stage I and II gastric cancer. J Surg Oncol. 2005;91:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Liu X, Qiu H, Huang Y, Xu D, Li W, Li Y, Chen Y, Zhou Z, Sun X. Impact of preoperative anemia on outcomes in patients undergoing curative resection for gastric cancer: a single-institution retrospective analysis of 2163 Chinese patients. Cancer Med. 2018;7:360-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Coquelle A, Toledo F, Stern S, Bieth A, Debatisse M. A new role for hypoxia in tumor progression: induction of fragile site triggering genomic rearrangements and formation of complex DMs and HSRs. Mol Cell. 1998;2:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Jung DH, Lee HJ, Han DS, Suh YS, Kong SH, Lee KU, Yang HK. Impact of perioperative hemoglobin levels on postoperative outcomes in gastric cancer surgery. Gastric Cancer. 2013;16:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | So JB, Yam A, Cheah WK, Kum CK, Goh PM. Risk factors related to operative mortality and morbidity in patients undergoing emergency gastrectomy. Br J Surg. 2000;87:1702-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Tang GH, Hart R, Sholzberg M, Brezden-Masley C. Iron deficiency anemia in gastric cancer: a Canadian retrospective review. Eur J Gastroenterol Hepatol. 2018;30:1497-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther. 2008;25:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Chang YC, Nagasue N, Kohno H, Taniura H, Uchida M, Yamanoi A, Kimoto T, Nakamura T. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85:1480-1485. [PubMed] |
| 36. | Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, Matsumoto Y. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002;19:359-65; discussion 365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Webb A, Scott-Mackie P, Cunningham D, Norman A, Andreyev J, O'Brien M, Bensted J. The prognostic value of serum and immunohistochemical tumour markers in advanced gastric cancer. Eur J Cancer. 1996;32A:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Oro S, Tatebe S, Tsujitani S, Ikeguchi M. Macroscopic tumor size as a simple prognostic indicator in patients with gastric cancer. Am J Surg. 2006;192:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |