Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1340
Peer-review started: December 12, 2022
First decision: December 27, 2022
Revised: January 3, 2023
Accepted: April 19, 2023
Article in press: April 19, 2023
Published online: July 27, 2023
Processing time: 221 Days and 2.7 Hours
Patients with combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC) are not traditionally considered eligible for liver transplantation (LT) due to poor outcomes.
To compare outcomes between living donor LT (LDLT) patients with hepatocellular carcinoma (HCC) and LT patients with cHCC-CC and to identify risk factors for tumor recurrence and death after LT in cHCC-CC patients.
Data for pathologically diagnosed cHCC-CC patients (n = 111) who underwent LT from 2000 to 2018 were collected for a nine-center retrospective review. Patients (n = 141) who received LDLT for HCC at Samsung Medical Center from January 2013 to March 2017 were selected as the control group. Seventy patients in two groups, respectively, were selected by 1:1 matching.
Cumulative disease-free survival (DFS) and overall survival (OS) in the cHCC-CC group were significantly worse than in the HCC group both before and after matching. Extrahepatic recurrence incidence in the cHCC-CC group was higher than that in the HCC group (75.5% vs 33.3%, P < 0.001). Multivariate analysis demonstrated that the cHCC-CC group had significantly higher rates of tumor recurrence and death compared to the HCC group. In cHCC-CC subgroup analysis, frequency of locoregional therapies > 3, tumor size > 3 cm, and lymph node metastasis were predisposing factors for tumor recurrence in multivariate analysis. Only a maximum tumor size > 3 cm was a predisposing factor for death.
The poor prognosis of patients diagnosed with cHCC-CC after LT can be predicted based on the explanted liver. Frequent regular surveillance for cHCC-CC patients should be required for early detection of tumor recurrence.
Core Tip: Cumulative disease-free survival and overall survival in the combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC) group were significantly worse than in the hepatocellular carcinoma group before and after matching. In cHCC-CC subgroup analysis, frequency of locoregional therapies > 3, tumor size > 3 cm, and lymph node metastasis were predisposing factors for tumor recurrence in multivariate analysis. Only a maximum tumor size > 3 cm was a predisposing factor for death. Poor prognosis of patients diagnosed with cHCC-CC after liver transplantation can be predicted based on explant liver. Frequent regular surveillance for cHCC-CC patients should be required for early detection of tumor recurrence.
- Citation: Kim J, Joo DJ, Hwang S, Lee JM, Ryu JH, Nah YW, Kim DS, Kim DJ, You YK, Yu HC. Liver transplantation for combined hepatocellular carcinoma and cholangiocarcinoma: A multicenter study. World J Gastrointest Surg 2023; 15(7): 1340-1353
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1340.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1340
Combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC) is rare, accounting for 0.5%-14% of primary liver malignancies and heterogeneous hepatic tumors with histological features of both hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC), respectively[1]. Surgical liver resection (LR) is the only curative option for patients with cHCC-CC[2-4]. However, LR for patients with cHCC-CC may not be safe in cases with prohibitive underlying liver cirrhosis or if the estimated future remnant liver volume is small. Even if LR proceeds safely, tumor recurrence is frequent (up to 80% at five years), and five-year survival rates do not exceed 30%[2].
Liver transplantation (LT) is the best treatment for small HCCs, but is contraindicated in CC due to its high recurrence and low overall survival (OS) rates[5]. Patients with cHCC-CC tumors are not traditionally considered for LT because single centers with few cases have previously reported poor outcomes[1,6-8]; however, several small single-center cohort studies showed satisfactory outcomes after LT for cHCC-CC equivalent to those attained for HCC[9,10]. The role of LT has been investigated in several retrospective studies that included patients diagnosed incidentally during pathological examination of the explant. The variation in results among patients with cHCC-CC suggests that LT should be considered only in select cases. Based on those limited experiences, some prognostic factors may include tumor diameter > 2 cm, lymph node invasion (present in 10%–20% of patients), beyond the Milan criteria, poor differentiation, multinodular tumors, presence of microvascular invasion, and high carbohydrate antigen (CA) 19-9 Level[5].
Data on clinicopathologic presentation, prognostic factors, and outcomes for LT in cHCC-CC patients are lacking because cHCC-CC is rare, and few studies have been published. To overcome the limitations of single-center and small-volume cases, we collected and analyzed data to evaluate the utility of LT for cHCC-CC from high-volume LT centers in Korea. We compared the characteristics between living donor LT (LDLT) patients with HCC and LT patients with cHCC-CC before and after propensity score matching and identified the risk factors for tumor recurrence and death after LT in cHCC-CC patients.
We performed a retrospective analysis of patients who were diagnosed with cHCC-CC in their postoperative pathology reports and who underwent LT at any of nine Korean medical centers between January 2000 and December 2018. The Samsung Medical Center Institutional Review Board (IRB) approved this study, No. SMC-2019-09-147-006, as did the IRB at each individual center. The requirement for written consent was waived by each center. Patients who received LDLT for HCC at Samsung Medical Center from January 2013 to March 2017 were selected as the control group. Recipients < 18 years, re-transplantation cases, and patients who received multiorgan grafts were excluded.
Data were collected at each center through retrospective medical records review. The following data were collected and evaluated: Donor and recipient sex; donor and recipient age at transplantation; donor and recipient body mass index (BMI) at transplantation; etiology of liver disease [hepatitis B virus (HBV), hepatitis C virus, non B non C, or alcoholism]; history of diabetes or hypertension; Child-Pugh class; history of locoregional therapy, including transarterial chemoembolization (TACE), LR, radiofrequency ablation (RFA), or radiation; serum levels of alpha-fetoprotein (AFP) and prothrombin induced by vitamin K absence-II (PIVKA-II); preoperative model for end-stage liver disease (MELD) score, status of donor (living or deceased); ABO-incompatibility; steatosis of liver graft; graft-to-weight ratio (GRWR); cold or warm ischemic time; operation time; hospitalization duration; in-hospital mortality; pathologic characteristics including maximum tumor size, tumor number, tumor grade, microvascular invasion, portal vein tumor thrombosis (PVTT), bile duct tumor thrombosis (BDTT), intrahepatic metastasis, multicentric occurrence, and lymph node involvement; tumor recurrence site; time to recurrence; and time to tumor-related death. In the cHCC-CC group, preoperative carcinoembryonic antigen (CEA), CA 19-9, tumor differentiation of adenocarcinoma, and dominant tumor type were also evaluated.
The original pathology slides were not re-reviewed. The frequency of locoregional therapy was defined as the total number of sequential treatments with locoregional treatments, including LR, RFA, TACE, or radiation therapy. Tumors were defined as either HCC or cHCC-CC based on the final surgical pathology report from the participating center. The Milan criteria were defined as a solitary lesion ≤ 5 cm in diameter or up to three lesions, each with a diameter ≤ 3 cm and no evidence of gross vascular invasion[11]. In-hospital mortality was defined as death within 30 d after LT or death without discharge after LT. Disease-free survival was defined as the time between LT and either local clinical recurrence or detectable distant metastasis. Tumor-related death was defined as patient death caused by tumor recurrence or tumor spreading.
All statistical analyses were performed using the Statistical Package for the Social Sciences version 22.0 (SPSS; IBM Corporation, Armonk, NY, United States). Categorical variables were compared using Fisher’s exact test or the chi-square test, as applicable. The Mann-Whitney U test was used for continuous variables. The cut-off values for significant or important continuous variables, including number of locoregional therapies before LT, AFP, PIVKA-II, tumor size, and tumor number, were found using the receiver operating characteristic curve. The differences between disease-free survival (DFS) and OS across the two groups were assessed using the Kaplan-Meier survival method with the log rank test.
Propensity score matching analysis was performed because there was a potential for confounding and selection biases between the two groups. Therefore, propensity score matching was conducted prior to comparisons of OS and DFS between the HCC and the cHCC-CC propensity score matched groups. Preoperative variables potentially affecting the outcomes were assigned propensity scores[12]. We employed a logistic regression model to estimate propensity scores, using age, AFP > 20 ng/mL, macrovascular invasion, tumor size > 3 cm, tumor grade 3 or 4, and a history of locoregional therapy before LT. Matching between the HCC group and the cHCC-CC group was achieved using nearest neighbor matching with a caliper width of 0.01 and without replacement[13]. Accordingly, 70 patients in each groups were selected by 1:1 matching.
We used generalized estimating equations for predicting factors for tumor recurrence and death after propensity score matching. From these results, variables with P < 0.05 were included in multivariate analyses. We used generalized estimating equations for predicting factors for patient survival after propensity score matching. Differences with P < 0.05 were considered statistically significant for every comparison, and all statistical tests were evaluated as two-sided.
The baseline characteristics of both groups are summarized in Table 1. Donor sex, age, and BMI did not differ between the two groups. In addition, sex, BMI, underlying liver disease, history of diabetes or hypertension, Child-Pugh class, and MELD score did not differ significantly between the two groups. Hemoglobin levels, platelet counts, serum albumin, alkaline phosphatase, and creatinine levels in the cHCC-CC group were significantly lower than those in the HCC group, while other blood parameters were not different between the two groups.
| Before PSM | After PSM | |||||
| HCC (n = 141) | cHCC-CC (n = 111) | P value | HCC (n = 70) | cHCC-CC (n = 70) | P value | |
| Donor | ||||||
| Sex (male) | 89 (63.1) | 69 (62.2) | 0.896 | 47 (67.1) | 45 (64.3) | 0.859 |
| Age (yr) | 30 (16-68) | 32 (11-60) | 0.217 | 27 (16-63) | 33 (11-58) | 0.048 |
| Body mass index (kg/m2) | 23.1 (17.3-36.3) | 23.0 (17.6-35.7) | 0.679 | 23.3 (17.3-36.3) | 23.5 (18.1-32.9) | 0.839 |
| Recipient | ||||||
| Sex (male) | 127 (90.1) | 95 (85.6) | 0.329 | 65 (92.9) | 60 (85.7) | 0.274 |
| Age (yr) | 56 (37-70) | 54 (31-66) | 0.027 | 55 (37-60) | 57 (31-66) | 0.125 |
| Body mass index (kg/m2) | 24.3 (17.3-36.7) | 24.0 (18.7-35.0) | 0.35 | 23.9 (18.3-34.6) | 24.1 (18.7-35.0) | 0.94 |
| Underlying liver disease | 0.639 | 0.223 | ||||
| HBV | 123 (87.2) | 91 (82.0) | 63 (90.0) | 56 (80.0) | ||
| HCV | 6 (4.3) | 5 (4.5) | 2 (2.9) | 2 (2.9) | ||
| NBNC | 6 (4.3) | 8 (7.2) | 1 (1.4) | 6 (8.6) | ||
| Alcoholic | 6 (4.3) | 7 (6.3) | 4 (5.7) | 6 (8.6) | ||
| Diabetes | 27 (19.1) | 25 (22.5) | 0.534 | 13 (18.6) | 16 (22.9) | 0.677 |
| Hypertension | 17 (12.1) | 14 (12.6) | 0.894 | 6 (8.6) | 7 (10.0) | 0.771 |
| Child-Pugh class | 0.613 | 0.914 | ||||
| A | 66 (46.8) | 46 (40.5) | 30 (42.9) | 30 (42.9) | ||
| B | 44 (31.2) | 43 (38.7) | 24 (34.3) | 25 (35.7) | ||
| C | 31 (22.0) | 23 (20.7) | 16 (22.9) | 15 (21.4) | ||
| MELD | 10 (6-35) | 11 (6-40) | 0.455 | 11 (6-33) | 11 (6-40) | 0.82 |
| WBC (/mL) | 3300 (1050-16120) | 3500 (1100-14300) | 0.15 | 3325 (1050-16120) | 3510 (1100-10700) | 0.098 |
| Hemoglobin (g/dL) | 12.4 (6.0-16.7) | 11.7 (5.9-16.4) | 0.026 | 12.2 (7.3-15.5) | 11.9 (7.0-15.9) | 0.461 |
| Platelets (1000/mL) | 72000 (16000-233000) | 43000 (26000-223000) | < 0.001 | 64500 (21000-233000) | 42000 (26000-200000) | < 0.001 |
| INR | 1.20 (093-5.21) | 1.25 (0.90-5.98) | 0.183 | 1.21 (0.94-3.68) | 1.23 (0.90-5.98) | 0.91 |
| Albumin (g/dL) | 3.7 (2.4-4.8) | 3.1 (1.8-4.7) | < 0.001 | 3.7 (2.4-4.8) | 3.4 (1.8-4.7) | 0.032 |
| Total bilirubin (mg/dL) | 1.2 (0.3-32.9) | 1.5 (0.3-42.1) | 0.078 | 1.3 (0.4-32.9) | 1.4 (0.3-42.1) | 0.778 |
| AST (U/L) | 37 (16-229) | 42 (10-1387) | 0.063 | 38 (16-192) | 44 (10-1387) | 0.137 |
| ALT (U/L) | 28 (7-205) | 28 (6-1249) | 0.437 | 26 (7-205) | 29 (6-1249) | 0.626 |
| ALP (U/L) | 90 (29-891) | 102 (30-653) | 0.013 | 100 (29-486) | 98 (30-653) | 0.423 |
| Creatinine (mg/dL) | 0.82 (0.44-1.75) | 0.77 (0.20-4.58) | 0.049 | 0.82 (0.57-1.38) | 0.76 (0.20-4.58) | 0.03 |
After propensity score matching, the median age of donors in the HCC group was significantly younger than that in the cHCC-CC group, and platelet counts and serum albumin and serum creatinine levels in the HCC group were significantly higher than those in the cHCC-CC group. There were no statistically significant differences in other variables between the two groups.
Patients with cHCC-CC are frequently misdiagnosed with HCC and deemed eligible for LT. In our study, 86.3% (n = 96/111) of the cHCC-CC group was preoperatively diagnosed with HCC; only 11 patients (9.9%) were suspected to have intrahepatic CC or cHCC-CC. One hundred twelve patients (79.4%) in the HCC group and 74 patients (66.7%) in the cHCC-CC group had a history of locoregional therapy before LT (Table 2). TACE and RFA incidences were significantly higher in the HCC group than in the cHCC-CC group. However, LR and radiation therapy did not differ between the two groups. No patients received chemotherapy before LT. The incidence of > 3 Locoregional therapies was 39.7% (n = 56) in the HCC group and 30.6% (n = 34) in the cHCC-CC group. The median AFP and PIVKA-II values in the HCC group were 6.0 ng/mL (range, 1.3-8367.7 ng/mL) and 26 mAU/mL/mL (range, 6-22462 mAU/mL), respectively, compared to 20.3 ng/mL (range, 1.1-7201.0 ng/mL) and 31 mAU/mL (range, 5-2428 mAU/mL) in the cHCC-CC group. Therefore, the AFP concentration in the cHCC-CC group was higher than that in the HCC group, while the PIVKA-II level did not differ between the two groups. In addition, those variables were not different between the two groups after propensity score matching.
| Before PSM | After PSM | |||||
| HCC (n = 141) | cHCC-CC (n = 111) | P value | HCC (n = 70) | cHCC-CC (n = 70) | P value | |
| Locoregional therapy prior to LT | 112 (79.4) | 74 (66.7) | 0.03 | 50 (71.4) | 52 (74.3) | 0.849 |
| TACE | 102 (72.3) | 65 (58.6) | 0.023 | 45 (64.3) | 46 (65.7) | 0.859 |
| Liver resection | 24 (17.0) | 20 (18.0) | 0.868 | 10 (14.3) | 14 (20.0) | 0.502 |
| RFA | 46 (32.6) | 17 (15.3) | 0.002 | 17 (24.3) | 12 (17.1) | 0.404 |
| Radiation therapy | 13 (9.2) | 8 (7.2) | 0.65 | 6 (8.6) | 6 (8.6) | 0.618 |
| Number of locoregional therapies before LT > 3 | 56 (39.7) | 34 (30.6) | 0.147 | 27 (38.6) | 26 (37.1) | 0.862 |
| AFP > 20 ng/mL | 37 (26.2) | 56 (50.5) | < 0.001 | 23 (32.9) | 25 (35.7) | 0.859 |
| PIVKA-II > 40 mAU/mL | 50 (35.5) | 40 (39.2) | 0.591 | 30 (42.9) | 19 (30.2) | 0.152 |
Perioperative and pathologic characteristics are outlined in Table 3. The proportions of LDLT, ABO-incompatibility, macro-steatosis, and micro-steatosis were higher in the HCC group than in the cHCC-CC group. The median GRWR, cold and warm ischemic times, and operation time were greater in the cHCC-CC group compared to in the HCC group because the cHCC-CC group included more deceased donor LT (DDLT) cases than the HCC group. However, the median length of hospitalization in the cHCC-CC group was shorter than that in the HCC group.
| Before PSM | After PSM | |||||
| HCC (n = 141) | cHCC-CC (n = 111) | P value | HCC (n = 70) | cHCC-CC (n = 70) | P value | |
| Perioperative | ||||||
| Operation (LDLT) | 141 (100) | 95 (85.6) | < 0.001 | 70 (100) | 57 (44.9) | < 0.001 |
| ABO-incompatibility | 35 (24.8) | 8 (7.2) | < 0.001 | 17 (24.3) | 7 (10.0) | 0.042 |
| Macro-steatosis (%) | 5 (0-20) | 3 (0-30) | < 0.001 | 5 (1-20) | 5 (0-30) | 0.062 |
| Micro-steatosis (%) | 5 (1-70) | 1 (0-90) | < 0.001 | 5 (1-40) | 3 (0-90) | < 0.001 |
| GRWR (%) | 1.00 (0.65-1.71) | 1.11 (0.67-3.89) | 0.001 | 0.94 (0.67-1.70) | 1.15 (0.67-3.89) | < 0.001 |
| Cold ischemic time (min) | 89 (45-168) | 97 (30-1414) | 0.029 | 95 (47-144) | 97 (30-1414) | 0.185 |
| Warm ischemic time (min) | 37 (16-81) | 44 (20-90) | < 0.001 | 37 (17-81) | 45 (22-87) | 0.002 |
| Operation time (min) | 550 (336-960) | 664 (270-1265) | < 0.001 | 544 (336-838) | 639 (270-1265) | 0.006 |
| Hospitalization stay (d) | 25 (17-445) | 23 (4-262) | 0.008 | 25 (17-94) | 24 (4-262) | 0.321 |
| In-hospital mortality | 2 (1.4) | 4 (3.6) | 0.41 | 1 (1.4) | 4 (5.7) | 0.366 |
| Pathology | ||||||
| Tumor size > 3 cm | 34 (24.3) | 43 (38.7) | 0.019 | 26 (37.1) | 22 (31.4) | 0.593 |
| Tumor number > 3 | 22 (15.6) | 28 (25.2) | 0.079 | 13 (18.6) | 20 (28.6) | 0.232 |
| Beyond Milan criteria | 47 (33.3) | 45 (40.5) | 0.292 | 29 (41.4) | 28 (40.0) | 0.863 |
| Tumor grade 3 or 4 | 19 (13.5) | 33 (29.7) | 0.002 | 10 (14.3) | 14 (20.0) | 0.502 |
| Encapsulation | 36 (25.5) | 31 (27.9) | 0.67 | 17 (24.3) | 18 (25.7) | 0.895 |
| Tumor necrosis | 55 (39.0) | 55 (49.5) | 0.098 | 24 (34.3) | 38 (54.3) | 0.027 |
| Microvascular invasion | 57 (40.4) | 32 (28.8) | 0.064 | 33 (47.1) | 15 (21.4) | 0.002 |
| PVTT | 7 (5.0) | 15 (13.5) | 0.023 | 4 (5.7) | 7 (10.0) | 0.532 |
| BDTT | 3 (2.1) | 3 (2.7) | 0.766 | 3 (4.3) | 2 (2.9) | 0.649 |
| Intrahepatic metastasis | 34 (24.1) | 19 (17.1) | 0.213 | 20 (28.6) | 13 (18.6) | 0.232 |
| Multicentric occurrence | 34 (24.1) | 27 (24.3) | 0.969 | 19 (27.1) | 16 (22.9) | 0.697 |
| Lymph node metastasis | 0 (0) | 3 (2.7) | 0.084 | 0 (0) | 1 (1.4) | 0.316 |
The median maximum tumor size was 2.4 cm (range, 0.2-8.5 cm) in the HCC group and 2.5 cm (range, 0.2-7.2 cm) in the cHCC-CC group (P = 0.777), but the proportion of patients with a maximum tumor size was > 3 cm was greater in the cHCC-CC group than in the HCC group (38.7% vs 24.3%, P = 0.019). The median number of tumors was two (range, 1-34 tumors) in the HCC group compared to one (range, 1-100 tumors) in the cHCC-CC group (P = 0.263). The proportion of patients beyond the Milan criteria did not differ between the two groups; however, proportions of patients with tumor grade 3 or 4 and of those with PVTT were higher in the cHCC-CC group than in the HCC group, while encapsulation, tumor necrosis, microvascular invasion, BDTT, intrahepatic metastasis, and multicentric occurrence did not differ between the two groups before propensity score matching. Three patients (2.7%) in the cHCC-CC group had lymph node metastases. Two cases (1.4%) in the HCC group and four cases (3.6%) in the cHCC-CC group suffered in-hospital mortality (P = 0.591).
After propensity score matching, the proportions of LDLT and ABO-incompatibility in the HCC group were significantly higher than in the cHCC-CC group. The median percentage of microsteatosis in the HCC group was significantly higher than that in the cHCC-CC group, but the median GRWR, warm ischemic time, and operation time in the HCC group were significantly smaller and shorter than those in the cHCC-CC group, respectively. The presence of tumor necrosis in the HCC group was significantly less frequently than that in the cHCC-CC group, but the presence of microvascular invasion in the HCC group was significantly higher than that in the cHCC group. There were no statistically significant differences in tumor size, tumor number, the proportion of patients beyond Milan criteria, tumor grade 3 or 4, encapsulation, PVTT, BDTT, intrahepatic metastasis, multicentric occurrence, and lymph node metastasis between the two groups.
The median follow-up duration was 44.5 mo (range, 1.4-72.5 mo) in the HCC group and 39.6 mo (range, 0.1-212.5 mo) in the cHCC-CC group (P = 0.521). Twenty-seven patients (19.1%) in the HCC group and 49 patients (44.1%) in the cHCC-CC group were diagnosed with tumor recurrence during the observation period. The initial recurrence sites in the HCC group were equally frequent among intrahepatic (n = 9, 33.3%), synchronous intrahepatic and extrahepatic (n = 9, 33.3%), and extrahepatic (n = 9, 33.3%), whereas the initial recurrence sites in the cHCC-CC group were more frequently extrahepatic (n = 37, 75.5%) than intrahepatic (n = 10, 22.4%) or synchronous intrahepatic and extrahepatic (n = 2, 4.1%).
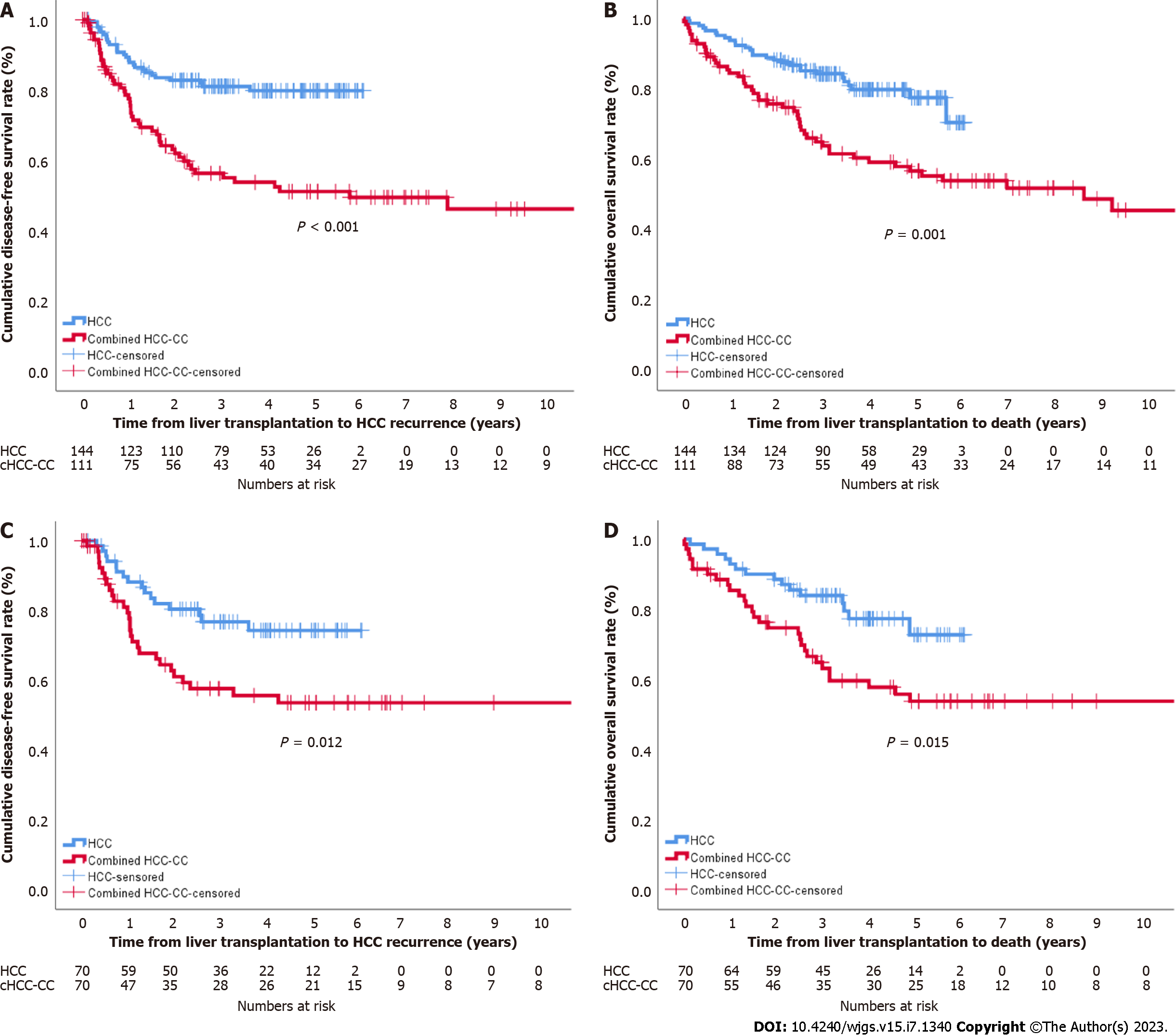
Cumulative DFS rates at one year, two years, and three years were 88.3%, 82.4%, and 80.6%, respectively, in the HCC group and 77.6%, 62.0%, and 56.3% in the cHCC-CC group. The OS rates at one year, two years, and three years were 93.6%, 87.9%, and 84.0%, respectively, in the HCC group and 84.4%, 75.7%, and 63.8% in the cHCC-CC group. Cumulative DFS and OS in the cHCC-CC group were significantly worse than those in the HCC group. Similarly, cumulative DFS and OS in the cHCC-CC group were significantly lower than those in the HCC group after propensity score matching (P = 0.012 and P = 0.015, respectively) (Figure 1).
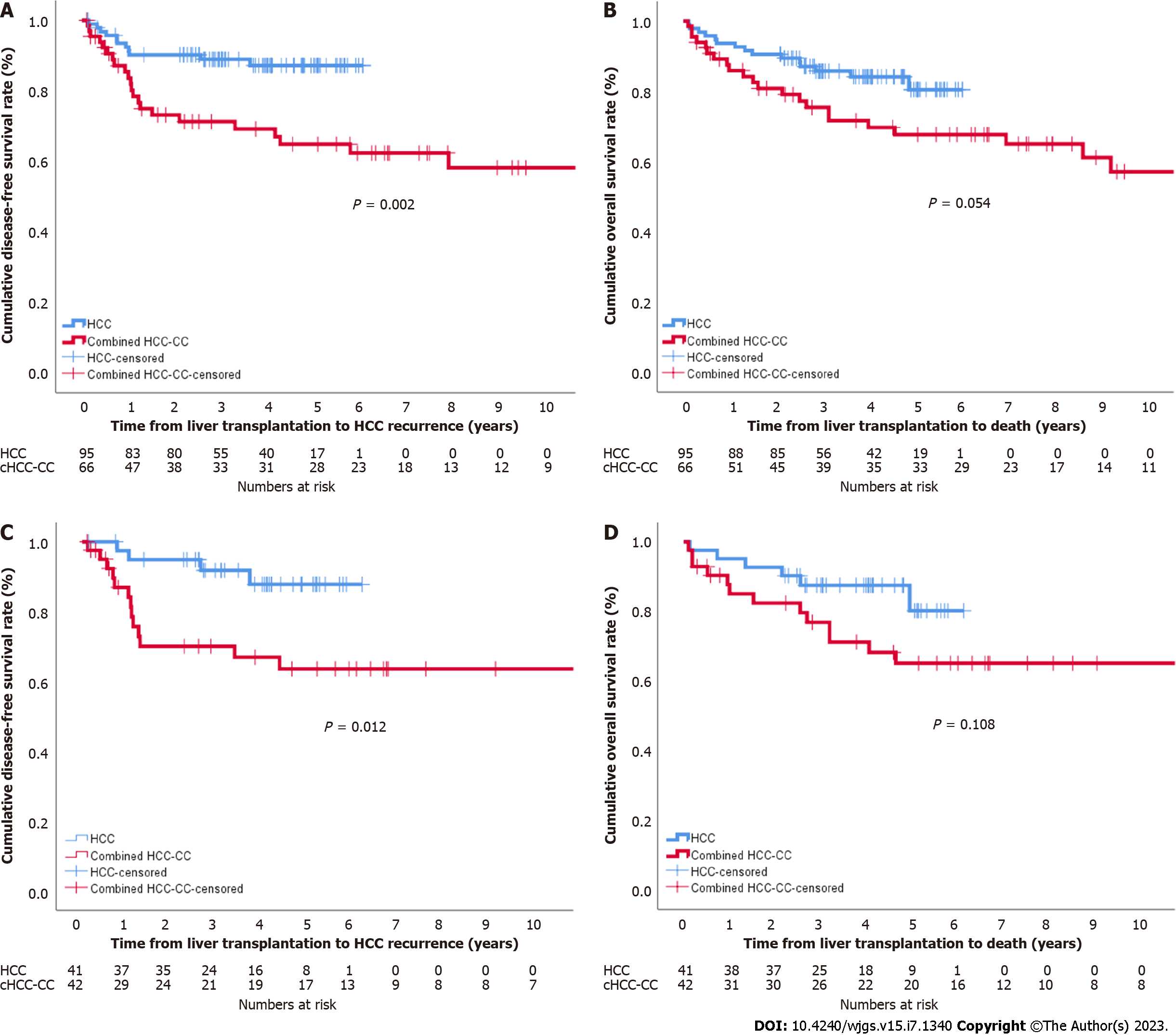
In patients within Milan criteria, DFS in the cHCC-CC group was significantly lower than that in the HCC group, but the difference in OS between the two groups did not reach a significant level (Figure 2A and B). After propensity score matching, the DFS and OS of the two groups showed similar patterns, but neither survival curve reached a significant level (Figure 2C and D). In patients beyond Milan criteria, the DFS and OS in the cHCC-CC group were significantly lower than those in the HCC group (P = 0.003 and P = 0.003, respectively) (Figure 3A and B), but no significant differences were noted between the two groups after propensity score matching (P = 0.263 and P = 0.050) (Figure 3C and D).
Multivariate analysis showed that the number of locoregional therapies before LT, tumor size > 3 cm, and lymph node metastasis were predisposing factors for tumor recurrence in the cHCC-CC group (Supplementary Table 1). Only a maximum tumor size > 3 cm was a predisposing factor for death (Table 4). In the propensity score matched set, significant risk factors for tumor recurrence included cHCC-CC, microvascular invasion, and number of locoregional therapies before LT > 3 in multivariate analysis. Death was closely associated with cHCC-CC, tumor size > 3 cm, and tumor number > 3 (Table 5).
| Tumor recurrence | Death | ||||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||
| Univariate | Univariate | ||||||
| Sex (male) | 4.763 | 1.155-19.640 | 0.031 | Sex (male) | 6.464 | 0.883-47.317 | 0.066 |
| Recipient age | 0.999 | 0.958-1.041 | 0.957 | Recipient age | 1.005 | 0.957-1.057 | 0.833 |
| Locoregional therapy before LT | 1.724 | 0.907-3.274 | 0.096 | Locoregional therapy before LT | 1.924 | 0.891-4.153 | 0.096 |
| TACE before LT | 1.826 | 0.989-3.371 | 0.054 | TACE before LT | 2.188 | 1.039-4.609 | 0.039 |
| Liver resection before LT | 2.594 | 1.387-4.852 | 0.003 | Liver resection before LT | 0.941 | 0.365-2.428 | 0.9 |
| RFA before LT | 1.616 | 0.781-3.340 | 0.196 | RFA before LT | 2.284 | 1.025-5.090 | 0.043 |
| Radiation therapy before LT | 0.718 | 0.174-2.962 | 0.646 | Radiation therapy before LT | 0.654 | 0.089-4.813 | 0.677 |
| Number of locoregional therapies before LT > 3 | 1.068 | 1.002-1.138 | 0.043 | Number of locoregional therapies before LT > 3 | 1.733 | 0.865-3.473 | 0.121 |
| MELD | 0.993 | 0.950-1.038 | 0.742 | MELD | 1.012 | 0.965-1.063 | 0.617 |
| Type of LT (DDLT) | 0.934 | 0.397-2.198 | 0.875 | Type of LT (DDLT) | 1.518 | 0.626-3.680 | 0.365 |
| ABO-incompatibility | 1.563 | 0.618-3.952 | 0.345 | ABO-incompatibility | 0.79 | 0.189-3.297 | 0.746 |
| Tumor size > 3cm | 3.013 | 1.707-5.317 | < 0.001 | Tumor size > 3 cm | 3.462 | 1.740-6.888 | < 0.001 |
| Tumor number > 3 | 1.35 | 0.723-2.520 | 0.346 | Tumor number > 3 | 1.463 | 0.694-3.084 | 0.318 |
| Milan criteria (beyond) | 2.495 | 1.403-4.436 | 0.002 | Milan criteria (beyond) | 2.813 | 1.395-5.670 | 0.004 |
| Tumor grade 3 or 4 | 1.465 | 0.809-2.651 | 0.208 | Tumor grade 3 or 4 | 1.229 | 0.586-2.580 | 0.585 |
| Microvascular invasion | 2.417 | 1.360-4.297 | 0.003 | Microvascular invasion | 2.28 | 1.153-4.507 | 0.018 |
| PVTT | 1.416 | 0.661-3.032 | 0.37 | PVTT | 1.302 | 0.502-3.376 | 0.587 |
| BDTT | 0.047 | 0.000-36.844 | 0.368 | BDTT | 0.048 | 0.000-333.805 | 0.5 |
| Intrahepatic metastasis | 1.357 | 0.676-2.722 | 0.39 | Intrahepatic metastasis | 1.182 | 0.515-2.716 | 0.693 |
| Multicentric occurrence | 1.148 | 0.607-2.170 | 0.671 | Multicentric occurrence | 1.125 | 0.526-2.403 | 0.762 |
| Encapsulation | 1.269 | 0.582-2.766 | 0.549 | Encapsulation | 0.952 | 0.367-2.471 | 0.92 |
| Tumor necrosis | 2.361 | 1.302-4.281 | 0.005 | Tumor necrosis | 3.22 | 1.531-6.773 | 0.002 |
| Dominant type (CC) | 0.995 | 0.495-2.003 | 0.989 | Dominant type (CC) | 0.823 | 0.375-1.805 | 0.627 |
| Lymph node metastasis | 13.954 | 3.065-63.526 | 0.001 | Lymph node metastasis | 24.719 | 4.683-130.472 | < 0.001 |
| AFP > 20 ng/mL | 1.563 | 0.880-2.777 | 0.128 | AFP > 20 ng/mL | 1.527 | 0.769-3.033 | 0.226 |
| PIVKA-II > 40 mAU/mL | 1.067 | 0.576-1.975 | 0.837 | PIVKA-II > 40 mAU/mL | 1.762 | 0.865-3.589 | 0.118 |
| Multivariate | Multivariate | ||||||
| Number of locoregional therapies before LT > 3 | 1.813 | 1.012-3.248 | 0.046 | Tumor size > 3 cm | 4.591 | 1.851-11.390 | 0.001 |
| Tumor size > 3 cm | 2.378 | 1.321-4.280 | 0.004 | ||||
| Lymph node metastasis | 8.585 | 1.822-40.453 | 0.007 | ||||
| Tumor recurrence | Death | ||||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||
| Univariate | Univariate | ||||||
| Group (cHCC-CC) | 2.15 | 1.162-3.977 | 0.015 | Group (cHCC-CC) | 2.134 | 1.142-3.987 | 0.018 |
| Sex (male) | 2.467 | 0.597-10.199 | 0.212 | Sex (male) | 1.536 | 0.475-4.964 | 0.474 |
| Recipient age | 0.973 | 0.928-1.021 | 0.266 | Recipient age | 1.009 | 0.962-1.057 | 0.725 |
| Locoregional therapy before LT | 1.78 | 0.855-3.706 | 0.123 | Locoregional therapy before LT | 2.304 | 1.026-5.171 | 0.043 |
| Number of locoregional therapies before LT > 3 | 2.185 | 1.207-3.957 | 0.01 | Number of locoregional therapies before LT > 3 | 1.241 | 0.683-2.254 | 0.478 |
| MELD | 0.944 | 0.889-1.002 | 0.056 | MELD | 0.993 | 0.947-1.041 | 0.758 |
| Type of LT (DDLT) | 0.771 | 0.239-2.493 | 0.664 | Type of LT (DDLT) | 1.999 | 0.891-4.488 | 0.093 |
| ABO-incompatibility | 1.094 | 0.526-2.277 | 0.81 | ABO-incompatibility | 0.439 | 0.157-1.228 | 0.117 |
| Tumor size > 3 cm | 2.541 | 1.406-4.592 | 0.002 | Tumor size > 3 cm | 2.426 | 1.341-4.386 | 0.003 |
| Tumor number > 3 | 1.81 | 0.957-3.422 | 0.068 | Tumor number > 3 | 1.458 | 0.749-2.839 | 0.267 |
| Milan criteria (beyond) | 2.893 | 1.573-5.322 | 0.001 | Milan criteria (beyond) | 2.261 | 1.242-4.119 | 0.008 |
| Tumor grade 3 or 4 | 1.247 | 0.599-2.596 | 0.554 | Tumor grade 3 or 4 | 1.268 | 0.610-2.639 | 0.525 |
| Microvascular invasion | 1.936 | 1.068-3.510 | 0.03 | Microvascular invasion | 1.873 | 1.032-3.400 | 0.039 |
| PVTT | 1.389 | 0.497-3.885 | 0.531 | PVTT | 1.836 | 0.722-4.669 | 0.202 |
| BDTT | 0.595 | 0.082-4.322 | 0.608 | BDTT | 2.035 | 0.487-8.504 | 0.33 |
| Intrahepatic metastasis | 2.192 | 1.172-4.101 | 0.014 | Intrahepatic metastasis | 1.742 | 0.923-3.289 | 0.087 |
| Multicentric occurrence | 1.313 | 0.687-2.512 | 0.41 | Multicentric occurrence | 0.967 | 0.489-1.915 | 0.924 |
| Encapsulation | 1.447 | 0.701-2.986 | 0.317 | Encapsulation | 1.305 | 0.626-2.723 | 0.478 |
| Tumor necrosis | 2.245 | 1.229-4.101 | 0.009 | Tumor necrosis | 2.56 | 1.383-4.740 | 0.003 |
| AFP > 20 ng/mL | 1.053 | 0.569-1.946 | 0.87 | AFP > 20 ng/mL | 1.333 | 0.733-2.425 | 0.346 |
| PIVKA-II > 40 mAU/mL | 1.202 | 0.639-2.263 | 0.568 | PIVKA-II > 40 mAU/mL | 1.422 | 0.762-2.651 | 0.268 |
| Multivariate | Multivariate | ||||||
| Group (cHCC-CC) | 2.531 | 1.191-5.376 | 0.016 | Group (cHCC-CC) | 2.281 | 1.057-4.922 | 0.036 |
| Microvascular invasion | 3.232 | 1.486-7.029 | 0.003 | Tumor size > 3 cm | 1.343 | 1.123-1.605 | 0.001 |
| Number of locoregional therapies before LT > 3 | 2.33 | 1.235-4.396 | 0.009 | Tumor number > 3 | 1.032 | 1.004-1.061 | 0.026 |
The survival benefit of LT for cHCC-CC patients has yet to be defined, and LR has been reported to be sufficient in patients with resectable cHCC-CC without underlying advanced liver cirrhosis. However, the ability to offer LR for patients with cHCC-CC is frequently limited or prohibited by the presence of underlying advanced liver cirrhosis[5]. LT is considered the only potentially curative option for cirrhotic patients with cHCC-CC[2,5,14,15], but the actual role of LT in therapeutic strategies for cHCC-CC is unclear. Therefore, we compared the outcomes of LT for cHCC-CC to those of LT for HCC. LT for cHCC-CC was associated with worse OS and DFS rates as well as worse recurrence rates than LT for HCC. Prognostic factors need to be identified to allow better patient selection and better outcomes after LT.
When comparing LT for cHCC-CC and HCC among patients within Milan criteria, the DFS in the cHCC-CC group was significantly worse than that in the HCC group before and after propensity score matching. OS in the cHCC-CC group was lower than in the HCC group before and after propensity score matching, but these differences were not significant. In patients beyond Milan criteria, both DFS and OS in the cHCC-CC group were significantly lower than those in the HCC group. However, there were no statistically significant differences in DFS and OS between the two groups after propensity score matching despite the shorter DFS and OS in the cHCC-CC group than those in the HCC group. Nearly all patients in our study had advanced liver cirrhosis or treatment-refractory HCC; thus, other treatment strategies could not be used. Patients with small cHCC-CC tumors (≤ 3 cm) showed a low recurrence rate after LT irrespective of tumor number. These findings suggest that cHCC-CC is not an absolute contraindication for LT.
Preoperative discrimination of cHCC-CC from HCC and CC as differential diagnoses of primary hepatic malignancies is important for therapeutic considerations. Unfortunately, accurate preoperative diagnosis of cHCC-CC prior to therapy initiation is difficult because the condition has few specific imaging characteristics[16]; thus, most cases are confirmed in postoperative histopathology. Patients with cHCC-CC are frequently misdiagnosed with HCC and deemed eligible for LT. In our study, 86.3% (n = 96/111) of cHCC-CC patients were preoperatively diagnosed with HCC. Korean liver cancer guidelines can diagnose HCC based on radiological images[17]; thus, liver biopsies are not routinely performed before or during several treatments. In atypical HCC cases, a biopsy is warranted to refine the diagnosis. However, biopsies not only lack sensitivity, but can be misleading because of the presence of different cellular components. In our study, 79.4% of the HCC group and 66.7% of the cHCC-CC group underwent locoregional therapy before LT without liver biopsy. In addition, liver biopsy was not performed prior to LT.
The median age at cHCC-CC diagnosis is 50-75 years, with the maximum incidence observed between 60 and 64 years for men and 75 and 79 years for women[18]. However, the median age of the cHCC-CC group in our study was 54 years, which was significantly younger than that in the HCC group. In the majority of Korean patients, HBV infection and cirrhosis are fundamental predisposing factors in the pathogenesis of cHCC-CC, similar to HCC[3,4]. Our study also found HBV (82.0%) to be the most common underlying liver disease. The tendency of cHCC-CC to emerge as multiple hepatic lesions is possibly associated with hepatocellular behavior[19]. Therefore, cHCC-CC is thought to arise from hepatic progenitor cells and occur in the presence of pre-existing abnormalities in the parenchymal architecture, such as advanced fibrosis and cirrhosis associated with HBV infection[2,10,16].
Preoperatively, serum AFP levels serve as an established tumor marker for HCC, whereas CA 19-9 is used as a tumor marker for CC[2,5]. Both markers are frequently elevated in cHCC-CC, depending on the proportion of either type of differentiation[3]. In the cases examined in this study, AFP was elevated in 56 patients (50.5%) in the cHCC-CC group. Therefore, the proportion of patients with AFP > 20 ng/mL was greater in the cHCC-CC group than in the HCC group. Elevated CA 19-9 was observed in 13 patients (11.7%) in the cHCC-CC group, but no other patients had detectable CA 19-9 preoperatively.
The long-term outcomes of LT for cHCC-CC are rarely reported, and existing data were obtained from small-volume studies with contradicting results[1,5]. Ninety-four LT patients with cHCC-CC from the United Network for Organ Sharing database had a significantly inferior OS rate of 40% at five years compared to HCC recipients[20]. However, a multicenter study from Spain reported that the cHCC-CC patients had outcomes similar to the HCC controls, with a 5-year survival rate of 78%[6]. A recent multicenter study showed that LT for cHCC-CC (n = 67) and HCC (n = 1,814) within the Milan criteria did not lead to a significant difference in terms of OS; the 5-year OS rate was 70.1% for cHCC-CC and 73.4% for HCC (P = 0.806), despite higher 5-year cHCC-CC recurrence rates (23.1% in cHCC-CC vs 11.5% in HCC, P < 0.001)[15]. Our study reported cumulative DFS at 3 years was 80.6% in the HCC group and 56.3% in the cHCC-CC group. Meanwhile, OS rates at three years were 84.0% in the HCC group and 63.8% in the cHCC-CC group. Therefore, our study revealed high DFS rates and low OS rates in the cHCC-CC group, suggesting that patients with a preoperative diagnosis of cHCC-CC should not be considered for LT. Our study showed that Milan criteria are an important prognostic factor for tumor recurrence and death after LT. The 5-year recurrence-free survival rate of 65% after LT is acceptable for unresectable hilar CC patients[21]. Our study showed 5-year DFS and OS rates in the cHCC-CC group of patients within Milan criteria ≥ 60% after LT.
Although our study covers a long period at multiple centers with many cases, it has several limitations. First, this study was retrospective; thus, it was difficult to obtain detailed information on eligible LT patients across nine institutions. Second, we included patients based on pathology reports, as one pathologist failed to review the entire pathology slide that included the matching criteria, and we could not collect pathologic slides due to the Personal Information Protection Act of Korea. The definition of cHCC-CC changed in a recently published classification[22] and might have a affected the patients in this study. Third, patients with cHCC-CC had significant disparities in pretransplant therapy and treatment strategies for recurrent tumors after LT; thus, detailed management included the use of mammalian target of rapamycin inhibitors as a potential source of bias in our analyses. Fourth, many patient records did not include information about preoperative tumor markers such as CEA or CA 19-9 or the granularity of preoperative radiologic details, which can affect tumor recurrence and posttransplant survival. Fifth, because our study is based on tumor burden in the pathology report of the explant liver, it was not possible to discuss the selection criteria before LT because candidacy was based on preoperative imaging. Finally, our results might not be applicable to Western patients.
In conclusion, our study shows that explant liver characteristics can predict a poor prognosis of patients diagnosed with cHCC-CC after LT. Among these patients, if maximum tumor size is ≤ 3 cm, number of locoregional therapies before LT is ≤ 3, or tumor number is ≤ 3, a good prognosis can be expected. In addition to these factors, if the liver recipient has lymph node metastasis or microvascular invasion, frequent regular surveillance is required for early detection of tumor recurrence.
Patients with combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC) tumors are not traditionally considered for liver transplantation (LT) because single centers with few cases have previously reported poor outcomes; however, several small single-center cohort studies showed satisfactory outcomes after LT for cHCC-CC equivalent to those attained for hepatocellular carcinoma (HCC). The role of LT has been investigated in several retrospective studies that included patients diagnosed incidentally during pathological examination of the explant. The variation in results among patients with cHCC-CC suggests that LT should be considered only in select cases.
Data on clinicopathologic presentation, prognostic factors, and outcomes for LT in cHCC-CC patients are lacking because cHCC-CC is rare, and few studies have been published. To overcome the limitations of single-center and small-volume cases, we collected and analyzed data to evaluate the utility of LT for cHCC-CC from high-volume LT centers in Korea.
We compared the characteristics between living donor LT (LDLT) patients with HCC and LT patients with cHCC-CC before and after propensity score matching and identified the risk factors for tumor recurrence and death after LT in cHCC-CC patients.
We performed a retrospective analysis of patients who were diagnosed with cHCC-CC in their postoperative pathology reports and who underwent LT at any of nine Korean medical centers between January 2000 and December 2018. Patients who received LDLT for HCC at Samsung Medical Center from January 2013 to March 2017 were selected as the control group. Recipients < 18 years, re-transplantation cases, and patients who received multiorgan grafts were excluded.
Cumulative disease-free survival and overall survival in the cHCC-CC group were significantly worse than in the HCC group both before and after matching. Extrahepatic recurrence incidence in the cHCC-CC group was higher than that in the HCC group (75.5% vs 33.3%, P < 0.001). Multivariate analysis demonstrated that the cHCC-CC group had significantly higher rates of tumor recurrence and death compared to the HCC group. In cHCC-CC subgroup analysis, frequency of locoregional therapies > 3, tumor size > 3 cm, and lymph node metastasis were predisposing factors for tumor recurrence in multivariate analysis. Only a maximum tumor size > 3 cm was a predisposing factor for death.
The poor prognosis of patients diagnosed with cHCC-CC after LT can be predicted based on the explanted liver. Frequent regular surveillance for cHCC-CC patients should be required for early detection of tumor recurrence.
Research is needed to determine how cHCC-CC patients are diagnosed and when to perform LT for the best outcome.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wei W, China; Zhao D, China S-Editor: Li L L-Editor: A P-Editor: Yuan YY
| 1. | Kim JM. Liver Transplantation in Mixed Hepatocellular Carcinoma and Cholangiocarcinoma. J Liver Cancer. 2019;19:85-90. [DOI] [Full Text] |
| 2. | Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: An update. J Hepatol. 2021;74:1212-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 3. | Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, Yoon JH, Lee HS, Yi NJ, Suh KS, Lee KU, Jang JJ, Kim YJ. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011;45:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Sapisochin G, Javle M, Lerut J, Ohtsuka M, Ghobrial M, Hibi T, Kwan NM, Heimbach J. Liver Transplantation for Cholangiocarcinoma and Mixed Hepatocellular Cholangiocarcinoma: Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl. 2011;17:934-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Panjala C, Senecal DL, Bridges MD, Kim GP, Nakhleh RE, Nguyen JH, Harnois DM. The diagnostic conundrum and liver transplantation outcome for combined hepatocellular-cholangiocarcinoma. Am J Transplant. 2010;10:1263-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Groeschl RT, Turaga KK, Gamblin TC. Transplantation versus resection for patients with combined hepatocellular carcinoma-cholangiocarcinoma. J Surg Oncol. 2013;107:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Sapisochin G, de Lope CR, Gastaca M, de Urbina JO, López-Andujar R, Palacios F, Ramos E, Fabregat J, Castroagudín JF, Varo E, Pons JA, Parrilla P, González-Diéguez ML, Rodriguez M, Otero A, Vazquez MA, Zozaya G, Herrero JI, Antolin GS, Perez B, Ciria R, Rufian S, Fundora Y, Ferron JA, Guiberteau A, Blanco G, Varona MA, Barrera MA, Suarez MA, Santoyo J, Bruix J, Charco R. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg. 2014;259:944-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Lunsford KE, Court C, Seok Lee Y, Lu DS, Naini BV, Harlander-Locke MP, Busuttil RW, Agopian VG. Propensity-Matched Analysis of Patients with Mixed Hepatocellular-Cholangiocarcinoma and Hepatocellular Carcinoma Undergoing Liver Transplantation. Liver Transpl. 2018;24:1384-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 12. | Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1444] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 13. | Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6382] [Cited by in RCA: 7528] [Article Influence: 537.7] [Reference Citation Analysis (0)] |
| 14. | Jung DH, Hwang S, Song GW, Ahn CS, Moon DB, Kim KH, Ha TY, Park GC, Hong SM, Kim WJ, Kang WH, Kim SH, Yu ES, Lee SG. Longterm prognosis of combined hepatocellular carcinoma-cholangiocarcinoma following liver transplantation and resection. Liver Transpl. 2017;23:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Dageforde LA, Vachharajani N, Tabrizian P, Agopian V, Halazun K, Maynard E, Croome K, Nagorney D, Hong JC, Lee D, Ferrone C, Baker E, Jarnagin W, Hemming A, Schnickel G, Kimura S, Busuttil R, Lindemann J, Florman S, Holzner ML, Srouji R, Najjar M, Yohanathan L, Cheng J, Amin H, Rickert CA, Yang JD, Kim J, Pasko J, Chapman WC, Majella Doyle MB. Multi-Center Analysis of Liver Transplantation for Combined Hepatocellular Carcinoma-Cholangiocarcinoma Liver Tumors. J Am Coll Surg. 2021;232:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Kim TH, Kim H, Joo I, Lee JM. Combined Hepatocellular-Cholangiocarcinoma: Changes in the 2019 World Health Organization Histological Classification System and Potential Impact on Imaging-Based Diagnosis. Korean J Radiol. 2020;21:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022;28:583-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 185] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 18. | Ramai D, Ofosu A, Lai JK, Reddy M, Adler DG. Combined Hepatocellular Cholangiocarcinoma: A Population-Based Retrospective Study. Am J Gastroenterol. 2019;114:1496-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Schizas D, Mastoraki A, Routsi E, Papapanou M, Tsapralis D, Vassiliu P, Toutouzas K, Felekouras E. Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management. Hepatobiliary Pancreat Dis Int. 2020;19:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Vilchez V, Shah MB, Daily MF, Pena L, Tzeng CW, Davenport D, Hosein PJ, Gedaly R, Maynard E. Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB (Oxford). 2016;18:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Tan EK, Taner T, Heimbach JK, Gores GJ, Rosen CB. Liver Transplantation for Peri-hilar Cholangiocarcinoma. J Gastrointest Surg. 2020;24:2679-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, Gouw A, Kagen A, Klimstra D, Komuta M, Kondo F, Miksad R, Nakano M, Nakanuma Y, Ng I, Paradis V, Nyun Park Y, Quaglia A, Roncalli M, Roskams T, Sakamoto M, Saxena R, Sempoux C, Sirlin C, Stueck A, Thung S, Tsui WMS, Wang XW, Wee A, Yano H, Yeh M, Zen Y, Zucman-Rossi J, Theise N. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (0)] |