Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1331
Peer-review started: March 20, 2023
First decision: April 13, 2023
Revised: April 20, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: July 27, 2023
Processing time: 123 Days and 11.8 Hours
In Japan, the transhiatal approach, including lower mediastinal lymph node dissection, is widely performed for Siewert type II esophagogastric junction adenocarcinoma. This procedure is generally performed in a magnified view using laparoscopy or a robotic system, therefore, the microanatomy of the lower mediastinum is important. However, mediastinal microanatomy is still unclear and classification of lower mediastinal lymph nodes is not currently based on fascia or other microanatomical structures.
To clarify the fascia and layer structures of the lower mediastinum and classify the lower mediastinal tissue.
We dissected the esophagus and surrounding organs en-bloc from seven cadavers fixed in 10% formalin. Organs and tissues were then cut at the level of the lower thoracic esophagus, embedded in paraffin, and serially sectioned. Tissue sections were stained with Hematoxylin-Eosin (all cadavers) and immunostained for the lymphatic endothelial marker D2-40 (three cadavers). We observed the periesophageal fasciae and layers, and defined lymph node boundaries based on the fasciae. Lymphatic vessels around the esophagus were observed on immunostained tissue sections.
We identified two fasciae, A and B. We then classified lower mediastinal tissue into three areas, paraesophageal, paraaortic, and intermediate, using these fasciae as boundaries. Lymph nodes were found to be present and were counted in each area. The dorsal part of the intermediate area was thicker on the caudal side than on the cranial side in all cadavers. On the dorsal side, no blood vessels penetrated the fasciae in six of the seven cadavers, whereas the proper esophageal artery penetrated fascia B in one cadaver. D2-40 immunostaining showed lymphatic vessel connections between the paraesophageal and intermediate areas on the lateral and ventral sides of the esophagus, but no lymphatic connection between areas on the dorsal side of the esophagus.
Histological studies identified two fasciae surrounding the esophagus in the lower mediastinum and the layers separated by these fasciae were used to classify the lower mediastinal tissues.
Core Tip: The transhiatal procedure including lower mediastinal lymph node (LN) dissection is widely performed to treat esophagogastric junction (EGJ) adenocarcinoma. However, microanatomy of the lower mediastinum is unclear and the classification of lower mediastinal LNs is obscure. Therefore, we performed a histological study to investigate the microanatomy of the lower mediastinum in seven cadavers. We identified two fasciae surrounding the esophagus in the lower mediastinum and classified the periesophageal lower mediastinal tissue into three areas based on these fasciae. LNs were found within all classified areas. These data provide useful landmarks for EGJ adenocarcinoma surgery.
- Citation: Saito T, Muro S, Fujiwara H, Umebayashi Y, Sato Y, Tokunaga M, Akita K, Kinugasa Y. Histological study of the structural layers around the esophagus in the lower mediastinum. World J Gastrointest Surg 2023; 15(7): 1331-1339
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1331.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1331
Cases of the esophagogastric junction (EGJ) adenocarcinoma are increasing worldwide, including in East Asia[1,2], and research on surgery for this disease has become increasingly important. EGJ adenocarcinoma is internationally classified according to the Siewert classification[3], however, there has been no consensus on the best procedure to treat Siewert type II EGJ adenocarcinoma. Recently in Japan, the transhiatal approach has been widely performed for Siewert type II cancers, while the right thoracic approach has been widely performed for surgical excision of Siewert type I cancers. Transhiatal or transthoracic resection of lower mediastinal lymph nodes (LNs) and resection of abdominal LNs are performed in both procedures. These procedures are often performed with a magnified view using laparoscopy, thoracoscopy, or robotic systems. Therefore, knowledge of the microanatomy of the layers of the lower mediastinum is essential for any approach. However, the microanatomy of the fascia and structural layers of the lower mediastinum is still unclear.
Furthermore, the Japan Esophageal Society defines the classification of LNs in the Japanese classification of Esophageal Cancer. Thus, the LNs of the lower mediastinum are classified as paraesophageal LNs, pulmonary ligament LNs, thoracic paraaortic LNs, and supradiaphragmatic LNs[4,5]. All these LNs may be dissected depending on the depth and LN metastasis in individual cases. However, this LN classification is not based on fascia or layer structures, and the distribution of each LN is unclear. To overcome these two problems, we performed a histological study of the fascia and layer structure around the esophagus in the lower mediastinum, with the aim of classifying the lower mediastinal tissue to identify clinically useful structural landmarks for surgery on EGJ adenocarcinoma.
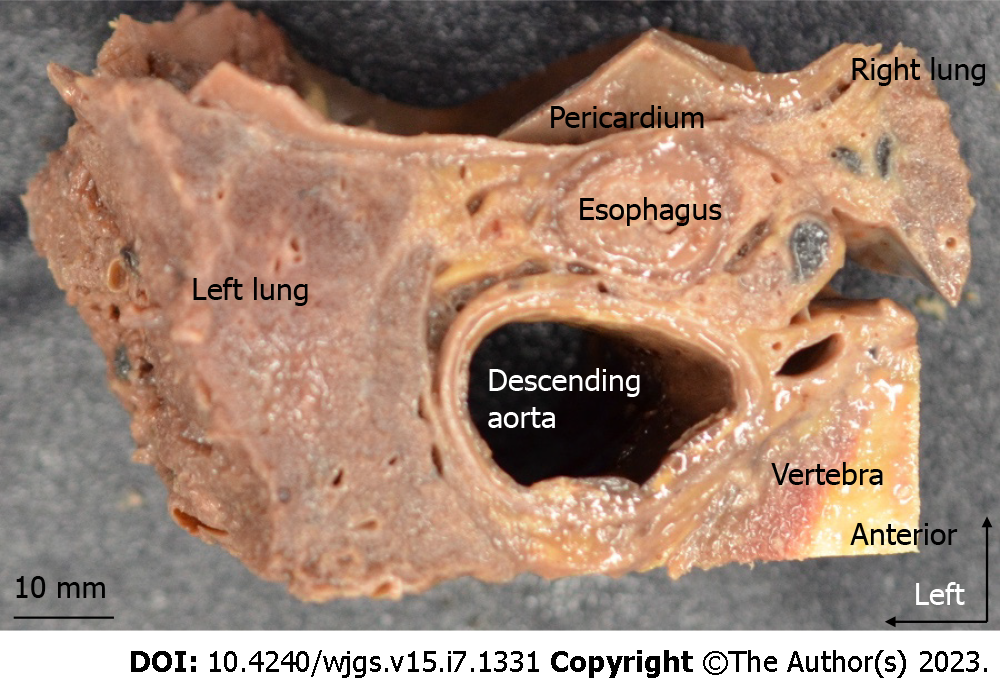
This study included seven cadavers (four males and three females) aged 75–99 years donated to the Department of Clinical Anatomy at the Tokyo Medical and Dental University. Cadavers with a history of gastric, esophageal, EGJ, or lung cancer, mediastinal tumor, or aortic aneurysm were excluded. Before death, all donors had signed documents agreeing to donate their bodies and had provided consent for use in anatomical studies. The consent document was consistent with the "Act on Body Donation for Medical and Dental Education" under Japanese law. This study protocol was approved by our Institutional Review Board, No. M2018-210. Cadavers were fixed in 10% formalin for a week and then preserved in 30% alcohol. At autopsy, the anterior thorax and abdomen were first resected, then the esophagus, trachea, bronchus, heart, aorta, vertebral body, lungs, stomach, and diaphragm were resected en-bloc. The block did not include vertebra in two cadavers because of osteosclerosis. The esophagus and surrounding organs were cut from the upper margin of the lower thoracic esophagus (Lt) to the esophageal hiatus (Figure 1A) using a diamond band saw. The esophagus and surrounding tissue above the middle thoracic esophagus (Mt) were not included in this study. Our classification of the esophagus was based on the 11th edition of the Japanese Classification of Esophageal Cancer[4,5]. The definitions of the thoracic esophagus and lower mediastinal LNs are described in Table 1. The Lt region generally coincided with the caudal level of the lower pulmonary vein. Specimens were fixed in formalin for 24 h, degreased for 2–3 d, decalcified in Plank-Rychlo solution for 5 d, and embedded in paraffin. Serial 5-μm sections were made without interruption every 1 mm in the horizontal plane. Hematoxylin-Eosin (HE) staining was performed every 1 mm.
| Definitions of the thoracic esophagus | |
| Upper thoracic esophagus (Ut) | From the sternal notch to the tracheal bifurcation |
| Middle thoracic esophagus (Mt) | The proximal half of the two equal portions between the tracheal bifurcation and the esophagogastric junction |
| Lower thoracic esophagus (Lt) | The thoracic part of the distal half of the two equal portions between the tracheal bifurcation and the esophagogastric junction |
| Definitions of lower mediastinal lymph nodes | |
| No. 110 | Lower thoracic paraesophageal lymph nodes |
| No. 111 | Supradiaphragmatic lymph nodes |
| No. 112aoA | Anterior thoracic paraaortic lymph nodes |
| No. 112aoP | Posterior thoracic paraaortic lymph nodes |
| No. 112pul | Pulmonary ligament lymph nodes |
We first observed the periesophageal layers and microanatomy, and defined LN boundaries based on fasciae. Then we counted the number of LNs in each region. The distance between the aorta and the esophageal wall was measured at the shortest distance perpendicular to the esophageal wall in order to examine the tissue thickness dorsal to the esophagus. Two cadavers were excluded from this measurement due to notable cracks that were caused during sectioning.
For three of the seven cadavers, we performed D2-40 (podoplanin) staining to identify lymphatic vessels. Immunostaining was performed on 5-μm tissue sections every 7–8 mm. Tissue sections were incubated overnight at room temperature with purified anti-podoplanin antibody (1.0 mg/mL, 1:1000, Clone D2-40, BioLegend Inc., San Diego, CA) as the primary antibody. Podoplanin is expressed by lymphatic endothelial cells. Tissue sections were incubated with peroxidase-conjugated anti-mouse IgG reagent (ready to use, ImmPRESS® HRP Goat Anti-Rabbit IgG Polymer, Vector Laboratories, CA, United States) as the secondary antibody for 30 min at room temperature. We used 3,3-diaminobenzidine (DAB) to detect immunocomplexes. During this process, we made several microscopic observations to confirm that the lymphatic vessels around the lymph nodes and the endothelial cells of the thoracic duct were stained, and then stopped the DAB reaction before any other cells were stained. We used these immunostained tissue sections to observe the lymphatic vessels around the esophagus.
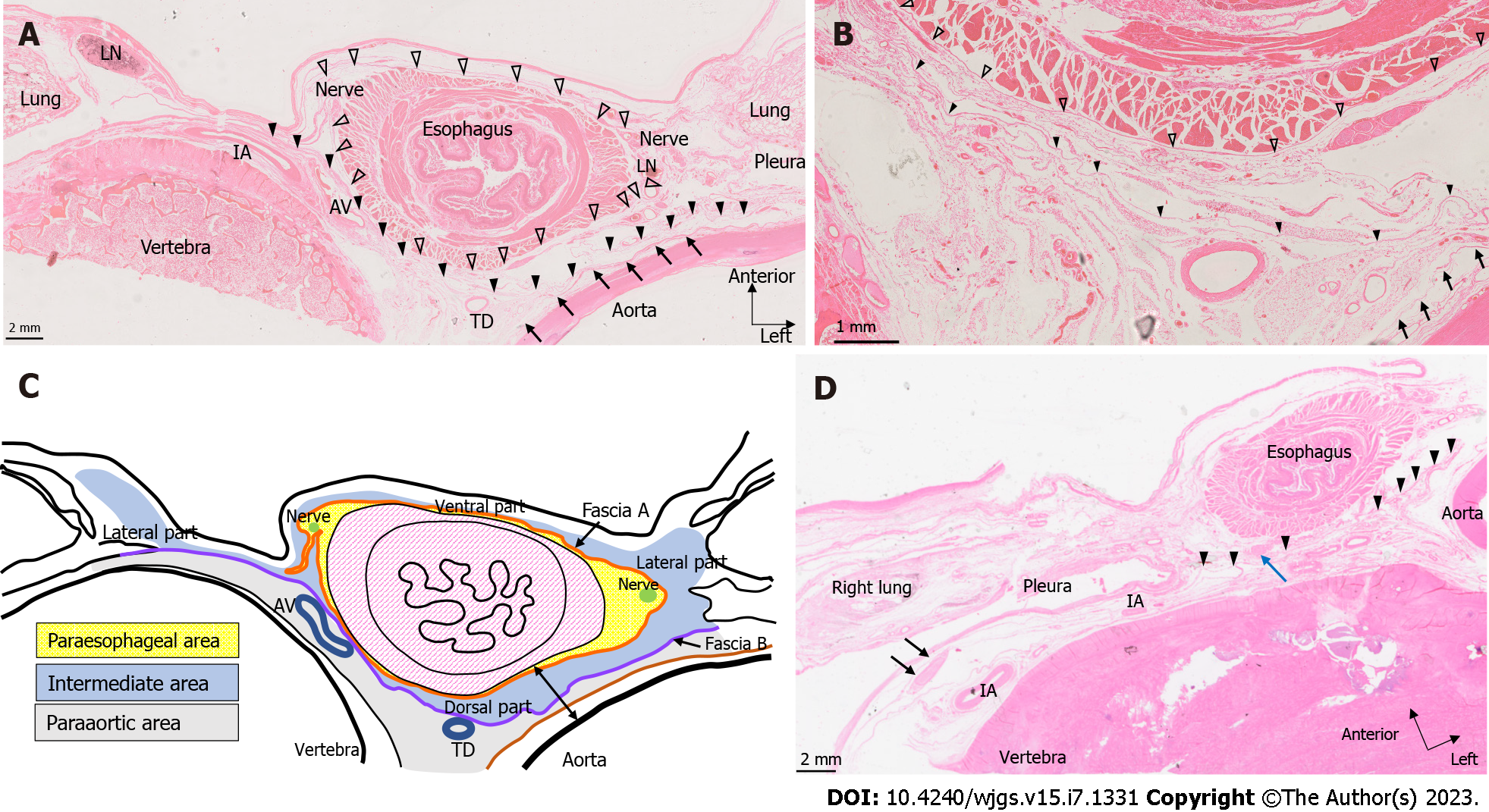
We microscopically examined the periesophageal anatomy in the lower mediastinum on HE-stained slides. As shown in Figure 2A and B, the pericardium, esophagus, bilateral vagus nerves, azygos veins, thoracic duct, descending aorta, pleura, lung, and intercostal artery were identified. We also identified two fasciae, A and B. These fasciae act as boundaries dividing the periesophageal tissue of the lower mediastinum into three areas. We defined the closest area to the esophagus encompassed by fascia A as the ‘paraesophageal area’, the area dorsal to fascia B as the ‘paraaortic area’, and the area between the two as the ‘intermediate area’. Within the paraesophageal area, tiny vessels feeding the esophagus, right and left vagus nerves, and LNs were observed. Fascia A (Figure 2A and B: White arrowhead), the boundary between the paraesophageal and intermediate areas, was close to the muscularis propria of the esophagus on the ventral and dorsal sides, but expanded transversely on both lateral sides, converging toward the left and right vagus nerves. The paraaortic area was located around the descending aorta and anterior vertebral body, containing the thoracic duct, azygos vein, and intercostal arteries. Dorsal to the paraaortic area, fascia was observed circumferentially surrounding the descending aorta (Figure 2A and B: Arrow). Between the paraesophageal and paraaortic areas, the intermediate area also surrounded the esophagus, contained the right and left pulmonary ligaments and pulmonary ligament LNs, and abutted the right and left pleura on the lateral sides. Dorsal to the intermediate area, fascia B (Figure 2A and B: Black arrowhead) ran anterior to the thoracic duct and azygos vein, and laterally contiguous to the right and left pleura. These three areas and fasciae were identified in all seven cadavers. We have shown the findings above as a schema in Figure 2C.
We then examined the LN distribution. Table 2 shows the number of LNs in each area. In the paraesophageal area, LNs were almost absent on the ventral and dorsal sides but were identified on the lateral side (Figure 2A). We subdivided the intermediate area into ventral, lateral (right and left), and dorsal parts. In the ventral part, a thin membranous structure was observed behind the pericardium, and LNs were identified on the dorsal side of this membrane. The lateral part coincided with the bilateral pulmonary ligaments. In the paraaortic area, LNs were identified in front of the vertebral body and around the thoracic duct (Figure 2D).
| Cadaver No. | Para-esophageal area | Intermediate area | Para-aortic area | |||
| Ventral | Lateral-right | Lateral-left | Dorsal | |||
| 1 | 3 | 0 | 1 | 2 | 0 | 1 |
| 2 | 3 | 1 | 1 | 3 | 0 | 2 |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 2 | 0 | 2 | 1 | 2 | 2 |
| 5 | 1 | 1 | 0 | 0 | 2 | 1 |
| 6 | 0 | 1 | 0 | 1 | 0 | 2 |
| 7 | 1 | 2 | 2 | 0 | 0 | 0 |
| Average | 1.6 | 0.9 | 1 | 1.1 | 0.7 | 1.3 |
The thickness of the dorsal part of the intermediate area varied depending on the distance from the esophageal hiatus. We measured the shortest distance between the aorta and esophagus in a direction perpendicular to the esophageal wall at the most cranial and caudal slides of each cadaver (Figure 2C, arrows at both ends). This allowed us to investigate the differences in tissue thickness of the dorsal part of the intermediate area. As shown in Table 2, the dorsal part was thicker caudally in all cadavers. The mean values were 673.9 μm for the cranial section and 1665.6 μm for the caudal section.
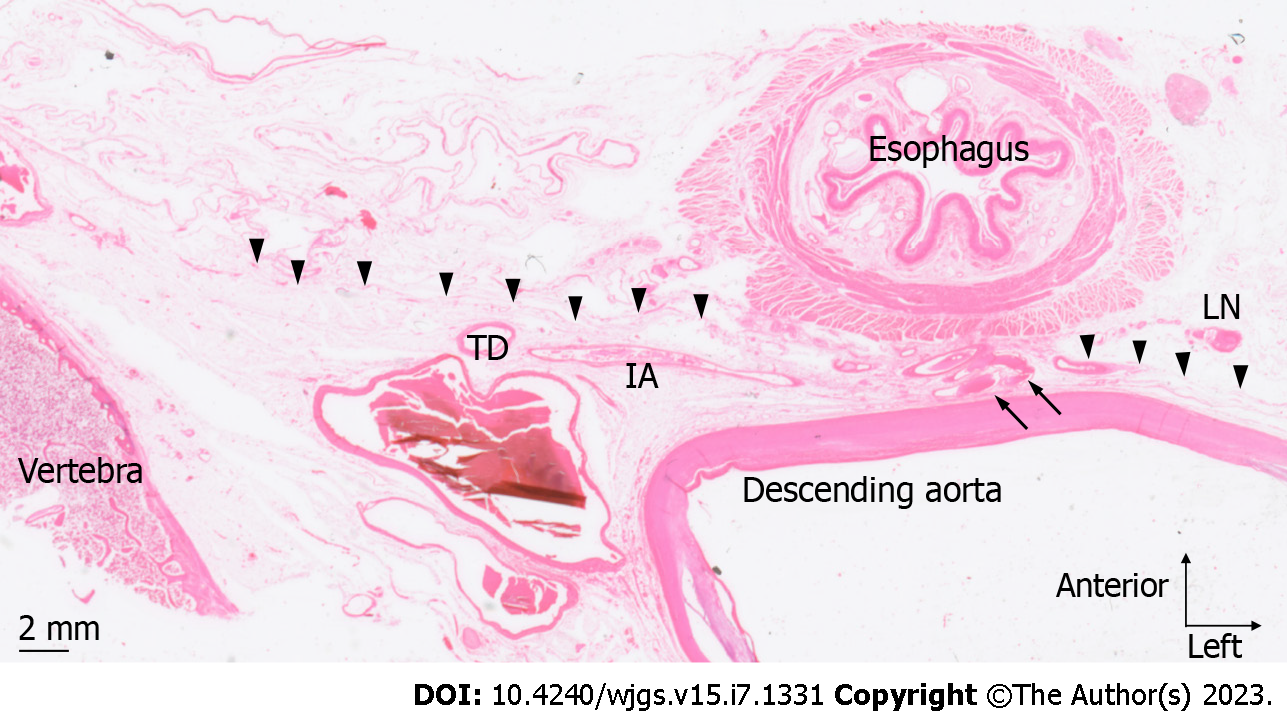
On the anterior region of the esophagus, no vessels were seen to penetrate fascia A and extend over the ventral region of the paraesophageal and intermediate areas. In the dorsal region of the esophagus, no structures penetrated fascia A and B in six of the seven cadavers, whereas the proper esophageal artery that branches from the descending aorta penetrated fascia B and the fascia surrounding the aorta in one cadaver (Figure 3).
We performed D2-40 staining on three cadavers. Lymphatic vessels ran alongside the arterioles within the paraesophageal area (Figure 4A). On the ventral and lateral sides of the esophagus (Figure 4A and B), we observed lymphatic vessels contiguous with the LNs in the lateral part of the intermediate area, which drained from or entered the ventral part. Some lymphatic vessels in the intermediate area ran toward lymphatic vessels in the paraesophageal area (seen around arterioles, Figure 4A). In the lateral and anterior regions of the esophagus, there was continuity between the lymphatic vessels of the paraesophageal and intermediate areas. In contrast, in the paraaortic area, lymphatic vessels ran along the thoracic duct in a cranio-caudal direction or transversely along the intercostal arteries and vertebra, and there were no lymphatic vessels extending over each area on the dorsal side of the esophagus in our limited specimens.
Summarizing the results of this anatomical study, we identified two fasciae, A and B, that surround the esophagus in the lower mediastinum, and classified the periesophageal lower mediastinal tissue into three areas based on these fasciae. LNs were found within all areas, not only in the paraesophageal area. Moreover, lymphatic vessel connections between the paraesophageal area and the intermediate area were observed, especially in the ventral region of the esophagus. The tissue thickness of the intermediate area on the dorsal side of the esophagus was greater on the caudal side than on the cranial side.
This study identified fascia A surrounding the esophagus. We used fascia A as a landmark to divide lower mediastinal tissue into an internal paraesophageal area close to the esophagus and an intermediate area. We considered the LNs within the paraesophageal area as corresponding to the paraesophageal LNs (110) defined by the Japanese classification of Esophageal Cancer. In addition, we considered fascia A to correspond to the previously reported visceral sheath. Tokairin et al[6] reported that the visceral sheath circumferentially covers the esophagus and trachea in the upper mediastinum but is obscured at the level of the tracheal bifurcation[6]. Around the tracheal bifurcation, the left and right bronchi, vagus nerves, and other vessels and nerves pass over this fascial structure, obscuring the layer structure. Thus, the fascial structure can be considered non-continuous around the tracheal bifurcation. Although we could not examine the continuity of fascia A in the upper mediastinum in this study, we did confirm the consistent presence of fascia A at the level of the Lt.
We also identified fascia B as a useful landmark that allows mediastinal dissection with preservation of the thoracic duct by dissecting along this layer. This fascia could correspond to the interpleural ligament reported by Meyer and Sublon[7]. Riddell et al[8] reported periesophageal anatomy using high-resolution magnetic resonance imaging and cadavers, including fascia from the anterior aortic surface to the pleura[8], which may represent the same structure as our fascia B. Although this study is based on a small number of cases, other reports showed the same structure as fascia B, indicating that fascia B is reproducible.
Our finding that the intermediate area on the dorsal side of the esophagus is thicker on the caudal side than the cranial side (Table 3) is clinically important. The intermediate area on the dorsal side of the esophagus is the intervening tissue between the descending aorta and an EGJ adenocarcinoma or lower thoracic esophageal carcinoma with the tumor center located in the lower mediastinum. Thus, this tissue possibly prevents aortic invasion by cancer, a hypothesis supported by published data showing that aortic invasion is more common in middle thoracic esophageal cancer than lower thoracic esophageal cancer[9,10]. Furthermore, the proper esophageal artery, which branches from the descending aorta into the esophagus, is relatively common in the Mt region, which is also likely due to the thickness of the intervening tissue between the esophagus and the aorta. In six of the seven cadavers in our study, there were no structures extending over each area on the dorsal side of the esophagus, and each area was separated by fascia. We only saw a proper esophageal artery penetrate fascia within the lower mediastinum in one of the seven cadavers. The proper esophageal artery branches from the descending aorta at the level of the 6th-9th thoracic vertebrae then descends and finally enters the dorsal surface of the esophagus[11]. Therefore, the origin of the artery should branch from the aorta at the Mt or Lt level, however, we observed few origins of the artery in the Lt region. The volume of intervening tissue between the esophagus and aorta plays a major role in the course of the proper esophageal artery and aortic invasion by the cancer.
| Cadaver No. | Cranial side (μm) | Caudal side (μm) |
| 1 | 289.7 | 1002.7 |
| 2 | 895.2 | 2337.9 |
| 3 | 928.6 | 3427.8 |
| 4 | 1573.6 | 3441.6 |
| 7 | 927.3 | 2688.9 |
| Average (μm) | 742.4 | 2070.2 |
D2-40-stained slides showed lymphatic connections between the lateral and ventral parts of the intermediate area. In addition, lymphatic connections were observed between the paraesophageal and intermediate areas on the lateral and ventral sides of the esophagus. Brotons et al[12] reported that the pulmonary ligament LNs collect lymph flow from the posterior mediastinum and drain it upward to the subcarinal nodes, that is, the LNs of the respiratory system[12]. Our study results are consistent with this report. In contrast, on the dorsal side of the esophagus, we found no lymphatic connection between the three areas bounded by fascia A and B: The paraesophageal, intermediate, and paraaortic areas. Although D2-40 staining was performed in only three cadavers, our data suggest that lymphatic vessel connections may be more infrequent on the dorsal side than on the ventral side. Our findings, although based on a small number of samples, are the same as those reported by Brotons et al[12].
This study has several limitations. Since our study was limited to the lower mediastinum, we did not examine the continuity of membrane or layer structures within the upper and middle mediastinum. We performed an anatomical study of non-cancerous tissue, and in patients with cancer, lymphatic proliferation may occur, which may alter the microanatomy. Furthermore, we did not study the direction of lymphatic flow. However, this study clarified the microstructure and LN boundaries based on two fasciae in the lower mediastinum, which may provide useful landmarks for performing surgery and for pathological diagnosis. These findings may also provide the basis for clinical studies to examine the frequency of LN metastasis and the effect of resection in EGJ adenocarcinoma and thoracic esophageal cancer patients.
We identified two fasciae surrounding the esophagus in the lower mediastinum and identified a layer structure separated by these fasciae. We used these fasciae to suggest a classification system for lower mediastinal tissues around the esophagus. These results could provide useful landmarks for the surgical treatment of EGJ adenocarcinoma.
Cases of the esophagogastric junction (EGJ) adenocarcinoma are increasing in number worldwide; however, there is no consensus on the surgical treatment for EGJ adenocarcinoma, especially Siewert II cases. In Japan, the transhiatal approach is widely performed for Siewert type II cases, and the right thoracic approach is widely performed for Siewert type I cases.
Because procedures for EJG adenocarcinoma are often performed with a magnified view, the microanatomy of the lower mediastinum is extremely important for surgeons. However, there is no consensus regarding the fascial and layer structures of the lower mediastinum. Furthermore, the boundaries of the mediastinal lymph nodes are unclear.
We examined the microanatomy, especially the fascial and layer structures, of the lower mediastinum and the boundaries of periesophageal tissue in the lower mediastinum in the present histological study of seven cadavers.
The esophagus and surrounding organs were resected at the level of the lower thoracic esophagus and embedded in paraffin, and serial 5-μm sections were made. We performed hematoxylin-eosin staining on all cadavers and D2-40 staining on three cadavers.
We identified two fasciae around the esophagus, and we classified the lower mediastinal tissue into three areas based on these two fasciae. The tissue on the dorsal side of the esophagus was thicker on the caudal side than on the cranial side. D2-40 staining revealed lymphatic connections between the paraesophageal tissue and the external area in the lateral and ventral regions of the esophagus; however, there were no lymphatic connections between areas in the dorsal region of the esophagus.
This histological study revealed two fasciae surrounding the lower thoracic esophagus and the layer structures separated by these fasciae. These findings will help to establish a new classification system for the lower mediastinal tissues.
These results can provide useful landmarks for treatment procedures in patients with EJG adenocarcinoma. Our research findings will also support further clinical studies, such as those focusing on the therapeutic value of mediastinal lymph node dissection.
The authors thank all the donors for this anatomical study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gajanan G, India; Senchukova M, Russia; Wang D, China S-Editor: Li L L-Editor: A P-Editor: Ma YJ
| 1. | Mariette C, Piessen G, Briez N, Gronnier C, Triboulet JP. Oesophagogastric junction adenocarcinoma: which therapeutic approach? Lancet Oncol. 2011;12:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Kurokawa Y, Hiki N, Yoshikawa T, Kishi K, Ito Y, Ohi M, Wada N, Takiguchi S, Mine S, Hasegawa S, Matsuda T, Takeuchi H. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery. 2015;157:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 912] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 704] [Article Influence: 88.0] [Reference Citation Analysis (1)] |
| 5. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus. 2017;14:37-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 6. | Tokairin Y, Nagai K, Kawamura Y, Nakajima Y, Kawada K, Hoshino A, Okada T, Muro S, Akita K, Kinugasa Y. Histological study of the thin membranous dense connective tissue around the middle and lower thoracic esophagus, caudal to the bifurcation of the trachea. Gen Thorac Cardiovasc Surg. 2021;69:983-992. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Meyer P, Sublon R. [Considerations on the interpleural ligament (De Morosow)]. Arch Anat Pathol (Paris). 1961;9:111-115. [PubMed] |
| 8. | Riddell AM, Davies DC, Allum WH, Wotherspoon AC, Richardson C, Brown G. High-resolution MRI in evaluation of the surgical anatomy of the esophagus and posterior mediastinum. AJR Am J Roentgenol. 2007;188:W37-W43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Tustumi F, Kimura CM, Takeda FR, Sallum RA, Ribeiro-Junior U, Cecconello I. Evaluation of lymphatic spread, visceral metastasis and tumoral local invasion in esophageal carcinomas. Arq Bras Cir Dig. 2016;29:215-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Tsujimoto H, Ichikura T, Aiko S, Yaguchi Y, Kumano I, Takahata R, Matsumoto Y, Yoshida K, Ono S, Yamamoto J, Hase K. Multidetector-computed tomography attenuation values between the tumor and aortic wall in response to induction therapy for esophageal cancer and its predictive value for aortic invasion. Exp Ther Med. 2012;3:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Kato T, Takase K, Ichikawa H, Satomi S, Takahashi S. Demonstration of the anatomy of the esophageal artery using multidetector-row helical computed tomography. J Comput Assist Tomogr. 2010;34:939-944. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Brotons ML, Bolca C, Fréchette E, Deslauriers J. Anatomy and physiology of the thoracic lymphatic system. Thorac Surg Clin. 2012;22:139-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |