Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1247
Peer-review started: February 17, 2023
First decision: February 28, 2023
Revised: March 3, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: June 27, 2023
Processing time: 118 Days and 10.3 Hours
Chemotherapy followed by gastrojejunostomy remains the main treatment for unresectable gastric cancer (GC) in the middle- or lower-third regions with gastric outlet obstruction (GOO). Radical surgery is performed as part of a multimodal treatment strategy for selected patients who respond well to chemotherapy. This study describes a case of successful radical resection with completely laparoscopic subtotal gastrectomy after a modified stomach-partitioning gastrojejunostomy (SPGJ) for obstruction relief, in a patient with GOO.
During the initial esophagogastroduodenoscopy, an advanced growth was detected in the lower part of the stomach, which caused an obstruction in the pyloric ring. Following this, a computed tomography (CT) scan revealed the presence of lymph node metastases and tumor invasion in the duodenum, but no evidence of distant metastasis was found. Consequently, we performed a modified SPGJ, a complete laparoscopic SPGJ combined with No. 4sb lymph node dissection, for obstruction relief. Seven courses of adjuvant capecitabine plus oxaliplatin combined with Toripalimab (programmed death ligand-1 inhibitor) were administered thereafter. A preoperative CT showed partial response; therefore, completely laparoscopic radical subtotal gastrectomy with D2 lympha
Laparoscopic SPGJ combined with No. 4sb lymph node dissection was an effective surgical technique for initially unresectable GC with GOO.
Core Tip: Radical resection, which may improve long-term survival, is often challenging in unresectable gastric cancer (GC) with gastric outlet obstruction (GOO) due to the management of complete gastrointestinal anastomoses and abdominal adhesions caused by gastrojejunostomy. We present a successful case of radical resection after laparoscopic stomach-partitioning gastrojejunostomy (SPGJ) combined with No. 4sb lymph node dissection, followed by conversion therapy; pathological complete remission was achieved. This case suggests that laparoscopic SPGJ combined with No. 4sb lymph node dissection is an option for initially unresectable GC with GOO.
- Citation: Shao XX, Xu Q, Wang BZ, Tian YT. Modified stomach-partitioning gastrojejunostomy for initially unresectable advanced gastric cancer with outlet obstruction: A case report. World J Gastrointest Surg 2023; 15(6): 1247-1255
- URL: https://www.wjgnet.com/1948-9366/full/v15/i6/1247.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i6.1247
Gastric cancer (GC) is one of the most common malignancies globally. In 2020, there were 768793 GC-related deaths, accounting for approximately 7.7% of total cancer deaths among 185 countries[1]. As GC is rarely detected at an early stage, the morbidity and mortality rates are high, with 5-year survival rates ranging from 20% to 40% in most countries[2,3]. Curative resection is essential for long-term survival. For patients with unresectable or metastatic GC, the median survival time is 4.3 mo with best supportive care[4], which can be extended to 10.5-11.6 mo with chemotherapy[5]. For unresectable GC in the middle- or lower-third with gastric outlet obstruction (GOO), chemotherapy followed by gastrojejunostomy remains the main therapeutic approach. Treatment modalities of adjuvant combination therapy, include combined targeted drugs or hyperthermic intraperitoneal perfusion chemotherapy[6-8]. For gastric adenocarcinoma, nivolumab plus chemotherapy demonstrated superior overall survival vs chemotherapy alone at the 12-mo follow-up in the randomized, global CheckMate 649 phase 3 trial[9]. The development of agents for GC has encouraged us to perform radical surgery for patients with initially unresectable GC who were converted to resectable status following their response to chemotherapy plus programmed death (PD)-1 inhibitor.
However, radical surgery is often challenging for patients who have undergone gastrojejunostomy. We report a successful case of radical resection utilizing completely laparoscopic subtotal gastrectomy with D2 lymph node dissection after completely laparoscopic stomach-partitioning gastrojejunostomy (SPGJ) combined with No. 4sb lymph node dissection, followed by adjuvant capecitabine plus oxaliplatin (CAPOX) combined with Toripalimab (PD-1 inhibitor), in a patient with GOO.
A 58-year-old woman presented with abdominal distension and vomiting.
The patient had previously attended a local hospital complaining of abdominal distension and vomiting. During the esophagogastroduodenoscopy (EGD), it was observed that there was an advanced growth located in the lower part of the stomach, causing a narrowing that made it difficult for the scope to pass through. Pathological examination of the biopsy confirmed the diagnosis of cancer. Afterwards, she was admitted to our hospital where she underwent a comprehensive medical examination and treatment.
The patient had been successfully treated for right breast cancer with surgery and adjuvant chemotherapy 17 years earlier.
There was no record of smoking or alcohol consumption in the patient’s medical history, and there was no pertinent family medical history.
Mild tenderness was noted in the upper abdomen.
No positive findings were obtained from standard laboratory examinations (including routine blood, and liver function, renal function, and tumor markers).
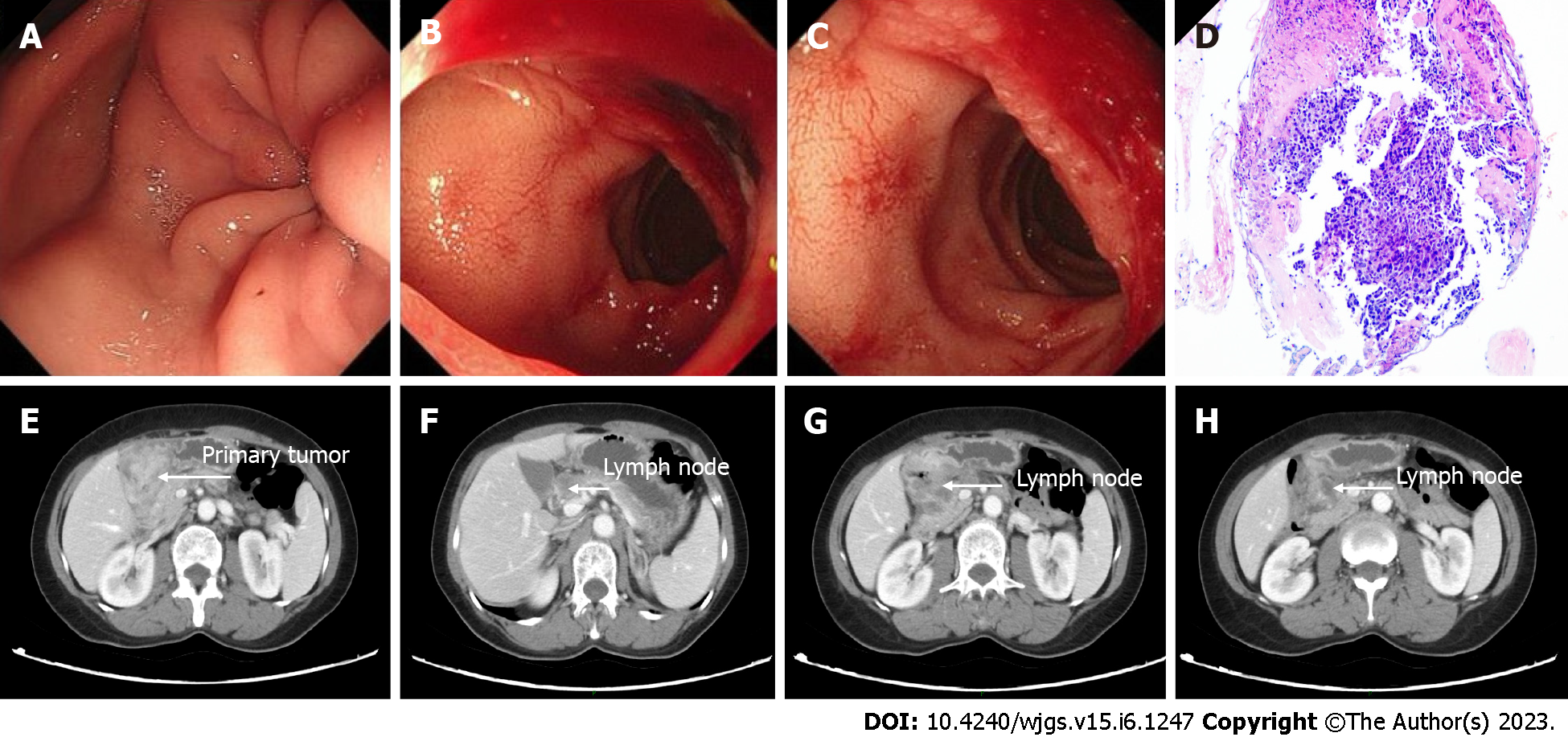
EGD detected an advanced lesion in the lower part of the stomach. The lesion was obstructing the pyloric ring and invading the duodenum (Figure 1A-C). Histological examination of the biopsies led to a diagnosis of poorly differentiated adenocarcinoma (Figure 1D). Computed tomography (CT) showed lymph node metastases and tumor invasion into the duodenum (Figure 1E-H) without distant metastases. Pathological examination revealed human epidermal growth factor receptor 2 negative, Ki-67 (+, 90%), and pMMR based on immunohistochemistry.
The final diagnosis was GC cT4aN2M0 c Stage III, according to the American Joint Committee on Cancer TNM Staging Classification for Carcinoma of the Stomach (8th ed, 2017).
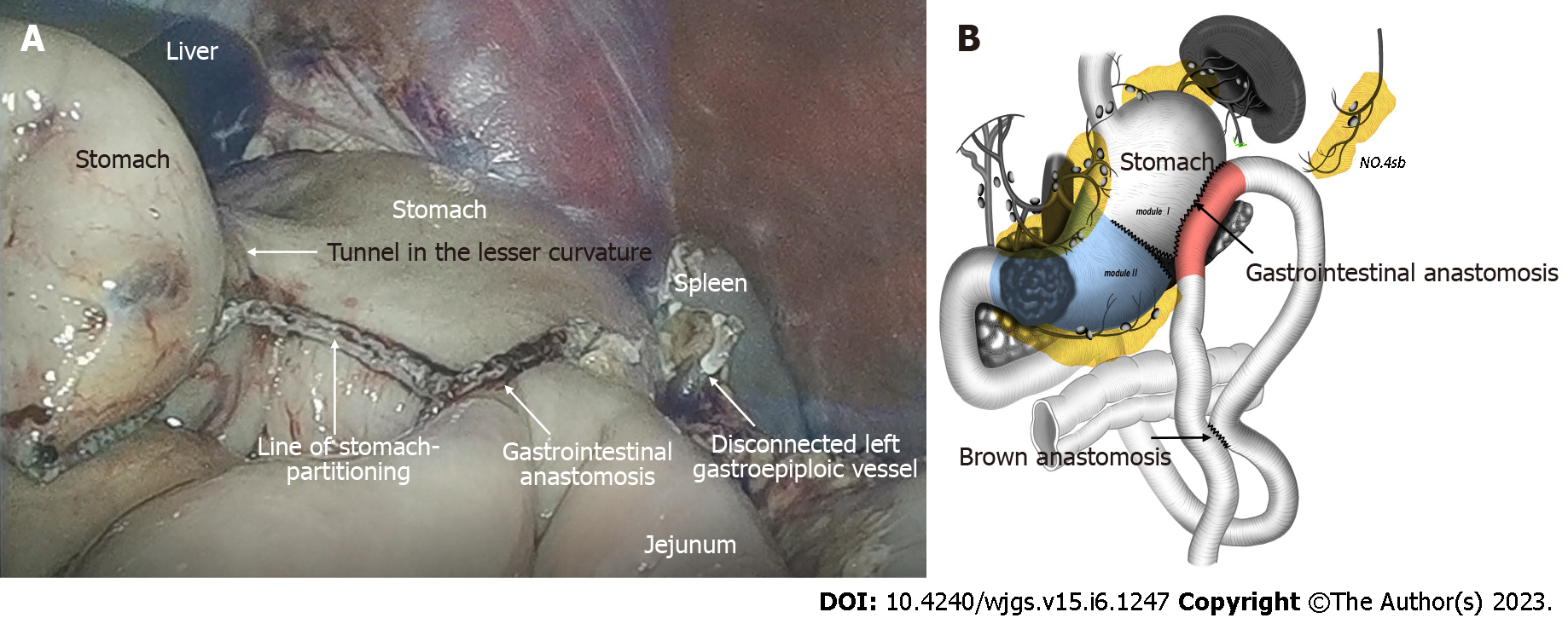
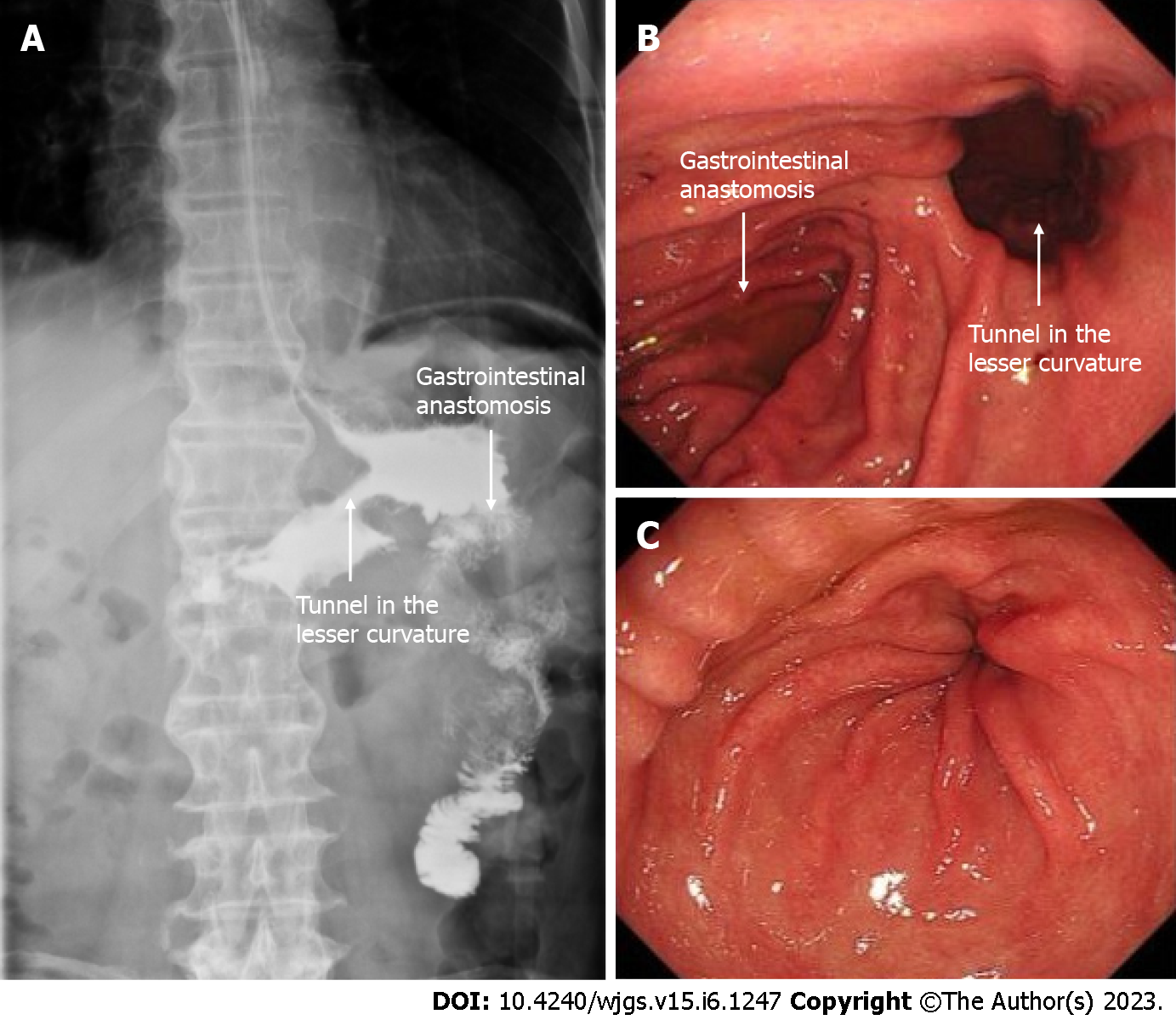
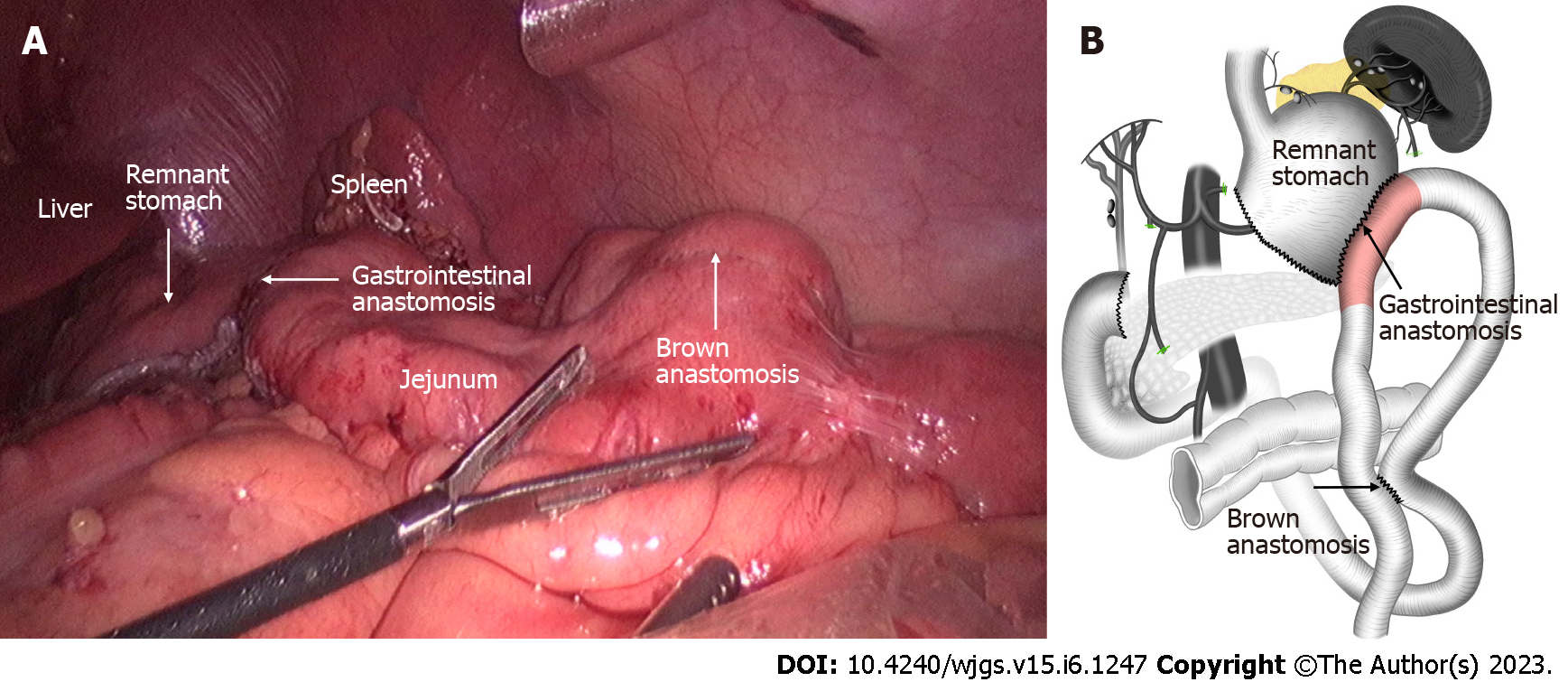
The staging laparoscopy indicated the absence of liver metastases or spreading, and the lavage cytology test results were negative. It was also confirmed that the lower part of the stomach body was immobile due to cancerous invasion into the duodenum. After the left gastroepiploic vessels were severed at their origin near the splenic tail, No. 4sb lymph nodes were dissected. The stomach was transected with a 60 mm linear stapler, from the greater curvature, extending 2 cm from the lesser curvature, at least 5 cm proximal to the tumor or site of obstruction. Thereafter, a small opening was made in the jejunum at the antimesenteric border, 25 cm from the ligament of Treitz. A second opening was made in the stapling line on the greater curvature side of the gastric stump. Next, gastrojejunostomy was performed using a laparoscopic linear stapler. Using the same equipment, the proximal and distal jejunum were anastomosed to form a jejunojejunostomy, 60 mm in length. The anastomotic stoma was established 15 cm from the ligament of Treitz and 25 cm from the gastrojejunostomy anastomosis (Figure 2). The surgery lasted for 1 h and 10 min, and the total amount of blood loss was 20 mL. No intra- and post-operative complications were observed. Post-operative upper digestive tract radiography indicated a good passage to the jejunum and drainage route in the lesser curvature (Figure 3A). The time to oral feeding was 3 d, and the post-operative hospital stay was 6 d.
Twenty days after laparoscopic SPGJ, seven courses of CAPOX combined with Toripalimab (capecitabine 1500 mg/d on days 1-14, oxaliplatin 100 mg/m2 on day 1, and Toripalimab 240 mg/d on day 1, Q3W) were performed. With this regimen, only grade 2 elevations in leukopenia were observed, and no serious adverse events of grade 3 or greater according to the Common Terminology Criteria for Adverse Events version 5.0 were detected.
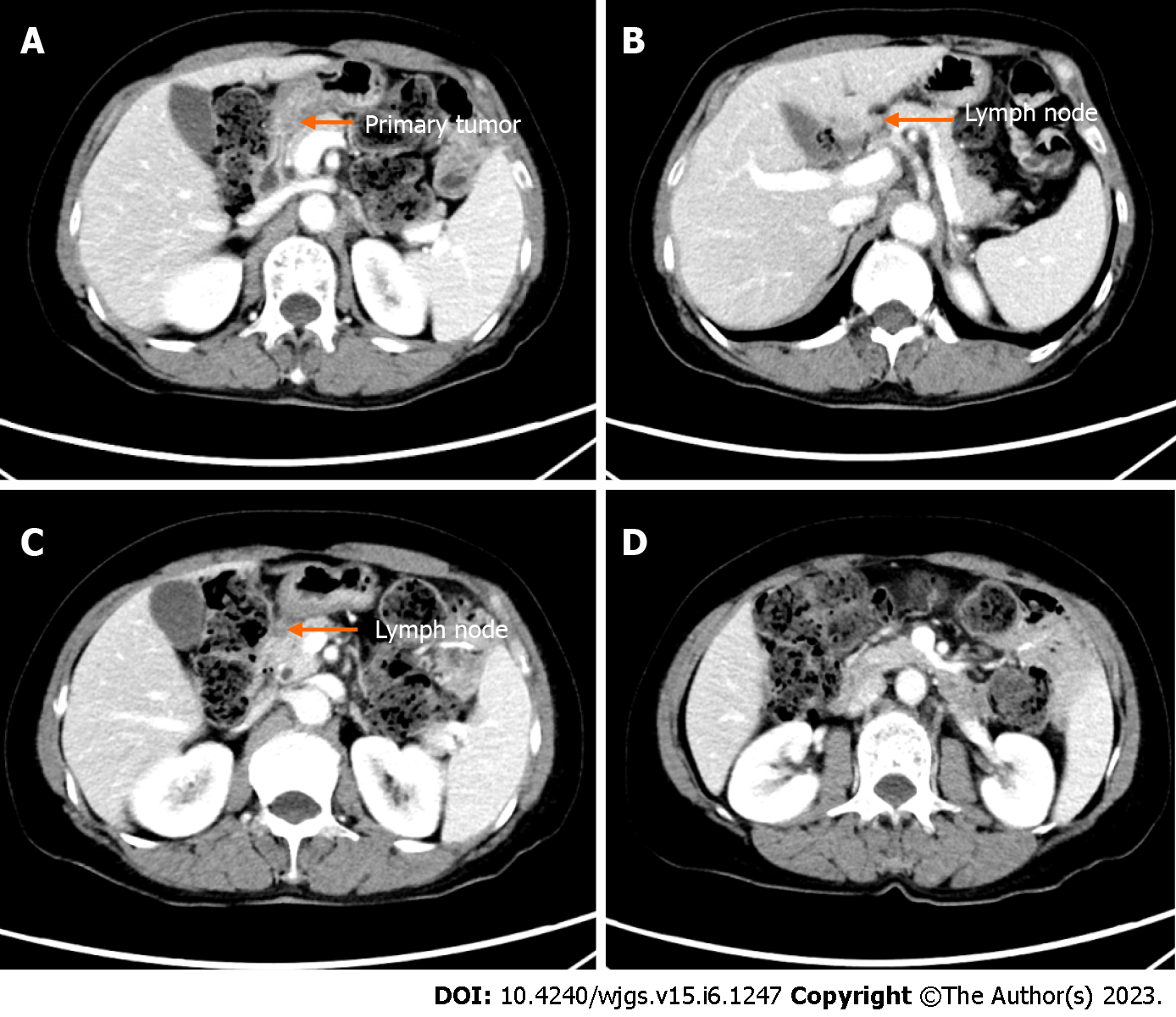
After conversion therapy, EGD showed a good drainage route in the lesser curvature and detected shrinkage of the primary tumor, allowing the endoscope to pass (Figure 3B and C). CT showed significant reduction in size of primary tumor and metastatic lymph nodes (Figure 4A-D). Clinical response was defined as a partial response on radiological examination according to the Response Evaluation Criteria in Solid Tumors criteria version 1.0. The preoperative diagnosis was ycT3N0M0 yc Stage IIB.
Following dissection of the adhesions between the omentum and the abdominal wall, it was confirmed that there were no liver metastases or spreading. Rapid lavage cytology results showed the absence of cancer cells in the ascites. Infra-pyloric dissection was performed by ligating the right gastro-epiploic vessels. The duodenum was divided at 2 cm below the pyloric ring using a 60 mm linear stapler. Negative margins were confirmed by intraoperative frozen pathology. The right gastric artery was ligated from its origin in the right hepatic artery. The peritoneum over the porta hepatis and the lesser omentum were excised. The supra-pancreatic dissection continued with the removal of No. 8a and No. 12a lymph nodes. After ligation of the right gastric vein, the pancreatic capsule was dissected from the root of the left gastric artery to the proximal splenic artery. The left gastric artery was then transected. The proximal splenic artery was skeletonized to dissect the No. 11 lymph nodes. The No. 3 and No. 1 lymph nodes were then dissected along the lesser curvature of the stomach. After D2 lymphadenectomy, the stomach was divided along the SPGJ line using a 60 mm linear stapler. The anastomoses created in previous operations were used throughout (Figure 5). The total operation time was 1 h and 55 min and the total blood loss was 50 mL. No intra- and post-operative complications were observed. The time to oral feeding was 1 d, and the post-operative hospital stay was 5 d.
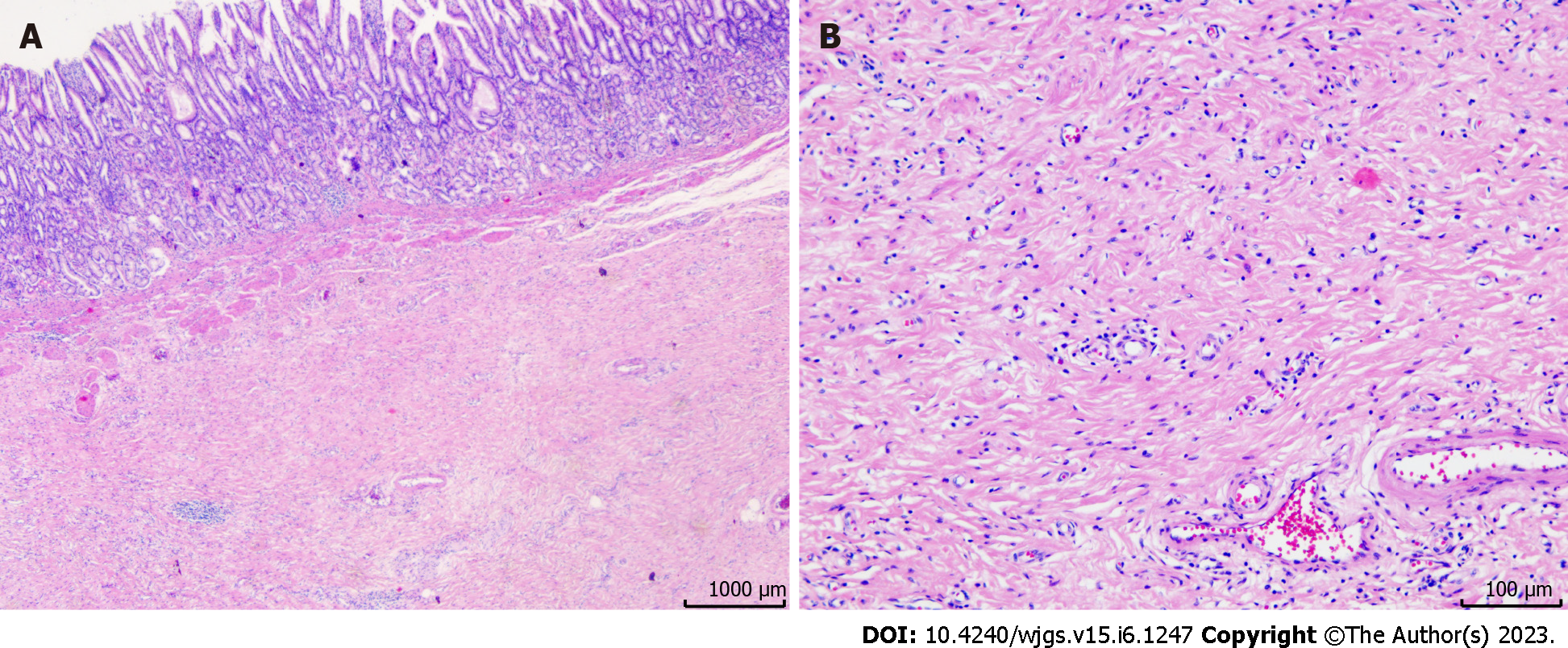
Pathological complete remission was achieved (Figure 6). Post-operative therapy was not performed due to the good response to the seven courses of preoperative therapy. No recurrences were observed 17 mo post-operatively. Table 1 shows the timeline from the onset of symptoms to the completion of treatment.
| Time series | Symptoms and treatment details |
| 9 mo ago | She presented with symptoms of abdominal distension and vomiting and visited a hospital |
| A gastric tube was inserted to drain food residue from the stomach | |
| 8 mo ago | Staging laparoscopy confirmed no liver metastasis, no dissemination, and negative lavage cytological results |
| Laparoscopic stomach-partitioning gastrojejunostomy and Braun anastomosis combined with No. 4sb lymph node dissection were performed | |
| 1-7 mo ago | CAPOX + Toripalimab 1-7 |
| The date of radical surgery | Completely laparoscopic subtotal gastrectomy with D2 lymph node dissection was done |
| 11 mo after surgery | CT shows no recurrence |
We present the successful case of a radical resection performed with a completely laparoscopic subtotal gastrectomy with D2 lymph node dissection after laparoscopic SPGJ combined with No. 4sb lymph node dissection, followed by CAPOX combined with Toripalimab for advanced GC with GOO.
Chemotherapy is one of the main treatments for advanced GC. GOO is a common condition among patients suffering from unresectable distal gastric tumors, which present with nausea and vomiting, and can cause dehydration, weight loss, and impaired chemotherapy tolerance for patients, hastening their demise. Traditionally, duodenal stent or gastrojejunostomy have been recommended as first-line treatment options for unresectable GC with GOO. For patients with good performance status and a favorable prognosis, the latter option is regarded as the preferred primary treatment[10].
Yamaguchi et al[11] reported long-term survivors in patients who underwent conversion surgery for stage IV GC. Therefore, radical surgery is performed during the treatment as part of a multimodal treatment strategy for selected patients who respond well to chemotherapy[11]. Many clinical trials have been performed for immunotherapy as a first-line treatment for advanced GC/gastroesophageal junction carcinoma and revealed encouraging results. The objective response rate was 60% and 67% in the cohort 2 study of KEYNOTE059 and ATTRACTION-04, respectively, when immune checkpoint inhibitors combined with chemotherapy were used as first-line therapy[12-14]. The development of agents for GC has encouraged us to perform radical surgery.
However, for patients who undergo gastrojejunostomy, radical surgery is often challenging for the following reasons: First, abdominal adhesions are often severe due to previous operations, especially laparotomy; this can cause significant interference in locating normal tissue spaces, dissecting lymph nodes, excising tumors, and anastomosing; Second, if the original anastomosis requires removal, the difficulty and risk of the operation greatly increases. Therefore, sufficient negative margins should be ensured. Moreover, No. 4sb lymph node dissection, especially in cases where the original anastomosis is retained, enables the short gastric vessels to be easily injured, which may cause gastric ischemia and spleen laceration, resulting in bleeding.
To solve these problems, we modified the operation based on the SPGJ[15] and named it the modular two-stage laparoscopic gastrectomy (MTLG). In MTLG, the gastrectomy and gastrointestinal anastomoses are divided into two modules and two stages. Module I was located on the upper side of the SPGJ line. Stage I surgery was performed for total laparoscopic SPGJ and Braun anastomosis combined with No. 4sb lymph node dissection. Module II was located inferiorly, and stage II surgery was offered for total laparoscopic subtotal gastrectomy with D2 lymphadenectomy (Figures 2B and 5B).
As a result, we observed the following advantages of MTLG: First, the incidence of post-operative complications was low, and operations could be completed using total laparoscopy to ensure less invasiveness, rapid post-operative recovery, and minimal abdominal adhesions. Second, SPGJ utilization: (1) Reduced the risk of bleeding from lesions caused by food stimulation; (2) Prevented the tumor from spreading to the gastrojejunal anastomosis; (3) Improved gastric emptying while maintaining endoscopic access to the region distal to the bypass; and (4) Obviated duodenal stump leaks. Third, it was conducive for SPGJ after No. 4sb lymph node dissection. Furthermore, patients only required subtotal gastrectomy with D2 lymphadenectomy in stage II surgery after conversion therapy to avoid anastomotic stomas, short gastric blood vessels, and splenic injuries caused by No. 4sb lymph node dissection. The anastomosis completed in the first stage continued to be used, and the two modules did not interfere with each other. Fourth, adding the Braun anastomosis to the gastrojejunostomy reconstruction could reduce the incidence of reflux gastritis. Fifth, even in the case of unsuccessful conversion, patients were able to eat soon after, and decreased post-operative morbidity, improved quality of life, and better prognosis after SPGJ were achieved[16].
Laparoscopic SPGJ combined with No. 4sb lymph node dissection is safe and effective for GOO. This procedure followed by chemotherapy and immunotherapy may be an effective treatment strategy before radical surgery.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hirasawa T, Japan; Wakabayashi H, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Ma YJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TÅ, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, De P, McClure C, Ramanakumar AV, Stuart-Panko H, Engholm G, Walsh PM, Jackson C, Vernon S, Morgan E, Gavin A, Morrison DS, Huws DW, Porter G, Butler J, Bryant H, Currow DC, Hiom S, Parkin DM, Sasieni P, Lambert PC, Møller B, Soerjomataram I, Bray F. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 727] [Article Influence: 121.2] [Reference Citation Analysis (0)] |
| 3. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3408] [Article Influence: 486.9] [Reference Citation Analysis (1)] |
| 4. | Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 420] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 5. | Shitara K. Chemotherapy for advanced gastric cancer: future perspective in Japan. Gastric Cancer. 2017;20:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Beeharry MK, Ni ZT, Yang ZY, Zheng YN, Feng RH, Liu WT, Yan C, Yao XX, Li C, Yan M, Zhu ZG. Study protocol of a multicenter phase III randomized controlled trial investigating the efficiency of the combination of neoadjuvant chemotherapy (NAC) and neoadjuvant laparoscopic intraperitoneal hyperthermic chemotherapy (NLHIPEC) followed by R0 gastrectomy with intraoperative HIPEC for advanced gastric cancer (AGC): dragon II trial. BMC Cancer. 2020;20:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Zheng Y, Yang X, Yan C, Feng R, Sah BK, Yang Z, Zhu Z, Liu W, Xu W, Ni Z, Beeharry MK, Hua Z, Yan M, Li C. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: A single-arm, open-label, phase II trial. Eur J Cancer. 2020;130:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, Harbeck N, Valero V, Stroyakovskiy D, Wildiers H, Campone M, Boileau JF, Beckmann MW, Afenjar K, Fresco R, Helms HJ, Xu J, Lin YG, Sparano J, Slamon D. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 9. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1877] [Article Influence: 469.3] [Reference Citation Analysis (1)] |
| 10. | Jang S, Stevens T, Lopez R, Bhatt A, Vargo JJ. Superiority of Gastrojejunostomy Over Endoscopic Stenting for Palliation of Malignant Gastric Outlet Obstruction. Clin Gastroenterol Hepatol. 2019;17:1295-1302.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, Tanabe K, Ohdan H. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21:315-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1459] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 13. | Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, Cho H, Kang WK, Komatsu Y, Tsuda M, Yamaguchi K, Hara H, Fumita S, Azuma M, Chen LT, Kang YK. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019;30:250-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 14. | Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, Borg C, Doi T, Yoon HH, Savage MJ, Wang J, Dalal RP, Shah S, Wainberg ZA, Chung HC. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22:828-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 15. | Kaminishi M, Yamaguchi H, Shimizu N, Nomura S, Yoshikawa A, Hashimoto M, Sakai S, Oohara T. Stomach-partitioning gastrojejunostomy for unresectable gastric carcinoma. Arch Surg. 1997;132:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Huang B, Sunde B, Tsekrekos A, Hayami M, Rouvelas I, Nilsson M, Lindblad M, Klevebro F. Partial stomach-partitioning gastrojejunostomy for gastric outlet obstruction: A cohort study based on consecutive case series from a single center. Asian J Surg. 2022;45:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |