Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1224
Peer-review started: December 25, 2022
First decision: February 28, 2023
Revised: March 10, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: June 27, 2023
Processing time: 172 Days and 2.9 Hours
Primary sclerosing cholangitis (PSC) is an extraintestinal manifestation of ulcerative colitis (UC). PSC is a well-known risk factor for intrahepatic cholangiocarcinoma (ICC), and ICC is known to have a poor prognosis.
We present two cases of ICC in patients with PSC associated with UC. In the first case, a tumor was found by magnetic resonance imaging (MRI) in the liver of a patient with PSC and UC who presented to our hospital with right-sided rib pain. The second patient was asymptomatic, but we unexpectedly detected two liver tumors in an MRI performed to evaluate bile duct stenosis associated with PSC. ICC was strongly suspected by computed tomography and MRI in both cases, and surgery was performed, but unfortunately, the first patient died of ICC recurrence 16 mo postoperatively, and the second patient died of liver failure 14 mo postoperatively.
Careful follow-up of patients with UC and PSC with imaging and blood tests is necessary for early detection of ICC.
Core Tip: Intrahepatic cholangiocarcinoma (ICC) commonly develops on top of primary sclerosing cholangitis (PSC) associated with ulcerative colitis (UC). Both of our patients died, although they were asymptomatic or mildly symptomatic at the time the ICC was discovered. Patients with long-term PSC coexisting with UC require regular follow-up with imaging such as magnetic resonance imaging even if they are asymptomatic.
- Citation: Miyazu T, Ishida N, Asai Y, Tamura S, Tani S, Yamade M, Iwaizumi M, Hamaya Y, Osawa S, Baba S, Sugimoto K. Intrahepatic cholangiocarcinoma in patients with primary sclerosing cholangitis and ulcerative colitis: Two case reports. World J Gastrointest Surg 2023; 15(6): 1224-1231
- URL: https://www.wjgnet.com/1948-9366/full/v15/i6/1224.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i6.1224
In the treatment of ulcerative colitis (UC), attention should be paid not only to intestinal lesions but also to extraintestinal complications, especially primary sclerosing cholangitis (PSC)[1]. The incidence of UC associated with PSC varies widely; it is reported to be 23% in Japan and 80% in Sweden[2]. In addition, UC associated with PSC often causes mild symptoms, and many cases show a good treatment response[3]. PSC is a chronic liver disease that causes cholestasis due to diffuse and multiple sites of inflammation and narrowing of the intrahepatic and extrahepatic bile duct[4]. Gastrointestinal cancer is reported to be complicated in patients with PSC, and cancer of the bile duct such as intrahepatic cholangiocarcinoma (ICC) in particular, is a poor prognostic factor for PSC[5]. ICC is known to have a poor prognosis, and it was reported that patients with multiple lymph node metastases did not survive more than 2 years after surgery[6].
We managed two cases of ICC resulting from PSC associated with long-standing UC. In both cases, the ICC was surgically resected, but the patients died relatively shortly after the operation. ICC, which develops in cases of long-term UC associated with PSC, is often asymptomatic early in its onset.
Case 1: A 34-year-old male patient with PSC presented to our hospital with right lower abdominal pain.
Case 2: A 47-year-old male patient presented to our hospital for follow-up of PSC.
Case 1: The patient’s symptoms started 2 mo prior.
Case 2: The patient was followed up regularly with abdominal ultrasound examination and magnetic resonance imaging (MRI). A liver mass was noted on a routine MRI and had increased in 4 mo, so additional close examination was performed and cancer was strongly suspected.
Case 1: He was diagnosed with UC (right-side significant pancolitis type) and PSC at the age of 20, and developed interstitial pneumonia caused by 5-aminosalicylate (5-ASA) one year later. His condition was maintained on steroids and 6-mercaptopurine. At the age of 32, the patient complained of right lower abdominal pain.
Case 2: At the age of 19, he was diagnosed with UC (pancolitis type) and PSC, and was administered oral 5-ASA to maintain mucosal healing. At the age of 31, he developed pancreatitis, which was diagnosed as UC-related autoimmune pancreatitis based on the diffuse parenchymal enlargement giving a sausage-like appearance on abdominal contrast enhanced (CE)-computed tomography (CT) examination. For the treatment of pancreatitis, he was given steroids, which were gradually reduced; however, they could not be stopped due to the appearance of signs of liver failure caused by the progression of PSC.
Case 1: The patient’s appetite was normal, and he had no weight loss. There was no increase in stool frequency, and jaundice was not observed.
Case 2: The patient had no subjective symptoms, no loss of appetite, and no weight loss. There was no increase in stool frequency and no jaundice.
Case 1: Laboratory results showed elevated alanine aminotransferase (ALT), alkaline phosphatase (ALP), and γ-glutamyl transferase (γ-GT), but no change from previous data. Bilirubin levels were also normal, but carbohydrate antigen 19-9 (CA19-9) was abnormally high at 1121 U/mL.
Case 2: Laboratory results showed elevations in aspartate aminotransferase, ALT, ALP, γ-GT, and elevated bilirubin with an indirect predominance, but no change from previous data. CA19-9 was below detection sensitivity.
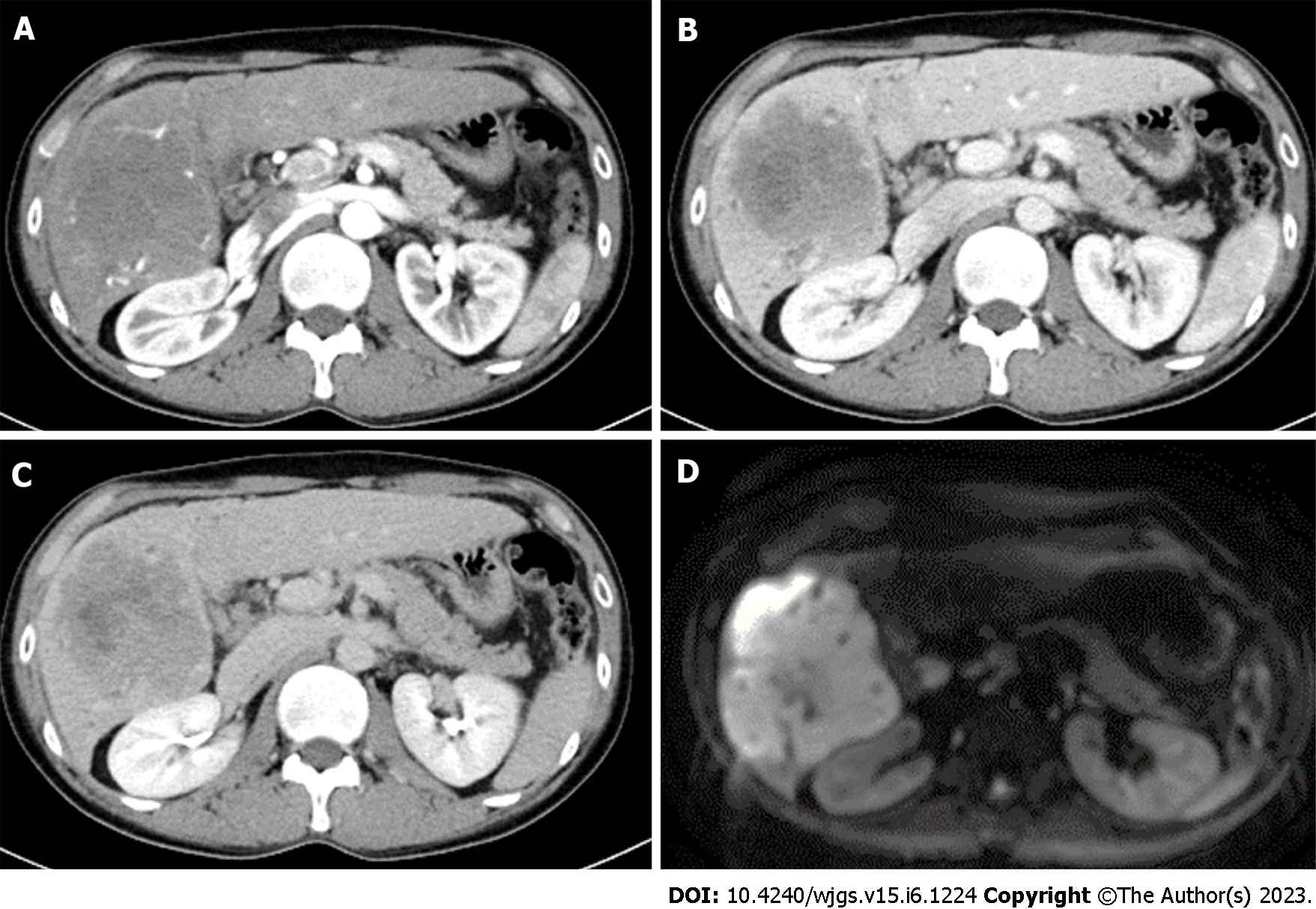
Case 1: Abdominal CE-MRI/CT examination revealed a huge mass (10 cm in size) in the right lobe of the liver, raising the suspicion of ICC (Figure 1).
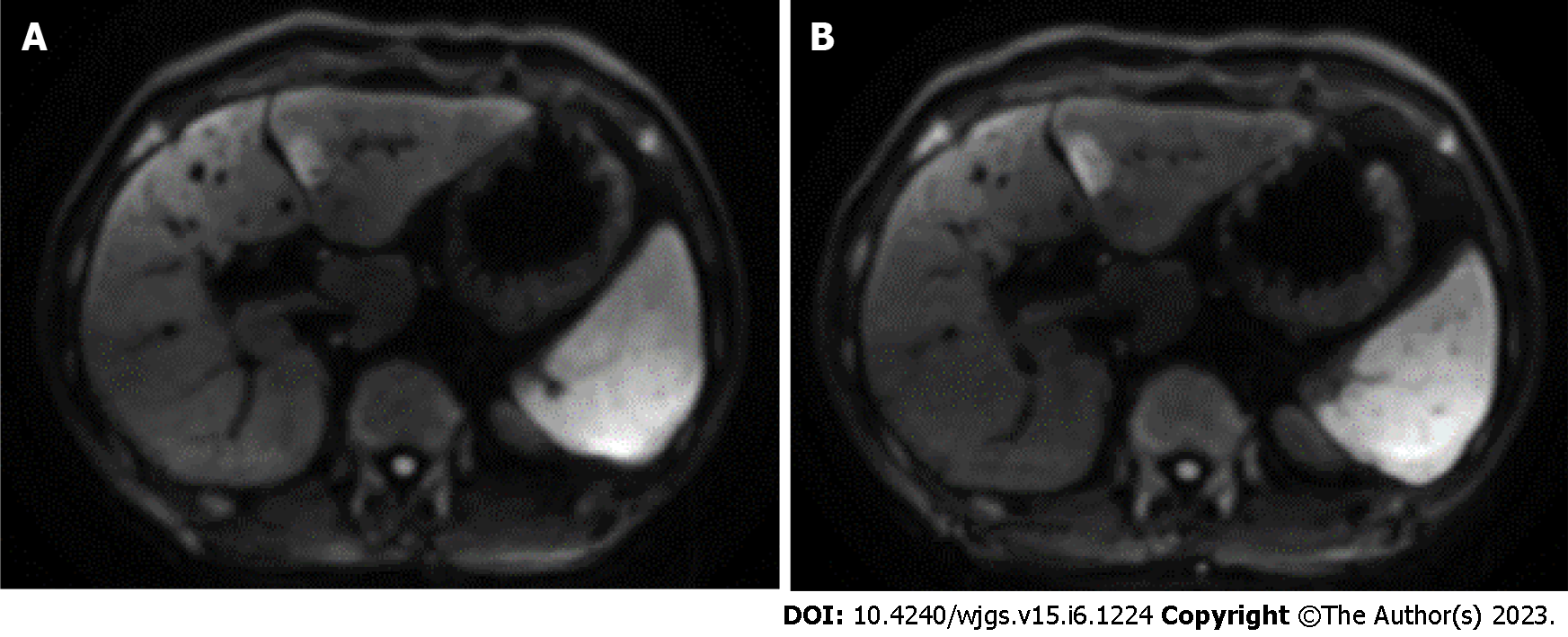
Case 2: At the age of 45, an abdominal CE-MRI examination was performed for PSC follow-up and showed a nodular lesion in the left lobe of the liver (Figure 2A). Reexamination 4 mo later showed that the lesion had increased in size (Figure 2B). Abdominal CE-CT examination was performed and ICC was strongly suspected.
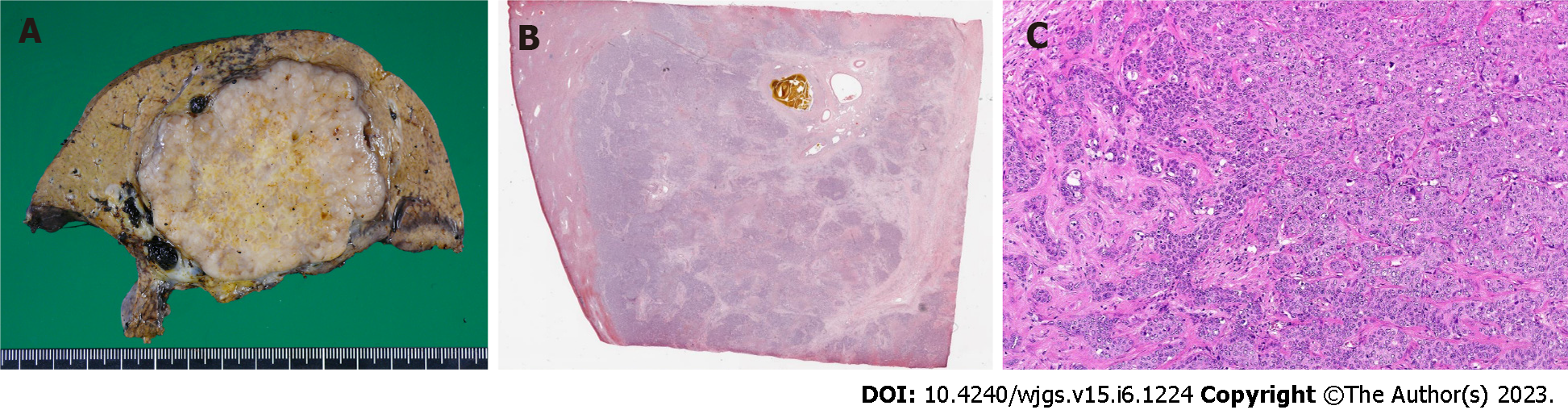
ICC (low to moderately differentiated adenocarcinoma, pT3N1M0 Stage IVB) was pathologically confirmed (Figure 3).
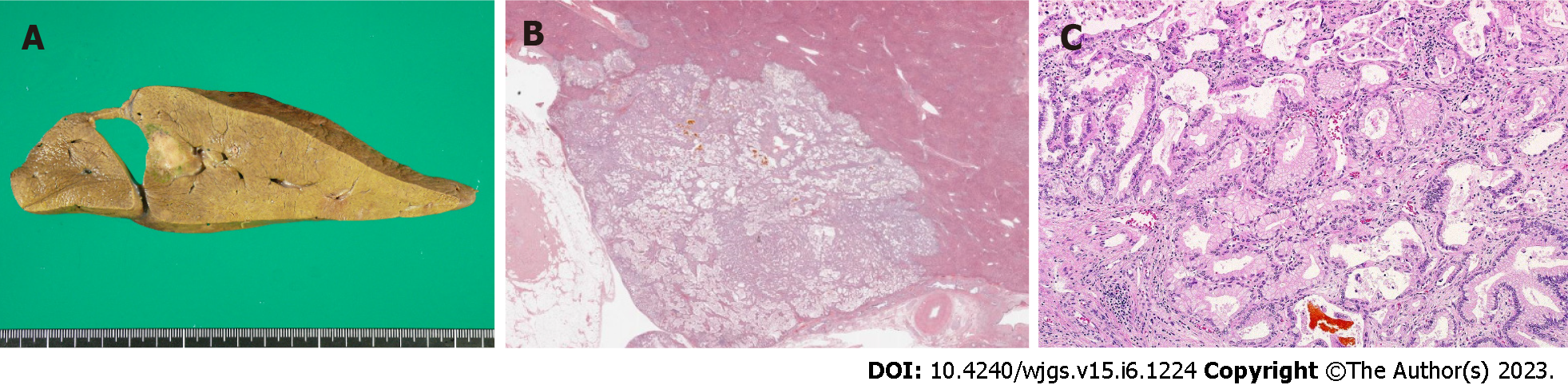
ICC was pathologically confirmed (highly to moderately differentiated adenocarcinoma, pT3N1M0 Stage IVB) (Figure 4). Immunohistochemical examination of tumor cells was performed. The tumor cells showed CK7 (+), Ck19 (+), MUC1 (partly+), CD10 (-), HepPar-1 (-), alpha-fetoprotein (-), Arginase-1 (-), Glypican-3 (-), CD117 (-), and CD56 (-).
Right hepatic lobectomy was performed, and the patient received S-1 therapy (120 mg) as postoperative adjuvant chemotherapy, but discontinued it in the middle of the second course due to liver damage.
Left hepatic lobectomy was performed, and the patient was administered Inchinkato (Chinese herbal medicine), ursodeoxycholic acid, and phenobarbital for postoperative jaundice, but the total bilirubin value did not fall below 10 mg/dL. As a result, postoperative adjuvant chemotherapy was not administered.
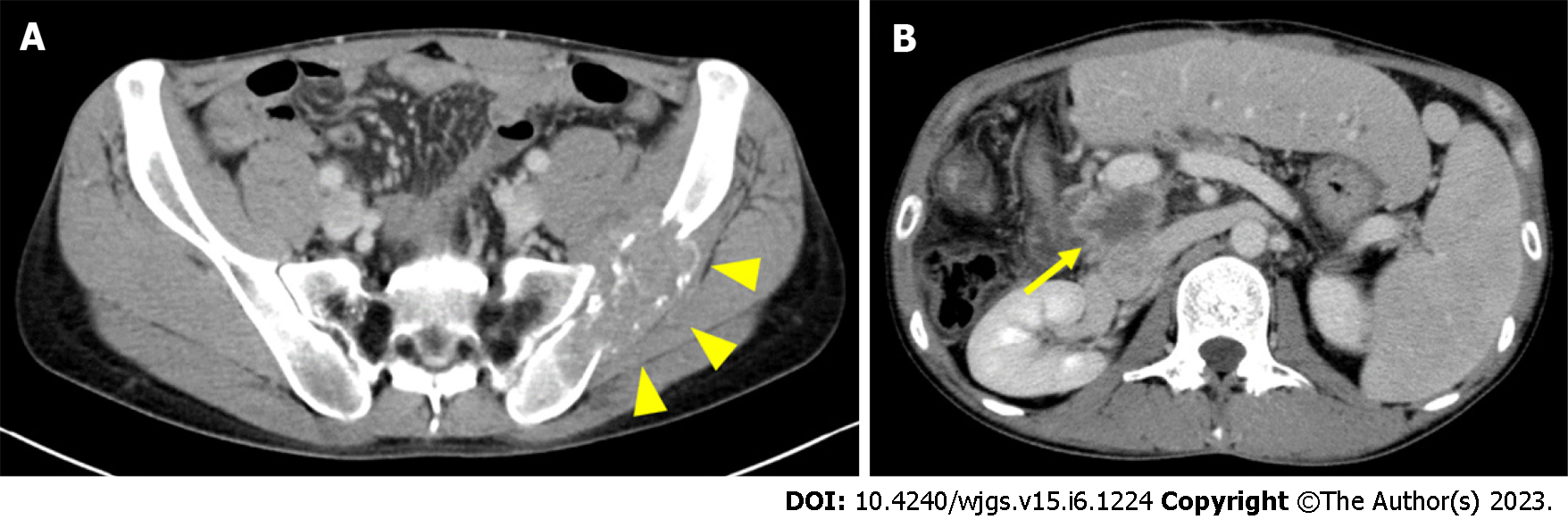
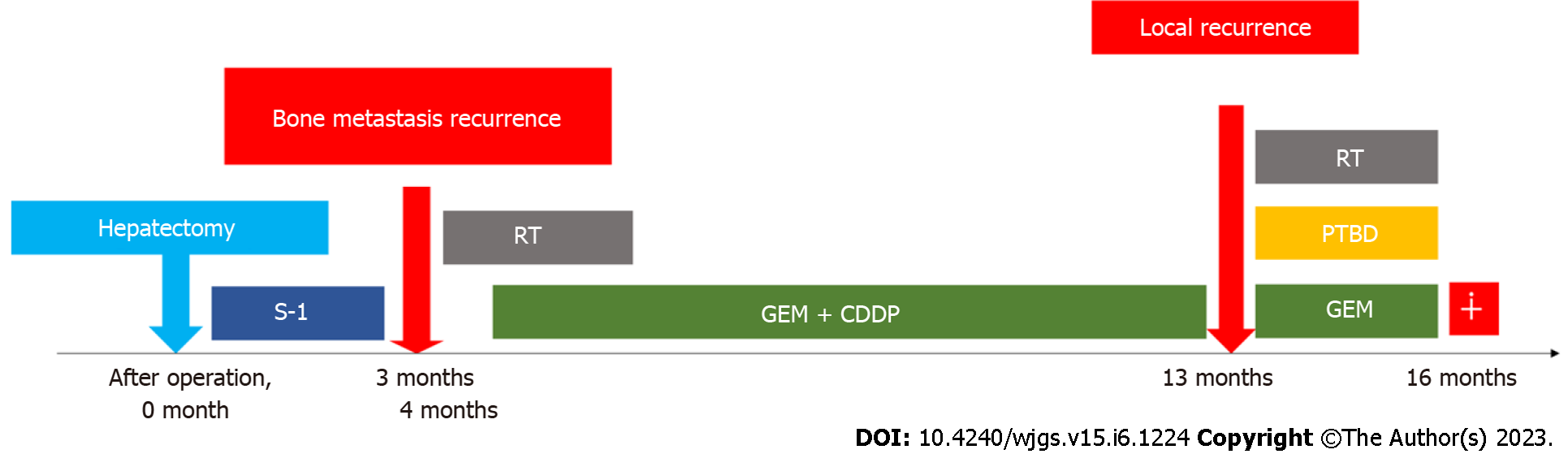
Abdominal CE-CT examination performed 3 mo postoperatively showed recurrence of bone metastasis in the left ilium (Figure 5A). Gemcitabine and cisplatin (GC) therapy (gemcitabine 1400 mg/cisplatin 35 mg; both 80% of their dose) was started 4 mo postoperatively with irradiation of the same site, and partial remission was sustained for over 10 mo. However, local recurrence was suspected on the dorsal side of the portal vein by abdominal CE-CT examination performed 13 mo postoperatively (at the end of 11 courses of GC therapy) (Figure 5B). The patient developed obstructive jaundice due to the appearance of recurrent lesions, and percutaneous transhepatic biliary drainage was performed. Irradiation and gemcitabine monotherapy were started after waiting for the improvement of jaundice, but systemic weakness progressed due to biliary tract infection. It became difficult to continue chemotherapy due to the deterioration of performance status, and the patient received palliative care. He died 16 mo postoperatively. The progress is shown in Figure 6.
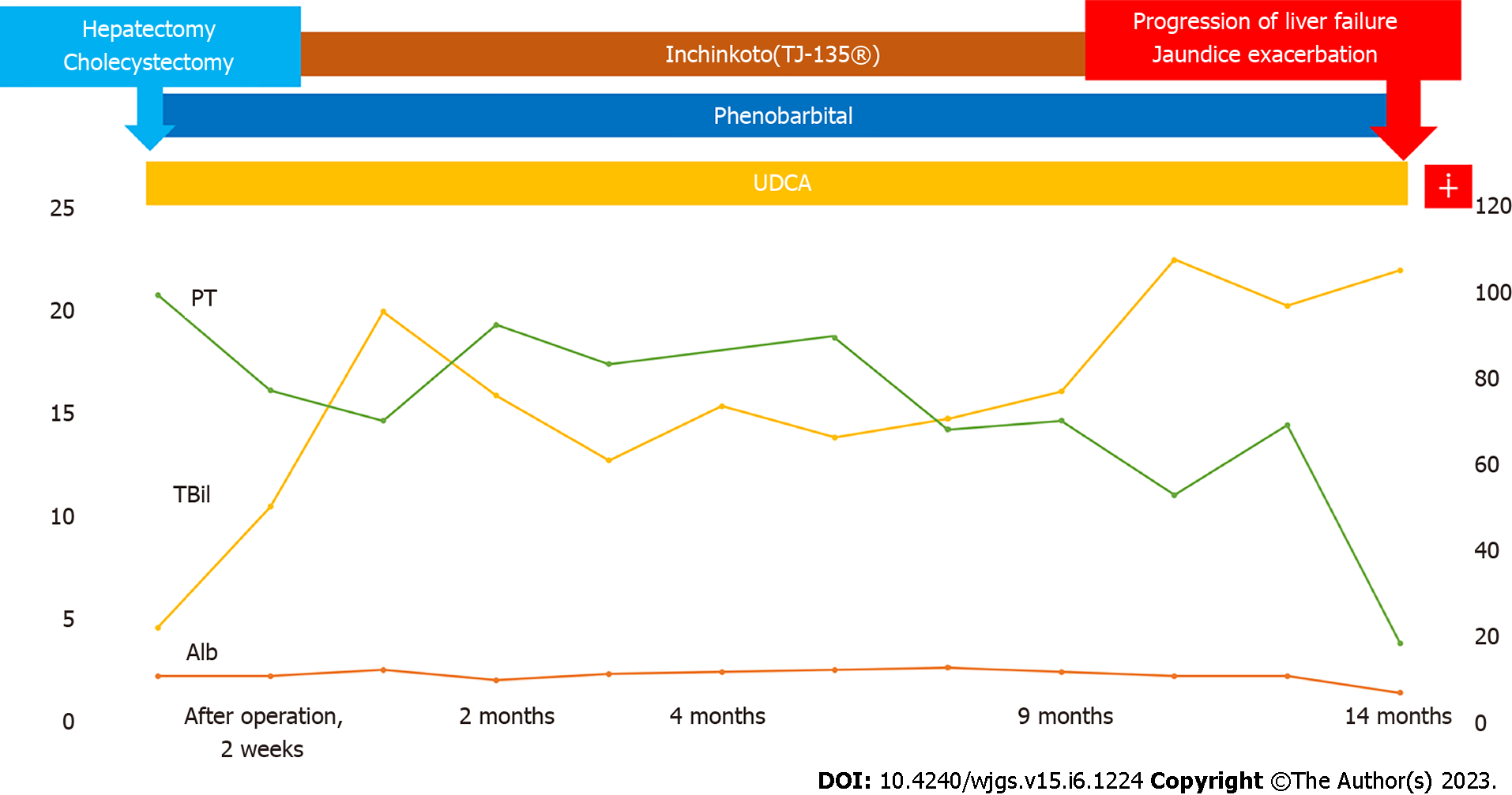
Jaundice with a total serum bilirubin level of around 20 mg/dL persisted postoperatively, and although oral treatment for jaundice was continued, jaundice persisted and liver failure progressed. Hepatic transplantation was also considered, but it was not indicated because the patient was in a cancer-bearing state. He was followed up while receiving symptomatic treatment for liver failure and jaundice, but died 14 mo postoperatively. The progress is shown in Figure 7.
PSC is characterized by the inflammation and destruction of intrahepatic and extrahepatic bile ducts, leading to progressive hepatic fibrosis, and its etiology remains unknown[7]. Inflammatory bowel diseases (IBD) such as Crohn's disease (CD) and UC are frequently complicated (66%-80% of cases), commonly by PSC[8,9]. Moreover, it is reported that 83% of IBDs associated with PSC are UC and 5% are CD[10]. However, the PSC complication rate in IBD patients is not high, and it has been reported that the complication rate in UC is 2%–7.5%[11,12]. The incidence of ICC in PSC cases is 8%–20%[13-15], and approximately 10% of cases with ICC have been reported to be related to PSC[16]. We found 94 case reports in the English literature since 2000 by using PubMed with the keywords: Ulcerative colitis, cholangiocarcinoma, and primary sclerosing cholangitis.
There have been several reports on the relationship between the prevalence of PSC and the prevalence of ICC. Case-control studies reported from Sweden[17] and the United States[18] did not show a significant difference between the prevalence and incidence of ICC. A cohort study of the population of PSC patients was conducted on the association between IBD and ICC, but many of the reports indicated that there was no association[19,20]. However, in a case-control study conducted by Welzel et al[21], both UC and CD were reported as risk factors for ICC. Nevertheless, they did not consider the coexistence of PSC with ICC in these IBD patients, and the association between IBD and ICC remained unclear. In our report, case 1 was diagnosed with ICC 12 years after the onset of UC, and case 2 was diagnosed with ICC 27 years after the onset of UC. The average duration of UC of these patients was 20.5 years, which is a relatively long period of time. Therefore, in patients with coexisting UC and PSC, long-term screening of the liver and bile duct by imaging tests such as MRI and CT is necessary.
Early detection of ICC is difficult, and the accuracy rates of ultrasonography, CT, MRI, and other diagnostic imaging methods in cases without mass formation are as low as 48%, 38%, and 40%, respectively. The correct diagnosis rate of ICC by endoscopic retrograde cholangiopancreatography and magnetic resonance cholangiopancreatography was 23% and 21%, respectively[5,22]. Nevertheless, examination by both tumor marker CA19-9 and diagnostic imaging are useful for the diagnosis of ICC[5]. At the time of diagnosis, CA19-9 was high in our first case, but was below the threshold for detection in our second case. Therefore, it is considered necessary to make a comprehensive diagnosis without relying solely on the value of tumor markers.
The 5-year survival rate for ICC is 39% for mass-forming lesions and 69% for intra-biliary lesions[23]. However, other reports state that no patients with tumor-forming lesions or peribiliary infiltrative lesions survived for more than 5 years[6,24]. Both our cases had mass-forming lesions and peribiliary infiltration, with a poor prognosis and an average survival duration of 15 mo from surgery to death.
As mentioned above, it is not uncommon for ICC to develop in cases of PSC with long-term follow-up. However, one of the patients presented here was largely asymptomatic, yet experienced a significant increase in tumor size at the time of discovery and ultimately died despite surgery and chemotherapy. Meanwhile, another patient had a relatively small lesion that could have been surgically removed; however, the patient died due to liver dysfunction which developed thereafter. With an unstable UC course, clinicians may be distracted by its progression and neglect to monitor the course of PSC. We have learned a valuable lesson – when CCC develops in patients with both IBD and PSC, the prognosis may be considerably worse. In both of our cases, there were tumor-forming lesions and periductal infiltration and the prognosis was poor, with an average survival time of 15 mo from surgery to death. Especially in the first case, the tumor was discovered when it was 10 cm in diameter, although the patient was largely asymptomatic until right lower abdominal pain began, suggesting that this tumor may grow relatively rapidly and almost asymptomatically.
Therefore, it is critical to perform regular imaging screening, such as MRI, during follow-up of patients with coexisting IBD and PSC, even if the patient is asymptomatic. This may aid ICC detection before periductal infiltration occurs.
We reported two patients with early-onset UC and PSC who developed ICC. Both cases followed an unfortunate course. Thus, in order to prevent similar scenarios from recurring, we should always consider ICC during the follow-up of patients with IBD and PSC and perform regular abdominal imaging examinations for its early detection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fabbri N, Italy; Zharikov YO, Russia S-Editor: Li L L-Editor: A P-Editor: Wu RR
| 1. | Wang R, Leong RW. Primary sclerosing cholangitis as an independent risk factor for colorectal cancer in the context of inflammatory bowel disease: a review of the literature. World J Gastroenterol. 2014;20:8783-8789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Broomé U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Sano H, Nakazawa T, Ando T, Hayashi K, Naitoh I, Okumura F, Miyabe K, Yoshida M, Takahashi S, Ohara H, Joh T. Clinical characteristics of inflammatory bowel disease associated with primary sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2011;18:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Nakanuma Y, Harada K, Katayanagi K, Tsuneyama K, Sasaki M. Definition and pathology of primary sclerosing cholangitis. J Hepatobiliary Pancreat Surg. 1999;6:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 6. | Suzuki S, Sakaguchi T, Yokoi Y, Okamoto K, Kurachi K, Tsuchiya Y, Okumura T, Konno H, Baba S, Nakamura S. Clinicopathological prognostic factors and impact of surgical treatment of mass-forming intrahepatic cholangiocarcinoma. World J Surg. 2002;26:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Mendes FD, Lindor KD. Primary sclerosing cholangitis. Clin Liver Dis. 2004;8:195-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, Rajaram R, Rauws EA, Mulder CJ, Reitsma JB, Heisterkamp SH, Tytgat GN. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, Loftus EV Jr, Yawn BP, Dickson ER, Melton LJ 3rd. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Okada H, Mizuno M, Yamamoto K, Tsuji T. Primary sclerosing cholangitis in Japanese patients: association with inflammatory bowel disease. Acta Med Okayama. 1996;50:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Loftus EV Jr, Sandborn WJ, Lindor KD, Larusso NF. Interactions between chronic liver disease and inflammatory bowel disease. Inflamm Bowel Dis. 1997;3:288-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Olsson R, Danielsson A, Järnerot G, Lindström E, Lööf L, Rolny P, Rydén BO, Tysk C, Wallerstedt S. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991;100:1319-1323. [PubMed] [DOI] [Full Text] |
| 13. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 544] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Kornfeld D, Ekbom A, Ihre T. Survival and risk of cholangiocarcinoma in patients with primary sclerosing cholangitis. A population-based study. Scand J Gastroenterol. 1997;32:1042-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Rosen CB, Nagorney DM, Wiesner RH, Coffey RJ Jr, LaRusso NF. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991;213:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 231] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Card TR, Solaymani-Dodaran M, West J. Incidence and mortality of primary sclerosing cholangitis in the UK: a population-based cohort study. J Hepatol. 2008;48:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Bergquist A, Glaumann H, Persson B, Broomé U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998;27:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 178] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, McCashland TM, Reddy KR, Zervos X, Anbari MA, Hoen H. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 208] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Boberg KM, Bergquist A, Mitchell S, Pares A, Rosina F, Broomé U, Chapman R, Fausa O, Egeland T, Rocca G, Schrumpf E. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 207] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 21. | Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 22. | Boberg KM, Lind GE. Primary sclerosing cholangitis and malignancy. Best Pract Res Clin Gastroenterol. 2011;25:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Yamamoto M, Takasaki K, Yoshikawa T, Ueno K, Nakano M. Does gross appearance indicate prognosis in intrahepatic cholangiocarcinoma? J Surg Oncol. 1998;69:162-167. [PubMed] [DOI] [Full Text] |
| 24. | Jiang BG, Sun LL, Yu WL, Tang ZH, Zong M, Zhang YJ. Retrospective analysis of histopathologic prognostic factors after hepatectomy for intrahepatic cholangiocarcinoma. Cancer J. 2009;15:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |