Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1178
Peer-review started: March 13, 2023
First decision: April 13, 2023
Revised: April 13, 2023
Accepted: April 25, 2023
Article in press: April 25, 2023
Published online: June 27, 2023
Processing time: 94 Days and 8 Hours
Growing evidence shows that pancreatic tumors in different anatomical locations have different characteristics, which have a significant impact on prognosis. However, no study has reported the differences between pancreatic mucinous adenocarcinoma (PMAC) in the head vs the body/tail of the pancreas.
To investigate the differences in survival and clinicopathological characteristics between PMAC in the head and body/tail of pancreas.
A total of 2058 PMAC patients from the Surveillance, Epidemiology, and End Results database diagnosed between 1992 and 2017 were retrospectively reviewed. We divided the patients who met the inclusion criteria into pancreatic head group (PHG) and pancreatic body/tail group (PBTG). The relationship between two groups and risk of invasive factors was identified using logistic regression analysis. Kaplan-Meier analysis and Cox regression analysis were conducted to compare the overall survival (OS) and cancer-specific survival (CSS) of two patient groups.
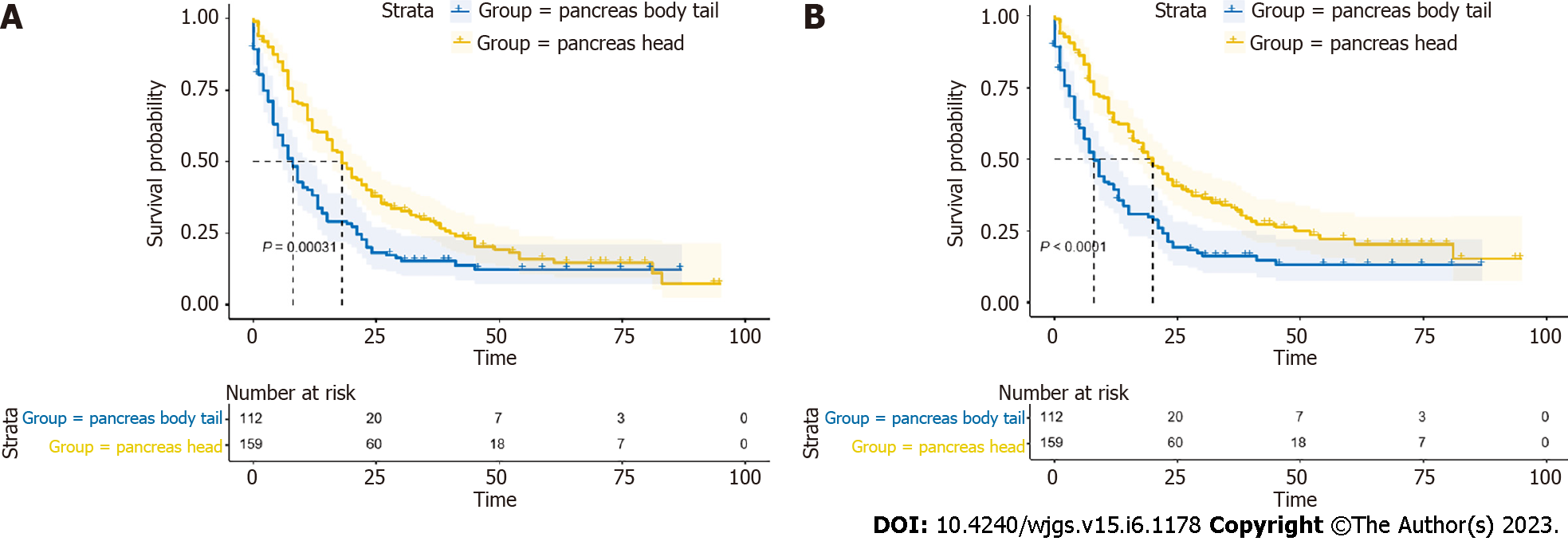
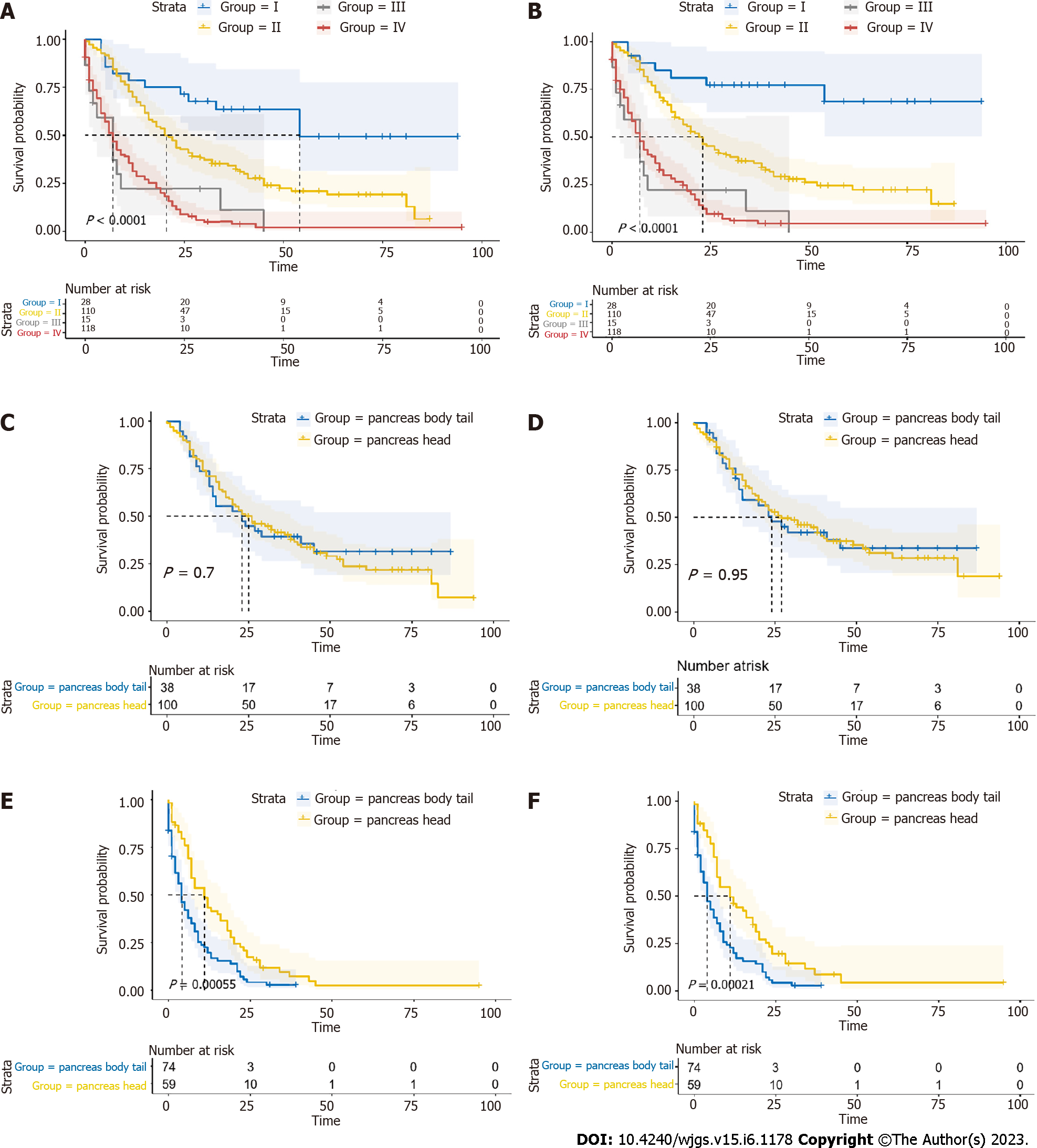
In total, 271 PMAC patients were included in the study. The 1-year, 3-year, and 5-year OS rates of these patients were 51.6%, 23.5%, and 13.6%, respectively. The 1-year, 3-year, and 5-year CSS rates were 53.2%, 26.2%, and 17.4%, respectively. The median OS of PHG patients was longer than that of PBTG patients (18 vs 7.5 mo, P < 0.001). Compared to PHG patients, PBTG patients had a greater risk of metastases [odds ratio (OR) = 2.747, 95% confidence interval (CI): 1.628-4.636, P < 0.001] and higher staging (OR = 3.204, 95% CI: 1.895-5.415, P < 0.001). Survival analysis revealed that age < 65 years, male sex, low grade (G1-G2), low stage, systemic therapy, and PMAC located at the pancreatic head led to longer OS and CSS (all P < 0.05). The location of PMAC was an independent prognostic factor for CSS [hazard ratio (HR) = 0.7, 95%CI: 0.52-0.94, P = 0.017]. Further analysis demonstrated that OS and CSS of PHG were significantly better than PBTG in advanced stage (stage III-IV).
Compared to the pancreatic body/tail, PMAC located in the pancreatic head has better survival and favorable clinicopathological characteristics.
Core Tip: Pancreatic tumors had different clinicopathological characteristics by anatomic location in the pancreas. We first investigated the different outcomes and characteristics between mucinous adenocarcinoma in the pancreatic head and body/tail using a variety of analytical methods. In conclusion, adenocarcinoma located at the pancreatic head tended to be characterized by longer survival and more favorable characteristics.
- Citation: Li Z, Zhang XJ, Sun CY, Li ZF, Fei H, Zhao DB. Dissimilar survival and clinicopathological characteristics of mucinous adenocarcinoma located in pancreatic head and body/tail. World J Gastrointest Surg 2023; 15(6): 1178-1190
- URL: https://www.wjgnet.com/1948-9366/full/v15/i6/1178.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i6.1178
Pancreatic cancer (PC) is a common malignancy with a poor prognosis. The incidence and mortality of PC have dramatically increased in recent decades. It has been estimated that PC will be the third leading cause of cancer-related mortality in the future[1,2]. In the subtype classification of PC, pancreatic mu
Recently, studies have suggested that there is diversity in the genetic and biological characteristics of pancreatic cancer depending on the localization of the tumor[5,6], which indicates that we can classify pancreatic cancer by anatomical location and develop targeted treatment strategies to achieve better outcomes. There is a burgeoning discussion on how the anatomical location of pancreatic cancer impacts its clinical outcomes and pathological characteristics, such as pancreatic ductal adenocarcinoma[7-10] and pancreatic neuroendocrine tumors[11]. However, no study has reported the differences in pancreatic mucinous adenocarcinoma (PMAC) in different pancreatic locations.
Given these considerations, we conducted the present study to compare the survival and clinicopathological features of PMAC in the head vs. the body/tail of the pancreas. A total of 271 PMAC patients from the Surveillance, Epidemiology, and End Results database (1992-2017) were reviewed.
Patients’ data in this population-based retrospective study were investigated from the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/), which is supported by National Cancer Institute. We screened the data “Incidence-SEER Research Plus Data, 13 Registries, Nov 2019 Sub (1992-2017)” using SEER*Stat 8.4.0.1. Furthermore, “8.6.4 Carcinoma of pancreas”, “8480/3: Mucinous adenocarcinoma”, and “Positive histology” were selected, and a total of 2058 pathologically confirmed patients with information of age, race, sex, grade, TNM, stage, primary malignancy, systemic therapy, and survival were collected. The exclusion criteria of this study were as follows: (1) Patients without TNM data (n = 1710); (2) Patients with incomplete information of cancer-specific survival (n = 2); (3) Patients with carcinoma located at ‘OthPancreas’ (n = 74); and (4) Patients with unknown race (n = 1). Then, we divided the eligible patients into pancreatic head group (PHG) and pancreatic body/tail group (PBTG) according to the location of PMAC. Additionally, we have to declare that the patients included in this study were not including those with cystic mucinous adenocarcinoma and intraductal papillary mucinous tumor, which could lead to a contaminated result.
Student’s t test, Mann–Whitney U test, chi-square test, and X2 test were properly utilized to compare the clinicopathological data and survival of the two groups of patients. Logistic regression analysis was applied to identify the relationship between tumor locations and pathological characteristics. The survival analyses were conducted using Kaplan-Meier analysis (log-rank test) and Cox regression analysis. Significance was considered as P < 0.05. All statistical analyses in the study were conducted using R software (version 4.2.0).
Finally, 271 patients met the inclusion criteria and were included in the study. According to the locations of tumor, these patients were divided into pancreatic head group (PHG) (n = 159) and PBTG (n = 112) (Table 1). In general, the median OS of 271 patients was 13 mo. Patients over 65 years old (61.3%) and white (74.5%) accounted the majority. Concerning the clinical characteristics, males in PHG were more than that in PBTG (P = 0.009), and the ratios of male to female of PHG and PBTG were 1.45 vs 0.67, while there was no significant difference of age and race between the two groups. Compared to PHG, PBTG patients were observed to have more metastatic tumors (P < 0.001) staged in advanced stage (P < 0.001). The differences in T, N, and primary malignancy of the two groups were not statistically significant. Moreover, patients in PHG were likely to have a longer OS than PBTG (median OS 18 vs 7.5 mo, P < 0.001).
| PBTG (n = 112) | PHG (n = 159) | Overall (n = 271) | P value | |
| Age, yr | ||||
| < 65 | 39 (34.8) | 66 (41.5) | 105 (38.7) | 0.538 |
| ≥ 65 | 73 (65.2) | 93 (58.5) | 166 (61.3) | |
| Race | ||||
| Black | 15 (13.4) | 17 (10.7) | 32 (11.8) | 0.443 |
| Other | 20 (17.9) | 17 (10.7) | 37 (13.7) | |
| White | 77 (68.8) | 125 (78.6) | 202 (74.5) | |
| Sex | ||||
| Female | 67 (59.8) | 65 (40.9) | 132 (48.7) | 0.009 |
| Male | 45 (40.2) | 94 (59.1) | 139 (51.3) | |
| Grade | ||||
| G1 + G2 | 35 (31.3) | 70 (44.0) | 105 (38.7) | 0.041 |
| G3 + G4 | 11 (9.8) | 26 (16.4) | 37 (13.7) | |
| Unknown | 66 (58.9) | 63 (39.6) | 129 (47.6) | |
| Stage | ||||
| I | 10 (8.9) | 18 (11.3) | 28 (10.3) | < 0.001 |
| II | 28 (25.0) | 82 (51.6) | 110 (40.6) | |
| III | 9 (8.0) | 6 (3.8) | 15 (5.5) | |
| IV | 65 (58.0) | 53 (33.3) | 118 (43.5) | |
| T | ||||
| T1 | 11 (9.8) | 15 (9.4) | 26 (9.6) | 0.209 |
| T2 | 26 (23.2) | 34 (21.4) | 60 (22.1) | |
| T3 | 49 (43.8) | 93 (58.5) | 142 (52.4) | |
| T4 | 26 (23.2) | 16 (10.1) | 42 (15.5) | |
| T0 | 0 (0) | 1 (0.6) | 1 (0.4) | |
| N | ||||
| N0 | 67 (59.8) | 83 (52.2) | 150 (55.4) | 0.462 |
| N1 | 45 (40.2) | 76 (47.8) | 121 (44.6) | |
| M | ||||
| M0 | 47 (42.0) | 106 (66.7) | 153 (56.5) | < 0.001 |
| M1 | 65 (58.0) | 53 (33.3) | 118 (43.5) | |
| Primary malignancy | ||||
| No | 23 (20.5) | 36 (22.6) | 59 (21.8) | 0.918 |
| Yes | 89 (79.5) | 123 (77.4) | 212 (78.2) | |
| OS, mo | ||||
| mean (SD) | 14.6 (18.5) | 24.1 (21.2) | 20.2 (20.6) | < 0.001 |
| Median [Min, Max] | 7.50 [0, 87.0] | 18.0 [0, 95.0] | 13.0 [0, 95.0] | |
By comparing the basic characteristics of the two groups, we identified that locations of the tumor were related to the metastasis and higher staging. After eliminating confounding factors, we included sex, age, race, location, and primary malignancy into the logistic regression models (Figure 1). It was shown that patients in PBTG have higher risk of metastasis [OR = 2.747, 95% confidence interval (CI): 1.628-4.636, P < 0.001] and high staging (III-IV) (OR=3.204, 95%CI: 1.895-5.415, P < 0.001) compared with PHG. Additionally, there was a higher risk of metastasis in patients over 65 years old (OR = 1.877, 95%CI: 1.079-3.264, P=0.026) with PMAC as the primary malignancy (OR = 2.317, 95%CI: 1.196-4.488, P = 0.013).
The 1-year, 3-year, and 5-year OS rates of all patients were 51.6%, 23.5%, and 13.6%, respectively. While the 1-year, 3-year, and 5-year CSS rates were 53.2%, 26.2%, and 17.4%, respectively. Univariate and multivariate Cox regression models of OS and CSS were further constructed (Table 2; Table 3), and the results could be drawn that age, grade, stage, and systemic therapy were independent factors for predicting both OS and CSS of these patients (all P < 0.05). Besides, tumor located at pancreatic head was considered as a favorable independent prognostic factor for CSS (HR = 0.7, 95%CI: 0.52-0.94, P = 0.017). Then, we depicted survival curves of the two groups using Kaplan-Meier analysis, which suggested that patients in PHG had longer OS and CSS than those in PBTG (all P < 0.05) (Figure 2A and B). Nevertheless, it is known that cancers of the body and especially of the tail are diagnosed at a more advanced stage or even metastatic than cancers of the head, which manifest themselves by jaundice at an earlier stage, probably being one of the contributors of "better prognosis" of pancreatic head cancer. Additionally, the rate of R1 surgery will be higher in PHG during cephalic resections because of the closer vascular relationships. Given these, we made a selection of PMAC without surgical resection treatment and compared the long-term survival of PHG (n = 81) and PBTG (n = 80), which avoided the imbalance in surgery thoroughness (non-surgery, R0 and R1 resection) of the two groups. The Kaplan-Meier curves elucidated that the long-term outcomes of PHG without surgery were better than PBTG without surgery (all P < 0.05) (Figure 3A and B).
| Characteristics | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | ||||||
| < 65 | Reference | Reference | ||||
| ≥ 65 | 1.62 | 1.23-2.14 | 0.001 | 1.42 | 1.06-1.89 | 0.017 |
| Race | ||||||
| Black | Reference | |||||
| Other | 0.91 | 0.54-1.53 | 0.725 | |||
| White | 0.94 | 0.62-1.41 | 0.751 | |||
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 0.68 | 0.52-0.89 | 0.004 | 0.81 | 0.61-1.07 | 0.134 |
| Location | ||||||
| Pancreas body/tail | Reference | Reference | ||||
| Pancreas head | 0.61 | 0.47-0.8 | < 0.001 | 0.76 | 0.57-1.01 | 0.057 |
| Grade | ||||||
| G1 + G2 | Reference | Reference | ||||
| G3 + G4 | 1.82 | 1.21-2.73 | 0.004 | 2.17 | 1.43-3.31 | < 0.001 |
| Unknown | 2.21 | 1.64-2.97 | < 0.001 | 1.23 | 0.89-1.69 | 0.216 |
| Stage | ||||||
| I | Reference | Reference | ||||
| II | 2.39 | 1.3-4.37 | 0.005 | 3.2 | 1.73-5.92 | < 0.001 |
| III | 6.2 | 2.81-13.68 | < 0.001 | 6.5 | 2.89-14.61 | < 0.001 |
| IV | 6.73 | 3.67-12.37 | < 0.001 | 6.2 | 3.34-11.5 | < 0.001 |
| Systemic therapy | ||||||
| No | Reference | Reference | ||||
| Yes | 0.32 | 0.24-0.44 | < 0.001 | 0.39 | 0.27-0.56 | < 0.001 |
| Characteristics | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | ||||||
| < 65 | Reference | Reference | ||||
| ≥ 65 | 1.56 | 1.17-2.08 | 0.002 | 1.37 | 1.02-1.84 | 0.038 |
| Race | ||||||
| Black | Reference | |||||
| Other | 0.91 | 0.54-1.55 | 0.739 | |||
| White | 0.89 | 0.58-1.34 | 0.568 | |||
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 0.64 | 0.48-0.84 | 0.001 | 0.77 | 0.58-1.03 | 0.082 |
| Location | ||||||
| Pancreas body/tail | Reference | Reference | ||||
| Pancreas head | 0.56 | 0.43-0.74 | < 0.001 | 0.7 | 0.52-0.94 | 0.017 |
| Grade | ||||||
| G1 + G2 | Reference | Reference | ||||
| G3 + G4 | 1.75 | 1.14-2.67 | 0.01 | 2.2 | 1.42-3.4 | < 0.001 |
| Unknown | 2.12 | 1.56-2.88 | < 0.001 | 1.1 | 0.79-1.54 | 0.559 |
| Stage | ||||||
| I | Reference | Reference | ||||
| II | 3.7 | 1.71-8.03 | 0.001 | 5.02 | 2.29-11 | < 0.001 |
| III | 10.3 | 4.09-25.95 | < 0.001 | 10.75 | 4.19-27.61 | < 0.001 |
| IV | 10.47 | 4.83-22.73 | < 0.001 | 9.81 | 4.47-21.51 | < 0.001 |
| Systemic therapy | ||||||
| No | Reference | Reference | ||||
| Yes | 0.3 | 0.22-0.42 | < 0.001 | 0.35 | 0.24-0.51 | < 0.001 |
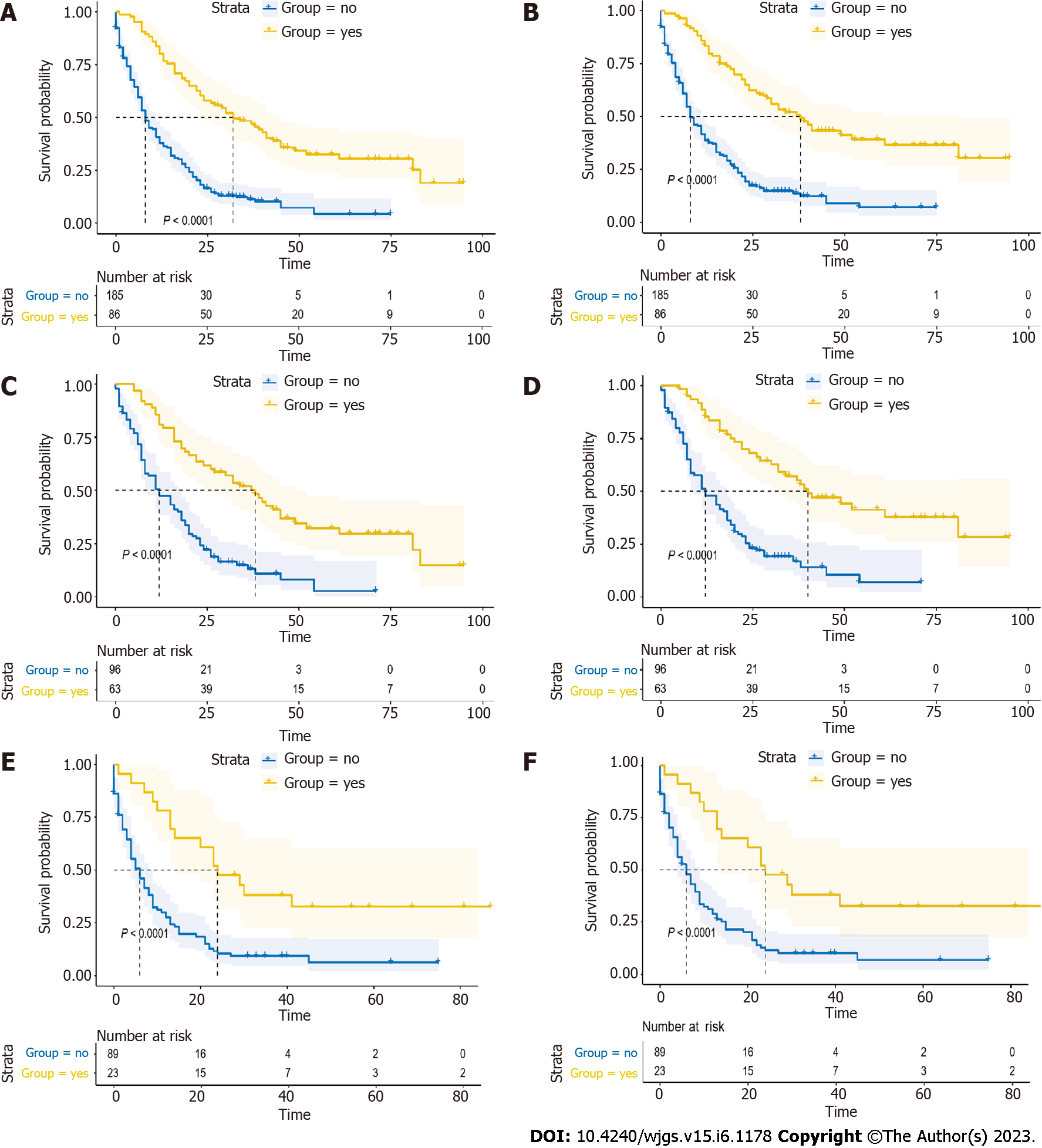
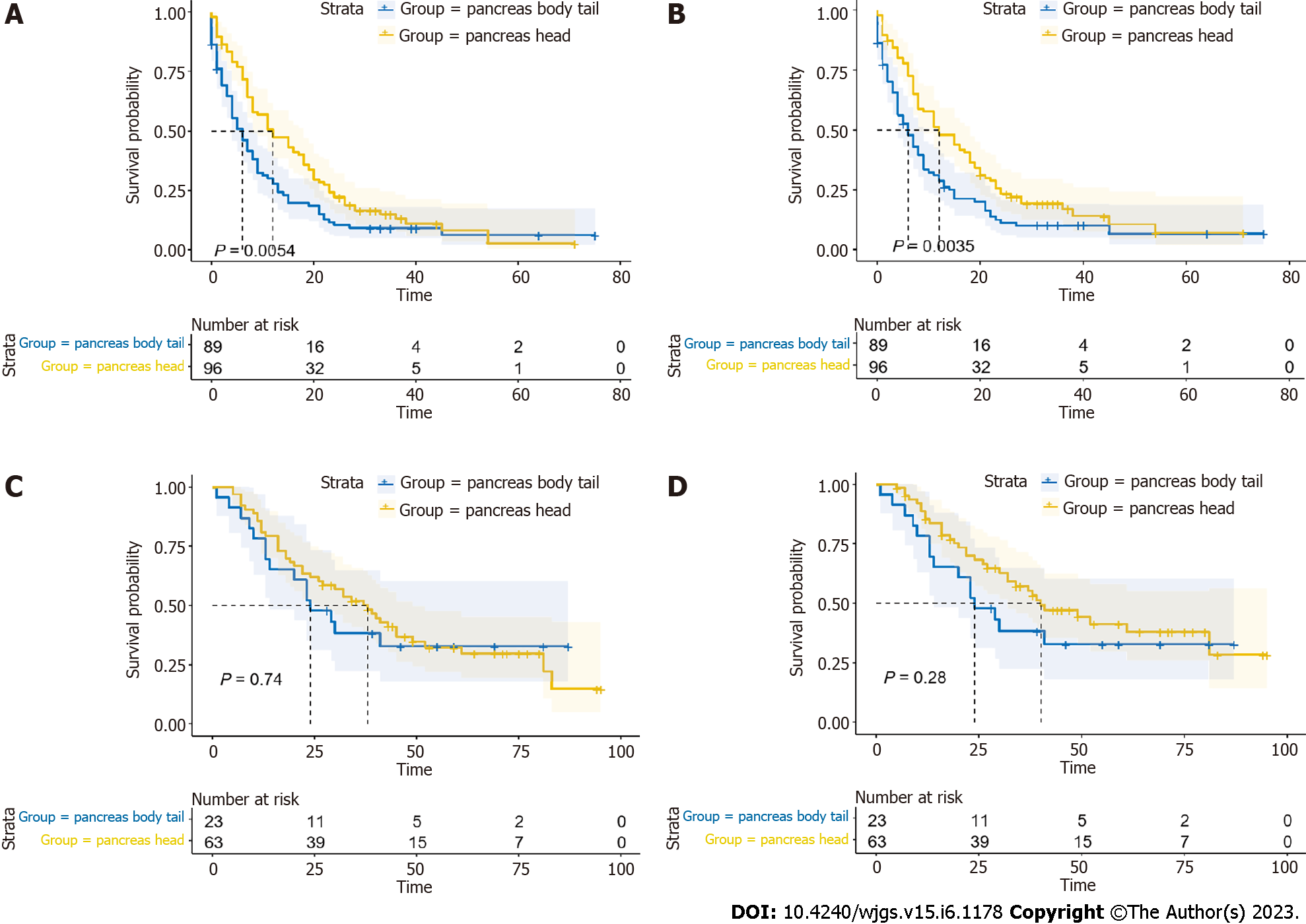
In this retrospective study, 86 patients (31.7%) received systemic therapy, while the remaining 185 (68.3%) patients did not. Patients who received systemic therapy had longer OS and CSS (all P < 0.05) (Figure 4A and B). Then, we conducted the analysis in PHG and PBTG, respectively. It demonstrated that regardless of which group the patients were in, patients who had received systemic therapy had better prognosis (all P < 0.05) (Figure 4C-F). Furthermore, we divided the patients into systemic therapy group and non-systemic therapy group and compared the survival of PHG and PBTG in each group. It showed that patients in PHG had a better survival in non-systemic therapy group (all P < 0.05) (Figure 5A and B), while there were no significant differences of survival in systemic therapy group (Figure 5C and D).
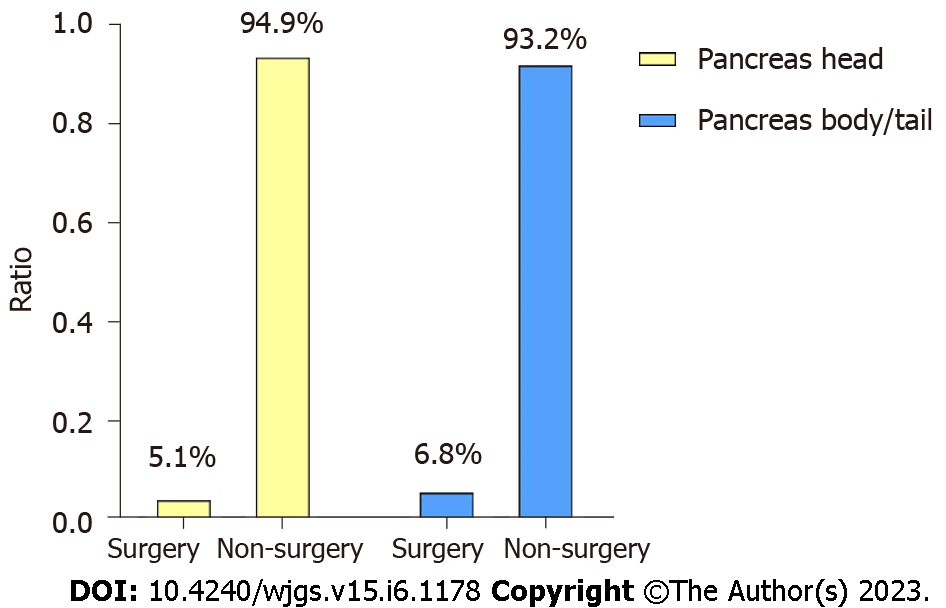
The significant differences of survival curves for all patients in stage I-IV were identified (P < 0.05) (Figure 6A and B). In early stage (stage I-II), there were no statistically significant differences between the survival of PHG and PBTG (Figure 6C and D). However, OS and CSS of PHG were significantly better than PBTG in advanced stage (stage III-IV) (Figure 6E and F). Moreover, surgical resection was considered as the best potential curative treatment for PMAC. The ratio of patients with advanced stage who received a surgery of two groups were calculated and depicted to avoid the impact of surgery on the results (Figure 7). From the ratio, we can see that more patients in PBTG received a surgery than PHG (6.8% vs 5.1%).
For pancreatic cancer (PC), there are various studies focusing on the characteristics of tumors occurring in different anatomical locations[6,8]. However, pancreatic mucinous adenocarcinoma (PMAC) is a rare type of PC. To the best of our knowledge, there is no study reported to discuss the characteristics of PMAC in different locations. Based on these viewpoints, this retrospective study was conducted to compare the survival and clinicopathological features of PMAC in pancreatic head and that in pancreatic body/tail. The new findings may provide novel insights for clinical workers to select appropriate strategies for pancreatic ductal adenocarcinoma (PDAC) management in the future.
Several previous studies had revealed that compared to pancreatic body/tail, patients with PC occurring in pancreatic head owned a better survival, especially for PDAC and pancreatic neuroendocrine tumors (PNETs)[6-8,12,13]. Not only that, anatomical locations of multiple cancer types produced a significant impact on cancer prognosis, such as gastric cancer[14-16], breast cancer[17], lung cancer[18], colorectal cancer[19-22]. These previous evidences provided support for our study through a broader cancer spectrum. However, there was also a study revealed that PDAC of pancreatic head had similar oncological outcomes with PDAC of pancreatic body/tail[10]. The divergence may be caused by different inclusion criteria of patients and various types of biases. In the present study, we firstly identified the better survival of PMAC located at pancreatic head compared to pancreatic body/tail, which was consistent with previous studies. Concerning the potential mechanisms underlying this situation, we believe that it is related to genetics and tumor biological diversity[5]. Pancreatic cancer cells in different anatomical positions have various embryonic origins and biological progresses[6], thereby leading to different clinical and pathological characteristics.
In the risk analysis for aggressive pathological factors, it was also shown that patients with PMAC of pancreatic body/tail had a greater risk for metastasis and higher staging compared to PMAC of pancreatic head. Such results were not contradictory to previous studies, which demonstrated that the pancreatic body/tail PDAC was larger, more frequently metastasized, and less likely to be resected compared to pancreatic head PDAC[8]. We thought the possible mechanisms were as follows: Firstly, the stemness of pancreatic tumor stem cells varies widely according to various embryonic origins and is related to the resistance to radiotherapy, chemotherapy, and tumor metastasis[23]. In this study, pancreatic body/tail PMAC was easy to metastasize, which may be caused by the high stemness of tumor cells in the body/tail of the pancreas. Secondly, the tumor microenvironment (TME) of different tumor sites is variable. TME is considered to play an important role in the process of pancreatic tumor metastasis, which can promote metastasis by stimulating angiogenesis/Lymphangiogenesis, epithelial-mesenchymal transition and so on[24]. Among these, pancreatic stellate cells (PSCs) were found to regulate angiogenesis and immune evasion, thereby promoting the resistance of therapy and tumor metastasis[25]. Thirdly, due to genetic and biological diversity, different tumor sites are characterized by variable gene communities. Alterations in these genes and characteristic signaling pathways are associated with tumor invasion and metastasis[26-29].
Systemic therapy is a combination of chemotherapy, radiotherapy, immunotherapy, targeted therapy and so on. Cancer patients rarely receive radical treatment, and more patients are treated with systemic therapy to control disease progression and prolong survival time[30]. In the survival analysis of this study, we revealed that patients treated with systemic therapy were prone to longer OS and CSS, regardless of the PMAC locations. In further investigation, non-systemic therapy patients with pancreatic head PMAC were observed to have a significant better survival compared to those with pancreatic body/tail PMAC. However, the survival of the two groups had no statistically significant difference after treated with systemic therapy. Although this was an observational analysis, without intervention experiments. Such results can also suggest that systemic therapy played an important role in prolonging the prognosis of patients. Meanwhile, systemic therapy has been paid attention to and applied to various cancer types, including cervical cancer[31], breast cancer[32], lung cancer[33], and even genitourinary malignancies of patients infected with COVID-19[34]. These consistent evidences from previous studies make our results easier to understand and more reliable.
There were also several limitations in this study that should be taken into account. Firstly, this was a retrospective study containing a relatively small simple size. Therefore, various biases existed in the study that may affect the results. Secondly, this study was unable to determine the exact mechanisms underlying the results, and further experiments are preferred to confirm our results. Thirdly, due to the limitations of SEER database, data of aggressive factors were incomplete including tumor size, tumor metastasis site and so on. In addition, typically pancreatic head cancer shows symptom in earlier stage than pancreatic body/tail ones and receives a surgical resection. That may be one of the contributors of "better prognosis" of pancreatic head cancer. Furthermore, in the group of patients who received curative surgery, the rate of R1 surgery will be higher during cephalic resections because of the closer vascular relationships, and such imbalance in surgery (R0 and R1) will lead to a compromised result. To solve there problems, we selected the PMAC located in pancreatic head (PHG) and body/tail (PBTG) without surgical resection treatment and compared the long-term outcomes of PHG and PBTG, which made the two groups comparable and drew more rigorous conclusions.
In summary, mucinous adenocarcinoma of pancreatic head has better survival and favorable clinicopathological characteristics compared to that of pancreatic body/tail. Moreover, systemic therapy was observed to effectively prolong the long-term survival of patients including OS and CSS.
Growing evidence shows that pancreatic tumors varied according to different anatomical locations, which produce a significant impact on the prognosis. However, there was no study reported to determine the differences between pancreatic mucinous adenocarcinoma (PMAC) in the head and body/tail of pancreas.
We aimed to investigate the differences in long-term outcomes (overall survival and cancer-specific survival) and clinicopathological characteristics between PMAC in the head and body/tail of pancreas.
A total of 2058 PMAC patients from the Surveillance, Epidemiology, and End Results database diagnosed between 1992 and 2017 were retrospectively reviewed.
We divided the patients who met the inclusion criteria into pancreatic head group (PHG) and pancreatic body/tail group (PBTG). The relationship between two groups and risk of invasive factors was identified using logistic regression analysis. Kaplan-Meier analysis and Cox regression analysis were conducted to compare the overall survival (OS) and cancer-specific survival (CSS) of two patient groups.
After selection, 271 PMAC patients were included in the study. The 1-year, 3-year, and 5-year OS rates of these patients were 51.6%, 23.5%, and 13.6%, respectively. While the 1-year, 3-year, and 5-year CSS rates were 53.2%, 26.2%, and 17.4%, respectively. The median OS of PHG was longer than that of PBTG (18 vs 7.5 mo, P < 0.001). Compared to PHG, patients in PBTG had a greater risk of metastases [odds ratio (OR) = 2.747, 95% confidence interval (CI): 1.628-4.636, P < 0.001] and higher staging (OR = 3.204, 95%CI: 1.895-5.415, P < 0.001). Survival analysis revealed that age < 65 years, male, low-grade (G1-G2), low-stage, systemic therapy, and PMAC located at pancreatic head led to longer OS and CSS (all P < 0.05). The location of PMAC was an independent prognostic factor for CSS [hazard ratio (HR)=0.7, 95%CI: 0.52-0.94, P = 0.017]. Further analysis demonstrated that OS and CSS of PHG were significantly better than PBTG in advanced stage (stage III-IV).
Compared to pancreatic body/tail, the PMAC located in pancreatic head have a better long-term outcomes and favorable clinicopathological characteristics.
The new findings may provide novel insights for clinical workers to select appropriate strategies for pancreatic ductal adenocarcinoma management in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dilek ON, Turkey; Elghali MA, Tunisia S-Editor: Ma YJ L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64456] [Article Influence: 16114.0] [Reference Citation Analysis (176)] |
| 2. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2113] [Article Influence: 150.9] [Reference Citation Analysis (3)] |
| 3. | Rosenberger LH, Stein LH, Witkiewicz AK, Kennedy EP, Yeo CJ. Intraductal papillary mucinous neoplasm (IPMN) with extra-pancreatic mucin: a case series and review of the literature. J Gastrointest Surg. 2012;16:762-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Conlon KC. Intraductal papillary mucinous tumors of the pancreas. J Clin Oncol. 2005;23:4518-4523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Ling Q, Xu X, Zheng SS, Kalthoff H. The diversity between pancreatic head and body/tail cancers: clinical parameters and in vitro models. Hepatobiliary Pancreat Dis Int. 2013;12:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Birnbaum DJ, Bertucci F, Finetti P, Birnbaum D, Mamessier E. Head and Body/Tail Pancreatic Carcinomas Are Not the Same Tumors. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Dreyer SB, Jamieson NB, Upstill-Goddard R, Bailey PJ, McKay CJ; Australian Pancreatic Cancer Genome Initiative, Biankin AV, Chang DK. Defining the molecular pathology of pancreatic body and tail adenocarcinoma. Br J Surg. 2018;105:e183-e191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | van Erning FN, Mackay TM, van der Geest LGM, Groot Koerkamp B, van Laarhoven HWM, Bonsing BA, Wilmink JW, van Santvoort HC, de Vos-Geelen J, van Eijck CHJ, Busch OR, Lemmens VE, Besselink MG; Dutch Pancreatic Cancer Group. Association of the location of pancreatic ductal adenocarcinoma (head, body, tail) with tumor stage, treatment, and survival: a population-based analysis. Acta Oncol. 2018;57:1655-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Winer LK, Dhar VK, Wima K, Morris MC, Lee TC, Shah SA, Ahmad SA, Patel SH. The Impact of Tumor Location on Resection and Survival for Pancreatic Ductal Adenocarcinoma. J Surg Res. 2019;239:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Ruess DA, Makowiec F, Chikhladze S, Sick O, Riediger H, Hopt UT, Wittel UA. The prognostic influence of intrapancreatic tumor location on survival after resection of pancreatic ductal adenocarcinoma. BMC Surg. 2015;15:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Mei W, Ding Y, Wang S, Jia Y, Cao F, Li F. Head and body/tail pancreatic neuroendocrine tumors have different biological characteristics and clinical outcomes. J Cancer Res Clin Oncol. 2020;146:3049-3061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Tomasello G, Ghidini M, Costanzo A, Ghidini A, Russo A, Barni S, Passalacqua R, Petrelli F. Outcome of head compared to body and tail pancreatic cancer: a systematic review and meta-analysis of 93 studies. J Gastrointest Oncol. 2019;10:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Lee M, Kwon W, Kim H, Byun Y, Han Y, Kang JS, Choi YJ, Jang JY. The Role of Location of Tumor in the Prognosis of the Pancreatic Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Feng F, Tian Y, Guo M, Liu S, Xu G, Liu Z, Zheng G, Lian X, Fan D, Zhang H. Comparison of clinicopathological features and prognosis of gastric cancer located in the lesser and greater curve. Clin Transl Oncol. 2017;19:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Jung YJ, Seo HS, Kim JH, Park CH, Lee HH. Cross-Sectional Location of Gastric Cancer Affects the Long-Term Survival of Patients as Tumor Invasion Deepens. Ann Surg Oncol. 2017;24:3947-3953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kim K, Cho Y, Sohn JH, Kim DH, Do IG, Lee HJ, Do SI, Ahn S, Lee HW, Chae SW. Clinicopathologic characteristics of early gastric cancer according to specific intragastric location. BMC Gastroenterol. 2019;19:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Perkins CI, Hotes J, Kohler BA, Howe HL. Association between breast cancer laterality and tumor location, United States, 1994-1998. Cancer Causes Control. 2004;15:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Bishawi M, Moore W, Bilfinger T. Severity of emphysema predicts location of lung cancer and 5-y survival of patients with stage I non-small cell lung cancer. J Surg Res. 2013;184:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Kim CW, Kim J, Park Y, Cho DH, Lee JL, Yoon YS, Park IJ, Lim SB, Yu CS, Kim JC. Prognostic Implications of Extranodal Extension in Relation to Colorectal Cancer Location. Cancer Res Treat. 2019;51:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Lee BC, Yu CS, Kim J, Lee JL, Kim CW, Yoon YS, Park IJ, Lim SB, Kim JC. Clinicopathological features and surgical options for synchronous colorectal cancer. Medicine (Baltimore). 2017;96:e6224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Li P, Xiao Z, Braciak TA, Ou Q, Chen G, Oduncu FS. A relationship to survival is seen by combining the factors of mismatch repair status, tumor location and age of onset in colorectal cancer patients. PLoS One. 2017;12:e0172799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Han NY, Kim MJ, Park BJ, Sung DJ. Location of rectal cancer as determined using rectal magnetic resonance imaging, and its relationship with pulmonary metastasis. Turk J Gastroenterol. 2014;25:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Fitzgerald TL, McCubrey JA. Pancreatic cancer stem cells: association with cell surface markers, prognosis, resistance, metastasis and treatment. Adv Biol Regul. 2014;56:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Ren B, Cui M, Yang G, Wang H, Feng M, You L, Zhao Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 415] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 25. | Xu Z, Pothula SP, Wilson JS, Apte MV. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol. 2014;20:11216-11229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Keleg S, Büchler P, Ludwig R, Büchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer. 2003;2:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Liang J, Yang Y, Bai L, Li F, Li E. DRP1 upregulation promotes pancreatic cancer growth and metastasis through increased aerobic glycolysis. J Gastroenterol Hepatol. 2020;35:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Purohit A, Saxena S, Varney M, Prajapati DR, Kozel JA, Lazenby A, Singh RK. Host Cxcr2-Dependent Regulation of Pancreatic Cancer Growth, Angiogenesis, and Metastasis. Am J Pathol. 2021;191:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Chen X, Liu F, Xue Q, Weng X, Xu F. Metastatic pancreatic cancer: Mechanisms and detection (Review). Oncol Rep. 2021;46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Schirrmacher V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int J Oncol. 2019;54:407-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 577] [Reference Citation Analysis (0)] |
| 31. | Liontos M, Kyriazoglou A, Dimitriadis I, Dimopoulos MA, Bamias A. Systemic therapy in cervical cancer: 30 years in review. Crit Rev Oncol Hematol. 2019;137:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 32. | Shien T, Iwata H. Adjuvant and neoadjuvant therapy for breast cancer. Jpn J Clin Oncol. 2020;50:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 33. | Chaft JE, Rimner A, Weder W, Azzoli CG, Kris MG, Cascone T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 2021;18:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 233] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 34. | Gulati S, Muddasani R, Gustavo Bergerot P, Pal SK. Systemic therapy and COVID19: Immunotherapy and chemotherapy. Urol Oncol. 2021;39:213-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |