Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1125
Peer-review started: January 10, 2023
First decision: February 20, 2023
Revised: February 21, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: June 27, 2023
Processing time: 156 Days and 8 Hours
Albumin-bilirubin (ALBI) score is an indicator of liver dysfunction and is useful for predicting prognosis of hepatocellular carcinomas. Currently, this liver function index has been used to predict prognosis in other neoplasms. However, the significance of ALBI score in gastric cancer (GC) after radical resection has not been elucidated.
To evaluate the prognostic value of the preoperative ALBI status in patients with GC who received curative treatment.
Patients with GC who underwent curative intended gastrectomy were retro
A total of 361 patients (235 males) were enrolled. The median ALBI value for the entire cohort was -2.89 (IQR -3.13; -2.59). The AUC for ALBI score was 0.617 (95%CI: 0.556-0.673, P < 0.001), and the cutoff value was -2.82. Accordingly, 211 (58.4%) patients were classified as low-ALBI group and 150 (41.6%) as high-ALBI group. Older age (P = 0.005), lower hemoglobin level (P < 0.001), American Society of Anesthesiologists classification III/IV (P = 0.001), and D1 lymphadenectomy P = 0.003) were more frequent in the high-ALBI group. There was no difference between both groups in terms of Lauren histological type, depth of tumor invasion (pT), presence of lymph node metastasis (pN), and pathologic (pTNM) stage. Major postoperative complication, and mortality at 30 and 90 days were higher in the high-ALBI patients. In the survival analysis, the high-ALBI group had worse disease-free survival (DFS) and overall survival (OS) compared to those with low-ALBI (P < 0.001). When stratified by pTNM, the difference between ALBI groups was maintained in stage I/II and stage III CG for DFS (P < 0.001 and P = 0.021, respectively); and for OS (P < 0.001 and P = 0.063, respectively). In multivariate analysis, total gastrectomy, advanced pT stage, presence of lymph node metastasis and high-ALBI were independent factors associated with worse survival.
The preoperative ALBI score is able to predict the outcomes of patients with GC, where high-ALBI patients have worse prognosis. Also, ALBI score allows risk stratification of patients within the same pTNM stages, and represents an independent risk factor associated with survival.
Core Tip: The present study evaluates the clinical impact of the preoperative albumin-bilirubin (ALBI) score in patients with gastric cancer who received curative treatment. We found that ALBI score is able to predict short-term and long-term outcomes of patients, and can be applied as a prognostic factor for gastric cancer. The ALBI is a simple and reproducible parameter that allows the risk stratification of patients within the pathologic stage stages, and may be an additional useful tool for decision-making regarding treatment and follow-up individualization.
- Citation: Szor DJ, Pereira MA, Ramos MFKP, Tustumi F, Dias AR, Zilberstein B, Ribeiro Jr U. Preoperative albumin-bilirubin score is a prognostic factor for gastric cancer patients after curative gastrectomy. World J Gastrointest Surg 2023; 15(6): 1125-1137
- URL: https://www.wjgnet.com/1948-9366/full/v15/i6/1125.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i6.1125
Gastric cancer (GC) is a solid gastrointestinal tumor with elevated incidence and mortality, represented by more than 1 million diagnosed cases and 768000 deaths reported worldwide in 2020[1]. Currently, staging is based only on TNM classification, which plays a crucial role in predicting the prognosis. However, it is well known that patients with same stage disease can have different outcomes[2], which indicate that additional parameters can play a role in staging and prognosis.
Thus, the search for alternative parameters that, integrated into staging systems, can provide further information about the prognosis are a constant target of investigation. Due to its potential to allow risk stratification and tailor individual treatment and follow-up, some of the research on additional prognostic variables has focused on inflammatory and nutritional-based biomarkers, based on the fact that tumor development is a complex process dictated by a series of intercellular and its sub product interactions[3]. Over the past years, many articles have been published demonstrating the correlation of inflammatory biomarkers, such as neutrophil-lymphocyte and platelet lymphocyte ratio, with prognosis of patients with GC[4]. The main advantages of these methods are the low cost, the need for simple tests such as a complete blood count, and the reproducibility between different centers.
It is also known that nutritional characteristics and liver function, represented by serum albumin and bilirubin, can interfere with prognosis and cancer survival. The decreased albumin level, which is produced in the liver, could be a sign of malnutrition or liver synthesis dysfunction. In turn, increased serum bilirubin levels usually suggests liver dysfunction[5]. Therefore, the albumin-bilirubin score (ALBI) was created to evaluate both levels together and estimate the extent of liver dysfunction[6,7]. It was first described by Johnson et al[6], where were evaluated patients with hepatocarcinoma (HCC) in a way to overcome the limitation of Child-Pugh grade on assessing hepatic function[7]. ALBI was initially developed to assess HCC, and represents a prognostic factor in these patients, irrespective of the degree of underlying liver fibrosis[8].
However, it has also been extensively investigated in patients who do not have HCC, and some studies demonstrated that ALBI score represents a prognostic factor even in patients without HCC, including patients with non-small cell lung carcinoma, pancreatic, colon, and esophageal cancer[9-12].
Despite the interest in ALBI score, few studies have considered its role in patients with GC. Kanda et al[13] were the first to recognize ALBI grade as a predictor of survival after radical gastrectomy. Furthermore, ALBI was also identified as a predictor of postoperative complications (POC) after gastrectomy for GC[14]. However, the clinical impact of the preoperative ALBI score in patients with GC who received curative treatment remains unclear.
Thus, the aim of this study was to investigate the prognostic value of the ALBI score in in patients with GC, and its clinical applicability for risk stratification. We also evaluated the clinicopathological characteristics associated with ALBI score groups.
This is a retrospective cohort. All GC treated with curative intent gastrectomy at our Hospital between 2009 and 2021 were evaluated from a prospectively maintained database. Only histologically proven gastric adenocarcinoma and patients who underwent gastrectomy with lymphadenectomy were considered eligible. Emergency gastrectomy or patients who had underlying chronic liver disease were excluded. The study was approved by the ethics committee of the hospital.
Preoperative staging consisted of upper gastrointestinal endoscopy with biopsy, chest, abdominal and pelvic computed tomography scans, and laboratory tests. The clinical characteristics evaluated included the American Society of Anesthesiologists (ASA) classification and the Charlson-Deyo Comorbidity Index (CCI)[15]. CCI was considered without including age and GC as comorbidity. The neutrophil-lymphocyte ratio was evaluated by the division between serum neutrophil and lymphocytes.
Peripheral blood was obtained after diagnosis and within 1 mo before surgery, at the time the patient had no sign of infection and was not under systemic chemotherapy. The ALBI score was calculated by the formula (log10 bilirubin × 0.660) + (albumin × -0.085), where bilirubin was expressed in μmol/L and albumin in g/L.
The extent of gastrectomy and lymph node dissection were performed in accordance with Japanese Gastric Cancer Association recommendations[16]. Tumor stage was determined based on the TNM/UICC (8th edition)[17]. Clavien-Dindo’s classification was applied to grade POC, when Clavien III-IV was considered as major POC[18].
Follow-up was performed every 3 mo in the first year and every 6 mo after this period, with clinical evaluation. Studies to detect relapse were performed based on the presence of symptoms.
Descriptive statistics included frequencies with percent for nominal variables, and mean (with standard deviation, SD) or median (with interquartile range, IQR) for continuous variables. Comparison of clinicopathological characteristics was performed using chi-square tests for categorical variables and t test or Mann-Whitney U test for continuous variables. The receiver operating characteristic (ROC) curves with area under the ROC curve (AUC) were used to evaluate the ability of ALBI in predicting disease-free survival (DFS) (recurrence/death). The optimal cutoff value was determined by maximizing Youden’s index (sensitivity + specificity - 1). Patients were divided into “low-ALBI” and “High-ALBI” groups based on the cutoff value.
Overall survival (OS) and DFS were estimated using the Kaplan-Meier method, and the comparison of curves was obtained through the log-rank test. Multivariate Cox proportional hazard analysis was performed to determine independent risk factors for survival. Only variables that were significant on univariate analysis (P < 0.05) were included as co-variable in the multivariate model. Survival time was calculated from the date of diagnosis until the date of death or recurrence for DFS, and until death for OS. The patients alive were censored at the date of the last contact. A P value of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software, version 20.0 (SPSS Inc, Chicago, IL, United States).
A total of 565 GC patients underwent curative intent gastrectomy in the referenced period. After excluding those with a lack of laboratory tests within one month before surgery, 361 patients met the inclusion criteria and were enrolled in the study. The mean age was 63.5 years-old (± 12.1), and the majority of patients were male (65%).
The mean value of albumin and bilirubin of all cases was 3.9 g/L (SD: 0.6; median of 4, IQR: 3.70-4.30) and 0.43 μmol/L (SD: 0.27; median of 0.37, IQR: 0.26-0.55), respectively. After ALBI calculation, the median ALBI value obtained was -2.89 (IQR: -3.13; -2.59; median of -2.82, SD: -0.48).
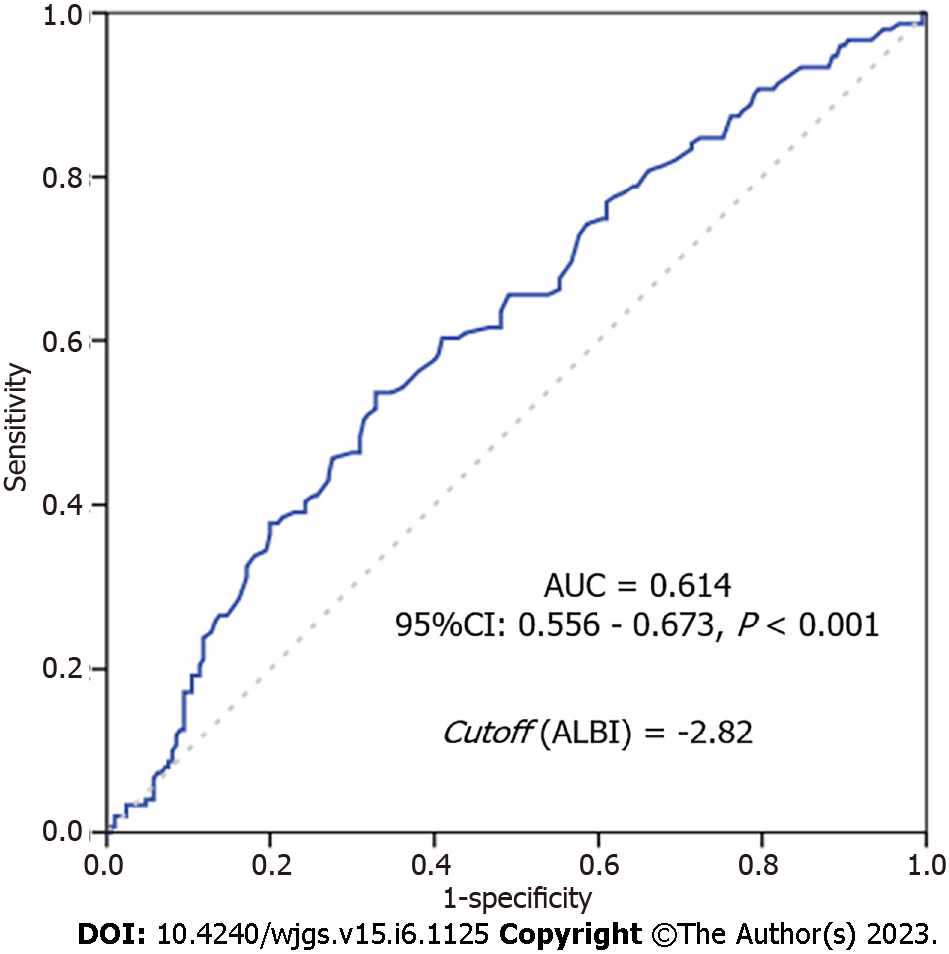
The ROC curve with the ALBI metric performance is shown in Figure 1. The AUC for ALBI score was 0.617 (95%CI: 0.556-0.673, P < 0.00171), and the optimal cutoff value was - 2.82.
Thus, based on the cutoff value determined by ROC curve, 211 (58.4%) patients were classified as low-ALBI group (ALBI < -2.82); and 150 (41.6%) as high-ALBI group (ALBI ≥ -2.82).
Clinical and surgical characteristics of both groups are presented in Table 1. Older age (P = 0.005), lower hemoglobin level (P < 0.001), and ASA III/IV (P = 0.001) were associated with the High-ALBI group. Also, D1 Lymphadenectomy was more frequent in the High-ALBI group (P = 0.003). There was no difference regarding sex, BMI, type of gastrectomy, and preoperative chemotherapy between the groups.
| Variables | Low-ALBI group (< -2.82), n = 211 | High-ALBI group (≥ -2.82), n = 150 | P value |
| Sex | 0.712 | ||
| Female | 72 (34.1) | 54 (36.0) | |
| Male | 139 (65.9) | 96 (64.0) | |
| Age (yr) | 0.005 | ||
| mean (SD) | 62.0 (12.0) | 65.6 (12.2) | |
| BMI (kg/cm²) | 0.856 | ||
| mean (SD) | 25 (4.5) | 25.2 (16.5) | |
| Hemoglobin (g/dL) | < 0.001 | ||
| mean (SD) | 12.7 (2.1) | 11.1 (2.1) | |
| Albumin (g/dL) | < 0.001 | ||
| mean (SD) | 4.3 (0.3) | 3.5 (0.5) | |
| Bilirubin (mg/dL) | 0.072 | ||
| mean (SD) | 0.41 (0.22) | 0.47 (0.33) | |
| Neutrophil to lymphocyte ratio | 0.092 | ||
| mean (SD) | 2.48 (2.29) | 2.91 (2.56) | |
| American Society of Anesthesiologists | 0.001 | ||
| I/II | 178 (84.4) | 105 (70.0) | |
| III/IV | 33 (15.6) | 45 (30.0) | |
| Charlson–Deyo Comorbidity Index1 | 0.344 | ||
| 0 | 141 (66.8) | 93 (62.0) | |
| ≥ 1 | 70 (33.2) | 57 (38.0) | |
| Type of gastrectomy | 0.562 | ||
| Subtotal | 126 (59.7) | 85 (56.7) | |
| Total | 85 (40.3) | 65 (43.3) | |
| Lymphadenectomy | 0.003 | ||
| D1 | 34 (16.1) | 44 (29.3) | |
| D2 | 177 (83.9) | 106 (70.7) | |
| Preoperative chemotherapy | 0.477 | ||
| No | 175 (82.9) | 120 (80) | |
| Yes | 36 (17.1) | 30 (20) | |
Regarding the pathological characteristics (Table 2), there was no significant difference between the ALBI groups in terms of histological type, depth of tumor invasion (pT), presence of lymph node metastasis (pN), and final pathologic (pTNM) stage.
| Variables | Low-ALBI group (< -2.82), n = 211 | High-ALBI group (≥ -2.82), n = 150 | P value |
| Lauren type | 0.367 | ||
| Intestinal | 124 (58.8) | 81 (54.0) | |
| Diffuse/mixed | 87 (41.2) | 69 (46.0) | |
| Histological differentiation | 0.993 | ||
| Well/moderately differentiated | 107 (59.7) | 76 (50.7) | |
| Poorly differentiated | 104 (49.3) | 74 (49.3) | |
| Lymphatic invasion | 0.132 | ||
| No | 121 (57.3) | 74 (49.3) | |
| Yes | 90 (42.7) | 76 (50.7) | |
| Venous invasion | 0.196 | ||
| No | 150 (71.1) | 97 (64.7) | |
| Yes | 61 (28.9) | 53 (35.3) | |
| Perineural invasion | 0.743 | ||
| No | 112 (53.1) | 77 (51.3) | |
| Yes | 99 (46.9) | 73 (48.7) | |
| T status | 0.105 | ||
| pT1/T2 | 91 (43.1) | 52 (34.7) | |
| pT3/T4 | 120 (56.9) | 98 (65.3) | |
| No of dissected lymph nodes | 0.543 | ||
| mean (SD) | 40.9 (17.7) | 39.8 (17.2) | |
| pN status | 0.239 | ||
| pN0 | 99 (46.9) | 61 (40.7) | |
| pN+ | 112 (53.1) | 89 (59.3) | |
| pTNM | 0.222 | ||
| I/II | 122 (57.8) | 77 (51.3) | |
| III | 89 (42.2) | 73 (48.7) |
The postoperative outcomes according to ALBI group are presented in Table 3. The occurrence of major POC (P = 0.029) and the mortality rate at 30 d and 90 d were more frequent in the high-ALBI group (P = 0.023 and P = 0.030, respectively). The frequency of patients undergoing adjuvant chemotherapy was similar between both groups (P = 0.917).
| Variables | Low-ALBI group (< -2.82), n = 211 | High-ALBI group (≥ -2.82), n = 150 | P value |
| Length of hospital stay (d) | 0.673 | ||
| Median (IQR) | 9 (6.0-13.3) | 10 (7.0-13.8) | |
| Postoperative complications (Clavien) | 0.029 | ||
| Non/minor POC (I-II) | 183 (86.7) | 117 (78) | |
| Major POC (III-IV) | 28 (13.3) | 33 (22) | |
| Postoperative chemotherapy | 0.917 | ||
| No | 117 (55.5) | 84 (56) | |
| Yes | 94 (44.5) | 66 (44) | |
| Chemotherapy-all (pre or postoperative) | 0.766 | ||
| No | 99 (46.9) | 68 (45.3) | |
| Yes | 112 (53.1) | 82 (54.7) | |
| 30-d motality | 0.023 | ||
| No | 206 (97.6) | 138 (92.6) | |
| Yes | 5 (2.4) | 11 (7.4) | |
| 90-d motality | 0.030 | ||
| No | 198 (94.7) | 130 (88.4) | |
| Yes | 11 (5.3) | 17 (11.6) | |
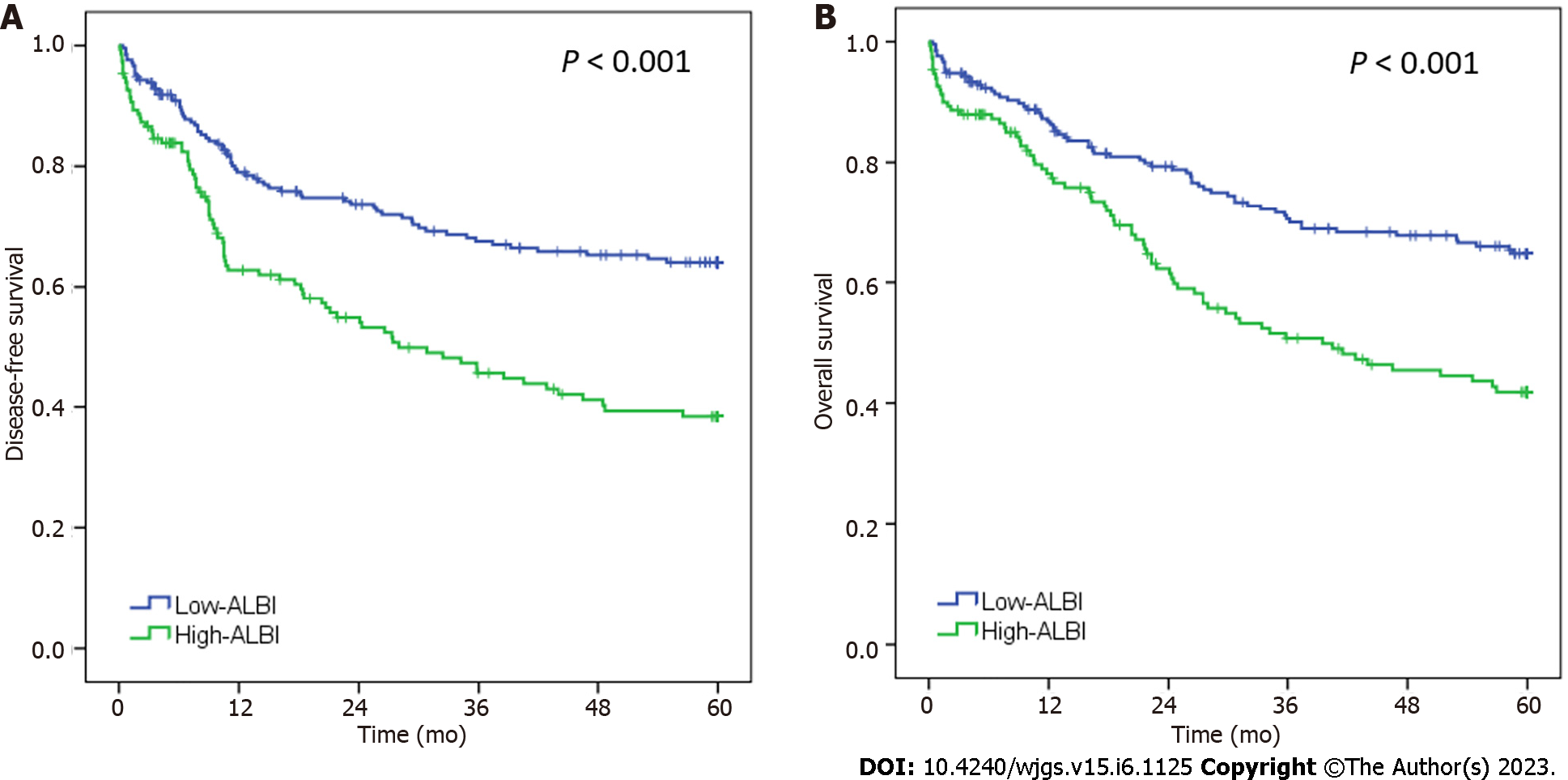
The median follow-up period was 40.1 mo. During this period, 81 patients had recurrence and 142 died. The 5-years DFS and OS rates for the entire cohort were 53.7% and 55.6%, respectively. In the survival analysis (Figure 2), DFS and OS rate was worse for patients with high-ALBI levels compared to the low-ALBI patients (P < 0.001 for both). The median DFS and OS for high-ALBI group were of 28.0 mo and 39.5 mo, respectively.
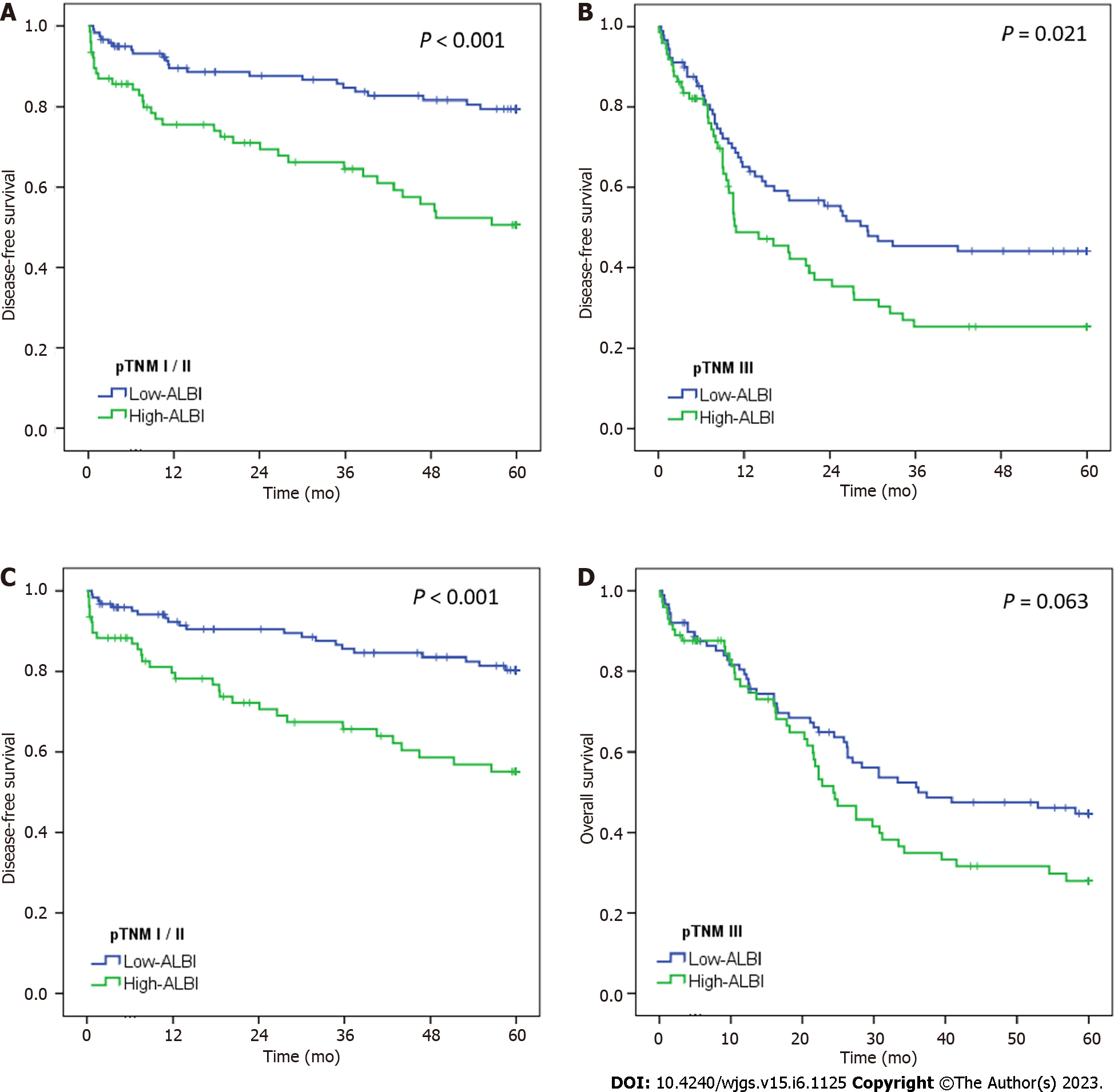
Similarly, when stratified by pTNM stage (Figure 3), pTNM I/II GC with high-ALBI had significantly worse DFS and OS compared to with low-ALBI pTNM I/II patients (P < 0.001).
Also, among pTNM III GC, DFS and OS in high-ALBI was shorter compared to low-ALBI group (P = 0.021 and P = 0.063, respectively).
In multivariate analysis, total gastrectomy, advanced pT stage, presence of lymph node metastasis and high-ALBI were independent factors associated with worse DFS (Table 4). For OS, ASA, type of gastrectomy, pT, pN, and ALBI-groups were factors significantly associated with survival in multi
| Disease-free survival | Univariate | Multivariate | ||||
| Variables | HR | 95%CI | P value | HR | 95%CI | P value |
| Male (vs female) | 1.15 | 0.81-1.61 | 0.438 | - | - | - |
| Age > 65 yr (vs < 65 yr) | 1.18 | 0.86-1.62 | 0.312 | - | - | - |
| Charlson > 1 (vs 0) | 1.48 | 1.07-2.04 | 0.019 | 1.39 | 0.94-2.06 | 0.102 |
| ASA III/IV (vs ASA I/II) | 1.84 | 1.29-2.64 | 0.001 | 1.43 | 0.93-2.21 | 0.106 |
| Total gastrectomy (vs distal) | 1.43 | 1.04-1.97 | 0.030 | 1.43 | 1.03-1.98 | 0.031 |
| Diffuse/mixed (vs others) | 1.21 | 0.87-1.67 | 0.255 | - | - | - |
| pT3/T4 (vs pT1/T2) | 2.57 | 1.76-3.76 | < 0.001 | 2.03 | 1.33-3.12 | 0.001 |
| pN+ (vs pN0) | 2.26 | 1.60-3.19 | < 0.001 | 1.54 | 1.04-2.27 | 0.030 |
| non-CMT (vs CMT) | 1.15 | 0.83-1.58 | 0.399 | - | - | - |
| Low-ALBI (vs High-ALBI) | 2.09 | 1.51-2.88 | < 0.001 | 1.83 | 1.32-2.53 | < 0.001 |
| Overall survival | Univariate | Multivariate | ||||
| Variables | HR | 95%CI | P value | HR | 95%CI | P value |
| Male (vs female) | 1.20 | 0.85-1.71 | 0.306 | - | - | - |
| Age > 65 yr (vs < 65 yr) | 1.28 | 0.94-1.79 | 0.137 | - | - | - |
| Charlson > 1 (vs 0) | 1.42 | 1.02-1.98 | 0.041 | 1.25 | 0.83-1.89 | 0.282 |
| ASA III/IV (vs ASA I/II) | 1.94 | 1.35-2.80 | < 0.001 | 1.60 | 1.02-2.52 | 0.041 |
| Total gastrectomy (vs distal) | 1.50 | 1.08-2.08 | 0.016 | 1.56 | 1.12-2.18 | 0.009 |
| Diffuse/mixed (vs others) | 1.29 | 0.93-1.79 | 0.133 | - | - | - |
| pT3/T4 (vs pT1/T2) | 2.44 | 1.65-3.61 | < 0.001 | 1.87 | 1.20-2.91 | 0.006 |
| pN+ (vs pN0) | 2.24 | 1.57-3.20 | < 0.001 | 1.58 | 1.06-2.37 | 0.026 |
| non-CMT (vs CMT) | 1.18 | 0.85-1.64 | 0.327 | - | - | - |
| Low-ALBI (vs High-ALBI) | 1.97 | 1.41-2.74 | < 0.001 | 1.68 | 1.20-2.35 | 0.003 |
The aim of the present study was to evaluate the impact of the ALBI status in GC patients who received curative treatment. Accordingly, we found that ALBI score was an independent prognostic factor for patients with GC, and may be useful in predicting patient survival after gastrectomy. Furthermore, ALBI groups were able to stratify survival of patients in the same pTNM stage.
ALBI score has been considered a useful marker for hepatic dysfunction based only the two variables, albumin and bilirubin, which are related to nutrition and liver function[6,19]. Classically, it is a biomarker intended to evaluate prognosis in patients with HCC, and the majority of available studies address this disease[20]. Even so, some articles report results of its application in other diseases[9,10,21]. For instance, Matsukane et al[22] reports that ALBI is an independent prognostic factor for patients with non-small cell lung cancer, and Lee et al[9] found that ALBI can predicted disease recurrence and survival in stage III colon cancer.
Considering the two parameters that comprise the ALBI score, albumin is synthesized in the liver, and its serum level is usually used to assess nutritional status and hepatic function[5]. Nutritional status is something that has already been shown to be related to the immune system and prognosis in GC, where a deficient nutritional condition can suppress the immune response against tumor, accelerating the cancer progression[5,23]. Thus, preoperative serum albumin level and prognostic nutritional index are factors already related to outcomes in GC[23]. But considering the evaluation of bilirubin levels alone, the association between serum levels of bilirubin and GC are poorly described[24].
The present study evaluated the ALBI through the ROC curve based on the DFS for GC, and we set the cut-off value for the ALBI score at -2.82. Our cutoff score was similar to reported by Ju et al[21], that include only curative GC patients, where based on OS rates at 3 years and 5 years they determined an ALBI cutoff value of -2.78. Interestingly, a similar value was also found in a study with patients with esophageal cancer, where the cutoff value for the ALBI score was -2.7[10]. This suggests that setting a single cut-off value for esophagogastric tumors may be appropriate.
In our cohort, ALBI groups were different in terms of some clinical characteristics, as age, hemoglobin levels and ASA, indicating a clinically impaired patient who might present a worse prognosis. However, no difference regarding the rate of comorbidity in relation to the ALBI groups were found in this study. Likewise, Kanda et al[13] evaluated 283 patients with pT2-4 resected GC and also demonstrated that high-ALBI group patients were older, but without reflecting on the comorbidity rate.
Considering the pathological characteristics, we expected that the high-ALBI group would have deeper gastric wall invasion and more nodal involvement, suggesting that a more advanced disease could cause impaired liver function and possible hepatic hilar compression due to nodal enlargement. However, similar than Kanda et al[13], we did not find this association.
Despite not influencing the pTNM stage, in the preset cohort ALBI score was related to patient survival. Patients with high-ALBI were related to both lower DFS and OS when compared to low-ALBI groups, even in the same TNM stages. Similar results have been reported by Zhu et al[14] that demonstrated worse OS for patients with GC and high ALBI values, especially those with stage disease II and III. Likewise, Ju et al[21] evaluating 244 patients with resected GC showed that increasing in ALBI levels was an independent factor related to OS, with a HR of 2.2. ALBI score was also related to recurrence in advanced GC stage as pT2-T4 after gastrectomy[13]. The association of ALBI with decreased survival can be explained by the prolonged damage caused by a deficient nutritional status, with lower albumin levels and anemia (both seen in our cohort). In addition to debilitating the patient, decreasing adherence to chemotherapy[25,26], malnutrition also affects cell-mediated immunity by T cells, which impairs anti-tumor response and accelerates tumor progression[5,23,27].
Noteworthy, some authors also demonstrated differences in relation to the incidence of POC according to the ALBI score, as described by Aoyama et al[10], where the incidence of postoperative anastomotic leakage in patients with esophageal cancer was 46.3% in the ALBI-high group compared with 27.5% in the ALBI-low group (P = 0.038). In patients with GC, some authors reported that there was no significant differences in morbidity and POC rate between ALBI groups[13]; whereas other found that in patients who underwent radical resection, the rate of POC were also higher in patients with high-ALBI than low-ALBI levels[14]. Similarly, in our cohort, we also observed that the incidence of major POC was higher in the high-ALBI group, including the mortality at 30 d and 90 d. One of the justifications for this result is probably related to the age of patients, since high-ALBI group are generally older, as seen in our study. So, it may represent a frail group of patients with a higher risk of complications[28]. Still, poor nutritional status can contribute to a higher risk of POC[29], since preoperative hypoproteinemia has already been reported as a risk factor for postoperative infection in gastrointestinal surgery[30]. So, in our study, ALBI had a clinical impact on both short-term and long-term outcomes.
Overall, we proposed a risk stratification in two groups based on ALBI values, which was independently associated with survival and may serve as an additional parameter to predict patient outcomes. Both DFS and OS were clearly separated according to the ALBI status, where those classified as high-ALBI had worse survival outcomes. In addition, the preoperative ALBI was found to be a promising marker for predicting disease relapse and survival even in GC with the same TNM stage. As well as other previously described pretreatment serum-based inflammatory indicators, such as the neutrophil to lymphocyte ratio[31], the ALBI score can be determined using routine tests and has potential to be an useful biomarker for patients with GC.
Some limitations should be mentioned in this study. Firstly, this is a retrospective research; therefore, some confounders and selection bias were not absolutely adjusted. Although ALBI grade has been related to the tolerability and introduction of postoperative adjuvant chemotherapy[21,32], since hepatic dysfunction are one of the main factors for adverse reactions to chemotherapy, it was not possible to assess the influences of the ALBI score on adherence to regimens and/or duration of chemotherapy. However, we believe that this limitation did not affect the results, since no difference in the frequency of patients treated with chemotherapy between low and high-ALBI groups was seen in our cohort. Also, the lack of a predefined cutoff value limits the comparison between studies. Instead, some studies utilized ALBI cutoff value set for patients with HCC, and maybe this is the reason that some results are different from ours. Nonetheless, as strengths, we included a homogeneous cohort consisted by patients who received curative surgery, minimizing the risk of impaired liver function due to the extension of the disease. To our knowledge, this is the first study to assess the prognostic impact of ALBI score in Western GC patients in the real-world setting, treated at a single referral center. Accordingly, our findings should be validated in other series of cases, and a larger scale multicentric validation study to confirm the relationship between ALBI score in GC is warranted.
Preoperative ALBI scores were able to predict short- and long-term outcomes in patients with GC who received curative treatment. High-ALBI patients had poor clinical conditions and worse outcomes compared to those with low-ALBI. Also, the ALBI status allowed the risk stratification of patients within the same pTNM stage, and was an independent risk factor associated with survival. Thus, it is a simple and reproducible parameter, which may serve as an additional prognostic factor for GC.
Albumin-bilirubin (ALBI) score is an indicator of liver dysfunction and is useful for predicting prognosis of hepatocellular carcinomas. Currently, this liver function index has been used to predict prognosis in other neoplasms.
The significance of ALBI score in gastric cancer (GC) after radical resection has not been elucidated.
To analyze the significance of ALBI score in GC after curative gastrectomy.
We retrospectively evaluated all GC patients who underwent gastrectomy between 2009 and 2021. ALBI score was calculated as follows: (log10 bilirubin × 0.660) + (albumin × -0.085). The receiver operating characteristic (ROC) curve with area under the curve (AUC) was plotted to evaluate the ability of ALBI score in predicting recurrence or death. Patients were divided into low-ALBI and high-ALBI groups for analysis, based on the optimal cutoff value determined by ROC curve.
A total of 361 patients were included. The AUC for ALBI score was 0.617, and the cutoff value was -2.82. Accordingly, 211 (58.4%) patients were classified as low-ALBI group and 150 (41.6%) as high-ALBI group. Older age, lower hemoglobin level, American Society of Anesthesiologists classification III/IV, and D1 lymphadenectomy were more frequent in the high-ALBI group. There was no difference between both groups in terms of Lauren histological type, depth of tumor invasion (pT), presence of lymph node metastasis (pN), and pathologic stage (pTNM). Major postoperative complication and 30- and 90-d mortality were higher in high-ALBI patients. In survival analysis, the high-ALBI group had worse disease-free survival and overall survival compared to those with low-ALBI. When stratified by pTNM, the survival difference between ALBI groups was maintained in stage I/II and stage III GC. Multivariate analysis demonstrated that high-ALBI was an independent factor associated to worse survival.
The preoperative ALBI score is able to predict the outcomes of patients with GC, where high-ALBI patients have worse prognosis. Also, ALBI score allows risk stratification of patients within the same pTNM stages, and represents an independent risk factor associated with survival.
ALBI score is able to predict short-term and long-term outcomes of patients, and can be applied as a prognostic factor for GC. The ALBI is a simple and reproducible parameter that allows the risk stratification of patients within the pTNM stages, and may be an additional useful tool for decision-making regarding treatment and follow-up individualization. Thus, our findings can be evaluated in other cohorts, and validated in other series of cases study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cesaretti M, Italy; Imai Y, Japan S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Kattan MW, Hess KR, Amin MB, Lu Y, Moons KG, Gershenwald JE, Gimotty PA, Guinney JH, Halabi S, Lazar AJ, Mahar AL, Patel T, Sargent DJ, Weiser MR, Compton C; members of the AJCC Precision Medicine Core. American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA Cancer J Clin. 2016;66:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Jiang Y, Xu D, Song H, Qiu B, Tian D, Li Z, Ji Y, Wang J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis. BMJ Open. 2021;11:e048324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 4. | Szor DJ, Dias AR, Pereira MA, Ramos MFKP, Zilberstein B, Cecconello I, Ribeiro-Júnior U. Prognostic Role of Neutrophil/Lymphocyte Ratio in Resected Gastric Cancer: A Systematic Review and Meta-analysis. Clinics (Sao Paulo). 2018;73:e360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 363] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2006] [Article Influence: 200.6] [Reference Citation Analysis (0)] |
| 7. | Hiraoka A, Kumada T, Kudo M, Hirooka M, Tsuji K, Itobayashi E, Kariyama K, Ishikawa T, Tajiri K, Ochi H, Tada T, Toyoda H, Nouso K, Joko K, Kawasaki H, Hiasa Y, Michitaka K; Real-Life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics). Albumin-Bilirubin (ALBI) Grade as Part of the Evidence-Based Clinical Practice Guideline for HCC of the Japan Society of Hepatology: A Comparison with the Liver Damage and Child-Pugh Classifications. Liver Cancer. 2017;6:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Toyoda H, Johnson PJ. The ALBI score: From liver function in patients with HCC to a general measure of liver function. JHEP Rep. 2022;4:100557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 78] [Reference Citation Analysis (1)] |
| 9. | Lee HG, Lim SB, Lee JL, Kim CW, Yoon YS, Park IJ, Kim JC. Preoperative albumin-bilirubin score as a prognostic indicator in patients with stage III colon cancer. Sci Rep. 2022;12:14910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Aoyama T, Ju M, Machida D, Komori K, Tamagawa H, Tamagawa A, Maezawa Y, Kano K, Hara K, Segami K, Hashimoto I, Nagasawa S, Nakazono M, Oshima T, Yukawa N, Rino Y. Clinical Impact of Preoperative Albumin-Bilirubin Status in Esophageal Cancer Patients Who Receive Curative Treatment. In Vivo. 2022;36:1424-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kinoshita F, Yamashita T, Oku Y, Kosai K, Ono Y, Wakasu S, Haratake N, Toyokawa G, Takenaka T, Tagawa T, Shimokawa M, Nakashima N, Mori M. Prognostic Impact of Albumin-bilirubin (ALBI) Grade on Non-small Lung Cell Carcinoma: A Propensity-score Matched Analysis. Anticancer Res. 2021;41:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Yagyu T, Saito H, Sakamoto T, Uchinaka EI, Morimoto M, Amisaki M, Watanabe J, Tokuyasu N, Honjo S, Ashida K, Fujiwara Y. Preoperative Albumin-Bilirubin Grade as a Useful Prognostic Indicator in Patients With Pancreatic Cancer. Anticancer Res. 2019;39:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Kanda M, Tanaka C, Kobayashi D, Uda H, Inaoka K, Tanaka Y, Hayashi M, Iwata N, Yamada S, Fujii T, Sugimoto H, Murotani K, Fujiwara M, Kodera Y. Preoperative Albumin-Bilirubin Grade Predicts Recurrences After Radical Gastrectomy in Patients with pT2-4 Gastric Cancer. World J Surg. 2018;42:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Zhu C, Wang X, Chen S, Yang X, Sun J, Pan B, Zhang W, Chen X, Huang Y. Efficacy of the Preoperative Albumin-Bilirubin Grade for Predicting Survival and Outcomes of Postoperative Chemotherapy for Advanced Gastric Cancer. Cancer Manag Res. 2020;12:11921-11932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38229] [Article Influence: 1006.0] [Reference Citation Analysis (0)] |
| 16. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1332] [Article Influence: 333.0] [Reference Citation Analysis (2)] |
| 17. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4396] [Article Influence: 549.5] [Reference Citation Analysis (4)] |
| 18. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24776] [Article Influence: 1179.8] [Reference Citation Analysis (0)] |
| 19. | Tanriverdi O. A discussion of serum albumin level in advanced-stage hepatocellular carcinoma: a medical oncologist's perspective. Med Oncol. 2014;31:282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Bannaga A, Arasaradnam RP. Neutrophil to lymphocyte ratio and albumin bilirubin grade in hepatocellular carcinoma: A systematic review. World J Gastroenterol. 2020;26:5022-5049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (2)] |
| 21. | Ju M, Aoyama T, Komori K, Tamagawa H, Tamagawa A, Maezawa Y, Morita J, Onodera A, Endo K, Hashimoto I, Kano K, Hara K, Cho H, Nakazono M, Segami K, Ishiguro T, Onuma S, Oshima T, Yukawa N, Rino Y. The Albumin-Bilirubin Score Is a Prognostic Factor for Gastric Cancer Patients Who Receive Curative Treatment. Anticancer Res. 2022;42:3929-3935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Matsukane R, Watanabe H, Hata K, Suetsugu K, Tsuji T, Egashira N, Nakanishi Y, Okamoto I, Ieiri I. Prognostic significance of pre-treatment ALBI grade in advanced non-small cell lung cancer receiving immune checkpoint therapy. Sci Rep. 2021;11:15057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Takama T, Okano K, Kondo A, Akamoto S, Fujiwara M, Usuki H, Suzuki Y. Predictors of postoperative complications in elderly and oldest old patients with gastric cancer. Gastric Cancer. 2015;18:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Seyed Khoei N, Wagner KH, Carreras-Torres R, Gunter MJ, Murphy N, Freisling H. Associations between Prediagnostic Circulating Bilirubin Levels and Risk of Gastrointestinal Cancers in the UK Biobank. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Ramos MFKP, Pereira MA, Dias AR, Yagi OK, Zaidan EP, Ribeiro-Júnior U, Zilberstein B, Cecconello I. Surgical outcomes of gastrectomy with D1 Lymph node dissection performed for patients with unfavorable clinical conditions. Eur J Surg Oncol. 2019;45:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Ramos MFKP, de Castria TB, Pereira MA, Dias AR, Antonacio FF, Zilberstein B, Hoff PMG, Ribeiro U Jr, Cecconello I. Return to Intended Oncologic Treatment (RIOT) in Resected Gastric Cancer Patients. J Gastrointest Surg. 2020;24:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Thurnham DI. Interactions between nutrition and immune function: using inflammation biomarkers to interpret micronutrient status. Proc Nutr Soc. 2014;73:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Silva FDA, Pereira MA, Ramos MFKP, Ribeiro-Junior U, Zilberstein B, Cecconello I, Dias AR. Gastrectomy in octogenarians with gastric cancer: is it feasible? Arq Bras Cir Dig. 2021;33:e1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Song GM, Tian X, Liang H, Yi LJ, Zhou JG, Zeng Z, Shuai T, Ou YX, Zhang L, Wang Y. Role of Enteral Immunonutrition in Patients Undergoing Surgery for Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2015;94:e1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 31. | Szor DJ, Roncon Dias A, Pereira MA, Ramos MFKP, Zilberstein B, Cecconello I, Ribeiro U Jr. Neutrophil-lymphocyte ratio is associated with prognosis in patients who underwent potentially curative resection for gastric cancer. J Surg Oncol. 2018;117:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Miwa T, Kanda M, Tanaka C, Kobayashi D, Hayashi M, Yamada S, Nakayama G, Koike M, Kodera Y. Albumin-Bilirubin Score Predicts Tolerability to Adjuvant S-1 Monotherapy after Curative Gastrectomy. J Gastric Cancer. 2019;19:183-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |