Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1093
Peer-review started: January 30, 2023
First decision: March 13, 2023
Revised: March 15, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: June 27, 2023
Processing time: 136 Days and 9.2 Hours
Preoperative evaluation of frailty is limited to a few surgical procedures. However, the evaluation in Chinese elderly gastric cancer (GC) patients remains blank.
To validate and estimate the prognostic value of the 11-index modified frailty index (mFI-11) for predicting postoperative anastomotic fistula, intensive care unit (ICU) admission, and long-term survival in elderly patients (over 65 years of age) undergoing radical GC.
This study was a retrospective cohort study which included patients who underwent elective gastrectomy with D2 Lymph node dissection between April 1, 2017 and April 1, 2019. The primary outcome was 1-year all-cause mortality. The secondary outcomes were admission to ICU, anastomotic fistula, and 6-mo mortality. Patients were divided into two groups according to the optimal grouping cutoff of 0.27 points from previous studies: High risk of frailty marked as mFI-11High and low risk of frailty marked as mFI-11Low. Survival curves between the two groups were compared, and univariate and multivariate regression analyses were performed to explore the relationship between preoperative frailty and postoperative complications in elderly patients undergoing radical GC. The discrimination ability of the mFI-11, prognostic nutritional index, and tumor-node-metastasis pathological stage to identify adverse postoperative outcomes was assessed by calculating the area under the receiver operating characteristic (ROC) curve.
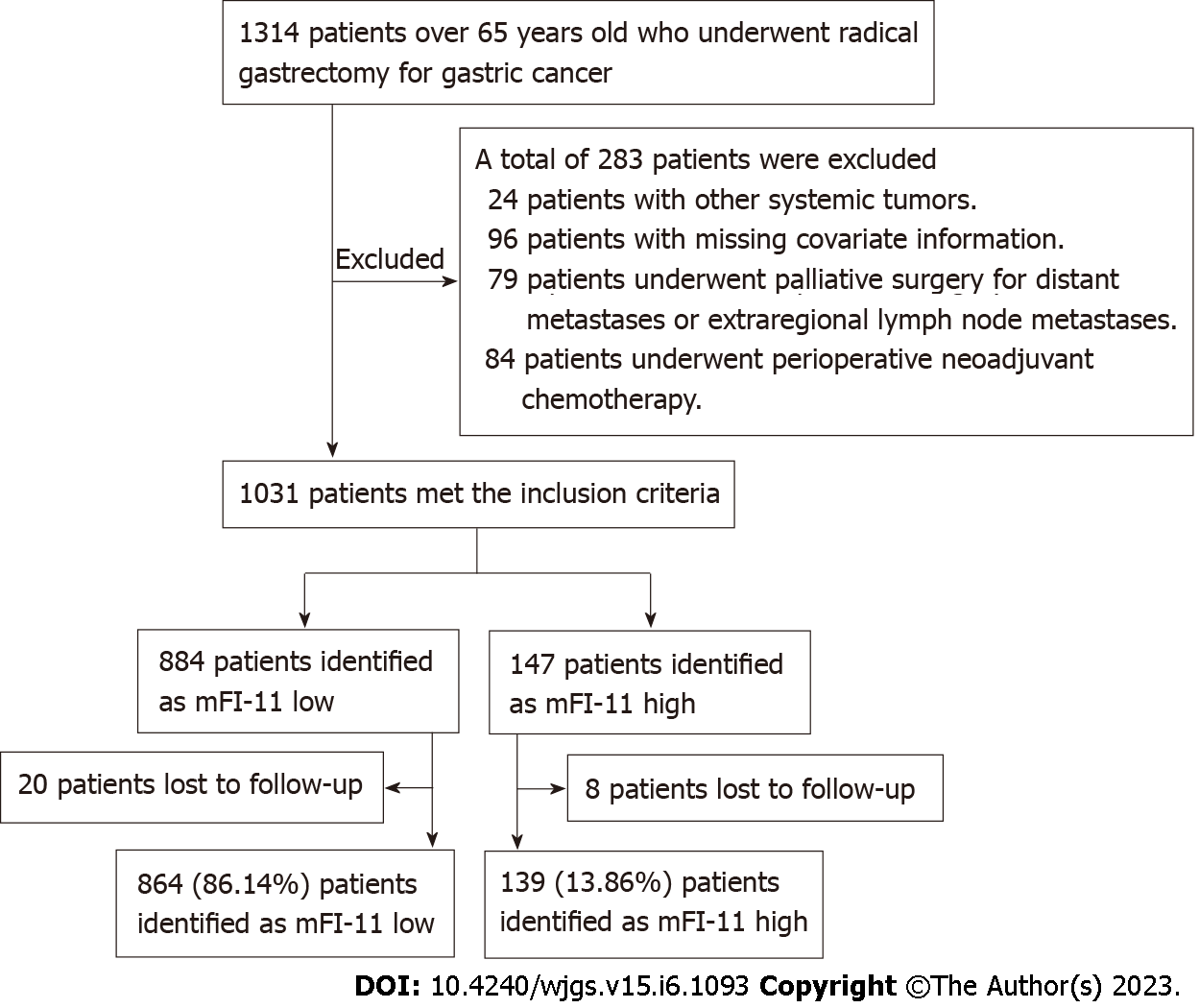
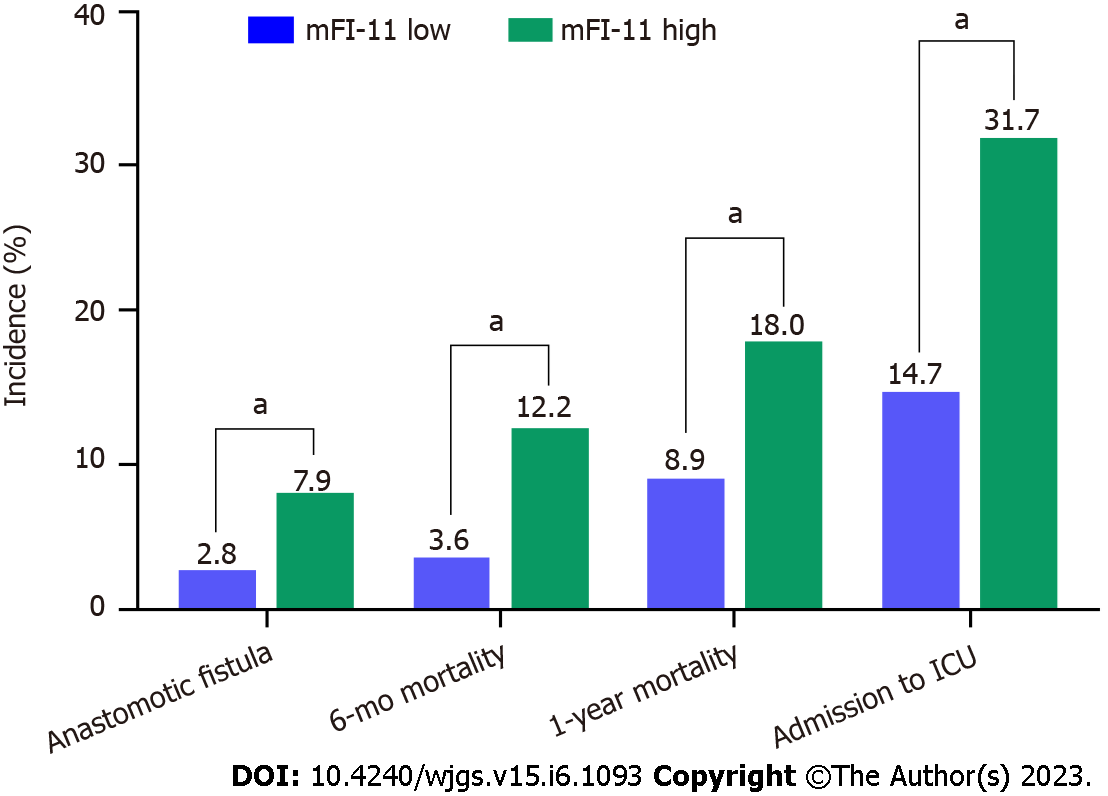
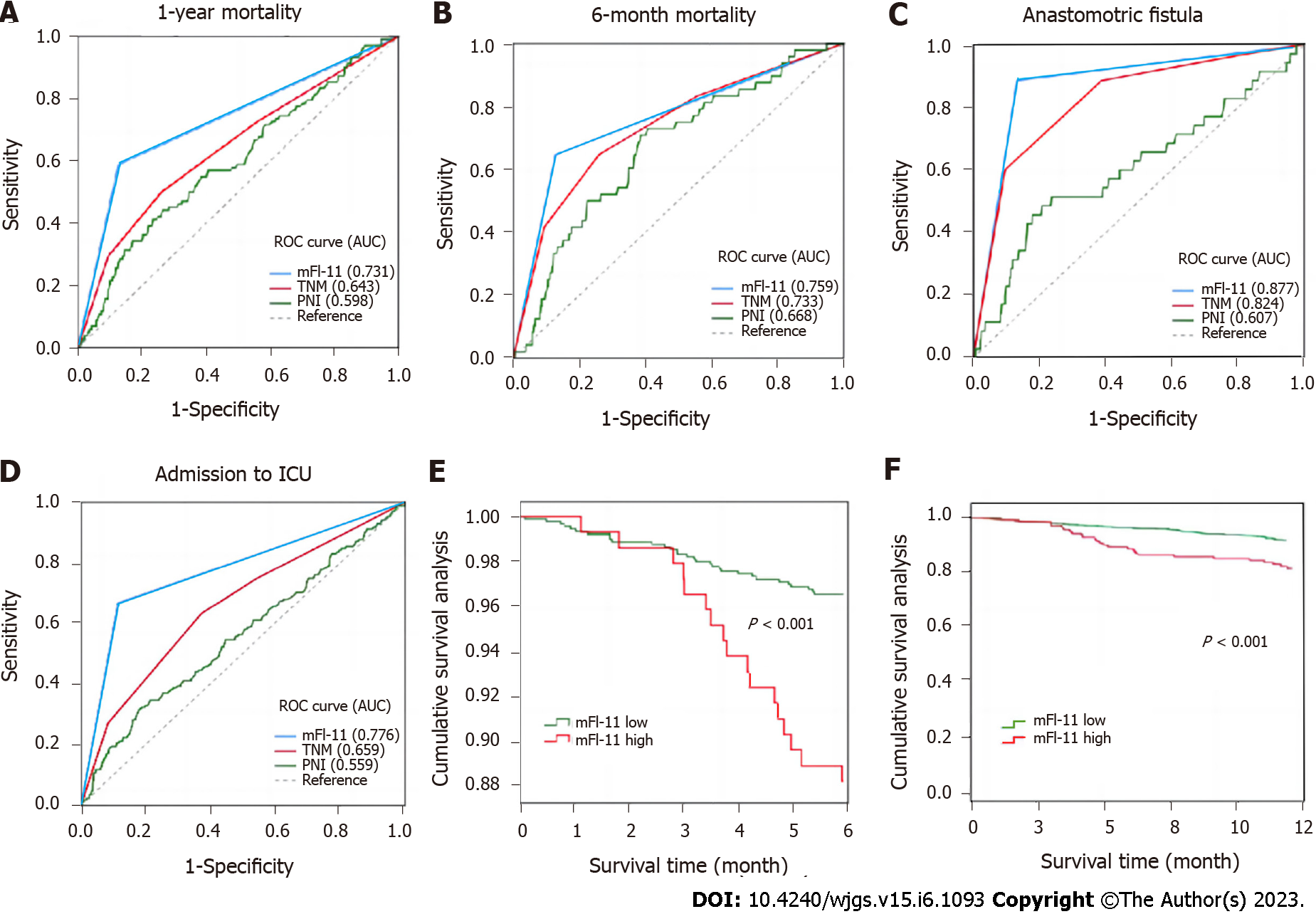
A total of 1003 patients were included, of which 13.86% (139/1003) were defined as having mFI-11High and 86.14% (864/1003) as having mFI-11Low. By comparing the incidence of postoperative complications in the two groups of patients, it was found that mFI-11High patients had higher rates of 1-year postoperative mortality, admission to ICU, anastomotic fistula, and 6-mo mortality than the mFI-11Low group (18.0% vs 8.9%, P = 0.001; 31.7% vs 14.7%, P < 0.001; 7.9% vs 2.8%, P < 0.001; and 12.2% vs 3.6%, P < 0.001). Multivariate analysis revealed mFI-11 as an independent predictive indicator for postoperative outcome [1-year postoperative mortality: Adjusted odds ratio (aOR) = 4.432, 95% confidence interval (95%CI): 2.599-6.343, P = 0.003; admission to ICU: aOR = 2.058, 95%CI: 1.188-3.563, P = 0.010; anastomotic fistula: aOR = 2.852, 95%CI: 1.357-5.994, P = 0.006; 6-mo mortality: aOR = 2.438, 95%CI: 1.075-5.484, P = 0.033]. mFI-11 showed better prognostic efficacy in predicting 1-year postoperative mortality [area under the ROC curve (AUROC): 0.731], admission to ICU (AUROC: 0.776), anastomotic fistula (AUROC: 0.877), and 6-mo mortality (AUROC: 0.759).
Frailty as measured by mFI-11 could provide prognostic information for 1-year postoperative mortality, admission to ICU, anastomotic fistula, and 6-mo mortality in patients over 65 years old undergoing radical GC.
Core Tip: Frailty is becoming an increasingly established risk factor for adverse postoperative outcomes. Given the innately high morbidity involved in radical gastric cancer and the propensity for comorbidities among this patient population, we sought to validate and estimate the prognostic value of the 11-index modified frailty index (mFI-11) in the postoperative period and long-term survival of those patients. The mFI-11 has proven to be a potential exponential tool that can easily stratify patients, predict long-term outcomes, and add value to future treatments.
- Citation: Xu ZY, Hao XY, Wu D, Song QY, Wang XX. Prognostic value of 11-factor modified frailty index in postoperative adverse outcomes of elderly gastric cancer patients in China. World J Gastrointest Surg 2023; 15(6): 1093-1103
- URL: https://www.wjgnet.com/1948-9366/full/v15/i6/1093.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i6.1093
Globally, gastric cancer (GC) is one of the most common cancers, accounting for more than 1 million cases a year, or 7 percent of all cancer diagnoses[1]. With the development of social aging, there is an increasing trend of patients with GC over the age of 65, and most of them are in the middle or late stages of diagnosis because of the hidden nature of GC[2]. As a general rule, gastrectomy + D2 lymph node dissection is the primary surgical procedure for advanced GC, which has been accepted in many countries[3]. Current perioperative management strategies are maturing; however, serious complications may still occur after radical resection of GC, affecting quality of life, tolerability, and outcome of subsequent management[4]. Thus, preoperative risk assessment and post-cancer symptom management in older patients remains critical.
Numerous studies have shown the predictive role of some indicators regarding postoperative complications, including tumor-node-metastasis (TNM) pathological stage and prognostic nutritional index (PNI)[5-7]. However, these indicators lack the ability to measure the physiological reserve of patients, so this paper introduces the concept of frailty in order to provide references for comprehensive preoperative assessment and risk stratification. Frailty is a complex clinical syndrome characterized by reduced physical strength, reduced metabolic and cognitive function, reduced resistance to adverse events, and reduced ability to deal with surgical blows[8]. Moreover, frailty has been investigated as a valuable predictor of adverse health events and poor postoperative outcomes in patients undergoing surgery. Frailty index (FI) is one of the tools for quantifying the degree of frailty in the clinic, and Velanovich and his colleagues summarized the frailty index with 11 variables, known as the 11-index modified frailty index (mFI-11)[9]. Previous studies have confirmed that frailty is an independent risk factor for perioperative complications in elderly patients. The more frailty the patient, the higher the incidence of postoperative adverse outcomes[10].
At this stage, preoperative evaluation of weakness is limited to a few surgical procedures such as arthroplasty, colorectal cancer, and urological tumors[11,12]. However, the evaluation of preoperative frailty in Chinese elderly GC patients remains blank. The purpose of this study was to evaluate the efficacy of mFI-11 applications in predicting adverse outcomes after radical GC surgery in elderly patients in China, and compare the efficacy of mFI-11, TNM stage, and PNI in predicting adverse outcomes after surgery.
Medical records and clinicopathologic data of patients aged 65 years and older who underwent radical GC surgery were retrospectively studied at the Department of Gastrointestinal Surgery of the First Medical Center of the People’s Liberation Army General Hospital (Beijing) between April 1, 2017 and April 1, 2019. Research design and data analysis were approved by the Committee of Medical Research Ethics (Approval No. S2021-342-01). The same committee waived the requirement of written informed consent for participation. The inclusion criteria were: (1) Patients over the age of 65 admitted to the study unit; (2) All patients had histologic confirmation of GC and underwent radical gastrectomy with D2 lymph node dissection; and (3) Patients and their families agreed to provide long-term follow-up information. The exclusion criteria were: (1) Patients had other systemic tumor diseases; (2) Patients who had missing covariate data or follow-up; (3) Patients underwent palliative surgery for distant metastasis and extraregional lymph node metastasis; and (4) Patients received perioperative neoadjuvant chemotherapy. Figure 1 shows the flow chart of this study. The main characteristics of 1003 people included in this study are summarized in Table 1.
| Variable | mFILow (n = 864) | mFIHigh (n = 139) | P value |
| Age (yr) | 0.0392 | ||
| 65-75 | 686 (79.4) | 102 (73.4) | |
| ≥ 75 | 178 (20.6) | 37 (26.6) | |
| Gender, male, n (%) | 656 (75.9) | 109 (78.4) | 0.5222 |
| BMI (kg/m2) | 23.47 ± 3.49 | 24.65 ± 2.97 | 0.0811 |
| Smokers, n (%) | 300 (34.7) | 54 (38.8) | 0.3452 |
| Drinkers, n (%) | 51 (5.9) | 18 (12.9) | 0.0022 |
| Serum albumin (g/L) | 36.05 ± 4.29 | 27.85 ± 4.56 | < 0.0011 |
| Lymphocytes (× 109/L) | 0.20 ± 0.11 | 0.19 ± 0.11 | 0.5271 |
| PNI | 37.03 ± 4.31 | 28.83 ± 4.58 | < 0.0011 |
| TNM stage, n (%) | < 0.0012 | ||
| I | 159 (18.4) | 12 (8.6) | |
| II | 405 (46.9) | 30 (21.6) | |
| II | 237 (27.4) | 61 (43.9) | |
| IV | 63 (7.3) | 36 (25.9) | |
| ASA physical stage, n (%) | < 0.0012 | ||
| I + II | 706 (81.71) | 78 (56.12) | |
| III + IV | 158 (18.29) | 61 (43.88) | |
| Gastrectomy, n (%) | 0.3612 | ||
| DG | 379 (43.9) | 52 (37.4) | |
| PG | 117 (13.5) | 21 (15.1) | |
| TG | 368 (42.6) | 66 (47.5) | |
| Surgical approach, n (%) | 0.0352 | ||
| Open | 85 (9.8) | 15 (10.8) | |
| Robotic surgery | 92 (10.6) | 25 (18.0) | |
| Laparoscopy | 687 (79.5) | 99 (71.2) | |
| Surgery duration (min) | 202.40 ± 63.07 | 214.02 ± 62.00 | 0.0411 |
| Diabetes mellitus | 111 (12.8) | 78 (56.1) | < 0.0012 |
| Myocardial infarction | 10 (1.2) | 18 (12.9) | < 0.0012 |
| Cardiac problems | 52 (6.0) | 27 (19.4) | < 0.0012 |
| Congestive heart failure | 1 (0.1) | 3 (2.2) | < 0.0012 |
| Cerebrovascular problems | 101 (11.7) | 44 (31.7) | < 0.0012 |
| Stroke | 1 (0.1) | 6 (4.3) | < 0.0012 |
| Decreased peripheral pulses | 21 (2.4) | 29 (20.9) | < 0.0012 |
| Respiratory problems | 12 (1.4) | 20 (14.4) | < 0.0012 |
| Non-independent functional status | 38 (4.4) | 27 (19.4) | < 0.0012 |
| Clouding or delirium | 43 (5.0) | 32 (23.0) | < 0.0012 |
| Arterial hypertension | 235 (27.2) | 119 (85.6) | < 0.0012 |
| Outcomes | |||
| 1-year mortality | 77 (8.9) | 25 (18.0) | 0.0012 |
| Admission to ICU | 127 (14.7) | 44 (31.7) | < 0.0012 |
| Anastomotic fistula | 24 (2.8) | 11 (7.9) | < 0.0012 |
| 6-mo mortality | 31 (3.6) | 17 (12.2) | < 0.0012 |
Data were obtained from electronic medical record systems using SQL server (Microsoft, United States). Demographic data were extracted from the Integrated Patient Records Management System (PRIDE 2.1.2.193, Heren Health, China), including age, sex, body mass index, hypertension, diabetes, cardiovascular and lung diseases, cerebrovascular diseases, delirium, independent functional status, and American Society of Anesthesiologists physical score (ASA PS). A non-independent functional state was defined when a patient was unable to perform basic life care alone prior to surgery, such as washing clothes, eating, simply exercising physically, or requiring a full-time escort from a family member, as noted in the care record. Laboratory indicators include serum albumin and lymphocytes. From the anesthesia information management systems (DoCare 3.1.0 build 153, MEDICALSYSTEM, China), intraoperative data were retrieved, including surgical procedures, duration of surgery, ASA PS, TNM stage, and pathologic types of GC. Primary outcome was 1-year of all-cause mortality. Secondary outcomes were 6-mo mortality, anastomotic fistula, and admission to intensive care unit (ICU).
We selected mFI-11, TNM stage, and PNI to predict adverse outcomes after radical GC resection in elderly patients, and compared the prognostic value of all three. Initially, because the FI scale contained more than 70 variables, which led to poor clinical outreach, we developed mFI-11 that mapped 70 variables from the original FI to 11 preexisting variables in the National Surgery Quality Improvement Program (NSQIP) database[13]. The 11 variables that were used to calculate the mFI-11 were functional status, history of diabetes, respiratory problems, congestive heart failure, myocardial infarction, cardiac problems, arterial hypertension, delirium, history related to cognitive impairment or loss, cerebrovascular problems, and history of stroke/decreased peripheral pulses[14]. Details of specific variables that match these factors are defined in Supplementary Table 1. mFI-11 score was calculated by dividing the number of positive variables in the patient by the number of total variables (11). Scores range from 0 to 1. High-risk frailty(mFI-11High) was defined when the mFI-11 score was ≥ 0.27 and low-risk frailty (mFI-11Low) was defined when the score was less than 0.27. PNI was calculated as 10 × peripheral serum protein (g/L) + 0.005 × peripheral blood lymphocyte count (mm3)[15]. PNI is a commonly used indicator to evaluate the nutritional and immune status of patients, which can predict the surgical risk and postoperative complications.
If the continuous data are normally distributed, they are shown as the mean ± SD, otherwise they are shown as median and interquartile range (IQR). The categorical data are presented as proportions. Categorical data are reported as frequencies and percentages and compared using the χ2 test or Fisher’s exact test as appropriate. Univariate and multivariate logistic regression analyses were performed to determine independent risk factors for postoperative mortality, anastomotic fistula, and admission to ICU. Receiver operating characteristic (ROC) curve analysis was used to evaluate the efficacy of different variables in predicting postoperative mortality, anastomotic fistula, and admission to ICU. The mFI-11High group and mFI-11Low group were compared using Kaplan-Meier (K-M) survival curves. A P value inferior to 0.05 was set to reach significance. Data analyses were performed using the SPSS software (version 26.0, IBM, Armonk, NY, United States).
A total of 1003 patients were included, of which 13.86% (139/1003) were defined as having mFI-11High and 86.14% (864/1003) as having mFI-11Low. Figure 2 compares the incidence of postoperative ICU admission, anastomotic fistula, death at 6 mo, and death at 1 year in both groups. By comparing the incidence of postoperative complications in the two groups of patients, it was found that mFI-11High patients had higher rates of 1-year postoperative mortality, admission to ICU, anastomotic fistula, and 6-mo mortality than the mFI-11Low group (18.0% vs 8.9%, P = 0.001; 31.7% vs 14.7%, P < 0.001; 7.9% vs 2.8%, P < 0.001; 12.2% vs 3.6%, P < 0.001).
Figure 3 shows the prognostic value of mFI-11, TNM stage, and PNI for postoperative adverse outcomes. In comparison to the other two measures, the mFI-11 scale showed the best predictive value with regard to the area under the curve. In predicting 1-year mortality after surgery, mFI-11 had the highest area under the curve (0.731), followed by TNM stage (0.643), and the lowest was PNI (0.598). In predicting 6-mo mortality after surgery, mFI-11 had the highest area under the curve (0.759), followed by TNM stage (0.733), and the lowest was PNI (0.668). In terms of admission to ICU after surgery, mFI-11 also had the highest area under the curve (0.776), followed by TNM stage (0.659), and the lowest was PNI (0.559). In predicting anastomotic fistula after surgery, mFI-11 still had the highest area under the curve (0.877), followed by TNM stage (0.824), and the lowest was PNI (0.607). Figure 3 also shows the K-M survival curves at 6 mo and 1 year after surgery between mFI-11High and mFI-11Low patients, and there was a significant difference between them (P < 0.001). Supplementary Table 2 shows the area under the curve values of different variables in predicting admission to ICU, anastomotic fistula, 6-mo mortality, and 1-year mortality.
Table 2 shows multivariate logistic regression analysis of adverse outcomes in elderly patients with GC after radical treatment. Multivariate analysis revealed mFI-11 as an independent predictive indicator for postoperative outcomes (1-year postoperative mortality: Adjusted odds ratio (aOR) = 4.432, 95% confidence interval (95%CI): 2.599-6.343, P = 0.003; admission to ICU: aOR = 2.058, 95%CI: 1.188-3.563, P = 0.010; anastomotic fistula: aOR = 2.852, 95%CI: 1.357-5.994, P = 0.006; 6-mo mortality: aOR = 2.438, 95%CI: 1.075-5.484, P = 0.033. Multivariate analysis also revealed TNM stage and PNI as independent predictive indicators for 1-year postoperative mortality (TNM stage III vs I: aOR = 1.423, 95%CI: 1.004-3.453, P = 0.005; TNM stage IV vs I: aOR = 2.422, 95%CI: 1.524-5.292, P = 0.032; PNI: aOR = 0.925, 95%CI: 0.902-0.964, P = 0.021).
| Variable | 1-year mortality | 6-mo mortality | Anastomotic fistula | Admission to ICU | ||||||||
| B | OR (95%CI) | P value | B | OR (95%CI) | P value | B | OR (95%CI) | P value | B | OR (95%CI) | P value | |
| Age, > 75 yr vs 65-75 yr | 0.883 | 2.418 (1.202-4.865) | 0.013 | 0.914 | 2.495 (1.723-3.613) | < 0.001 | ||||||
| Serum albumin, g/L | -0.532 | 0.923 (0.900-0.954) | 0.023 | 0.013 | 0.936 (0.325-0.999) | 0.002 | -0.881 | 0.907 (0.484-1.696) | 0.759 | -0.018 | 0.718 (0.439-0.909) | 0.012 |
| PNI | -0.251 | 0.925 (0.902-0.964) | 0.021 | 0.041 | 0.932 (0.554-0.942) | 0.014 | 0.062 | 0.567 (0.214-1.481) | 0.846 | -0.019 | 0.719 (0.438-0.902) | 0.041 |
| TNM stage, III vs I | 0.324 | 1.423 (1.004-3.453) | 0.005 | 0.365 | 1.122 (0.798-2.525) | 0.424 | ||||||
| TNM stage, IV vs I | 0.683 | 2.422 (1.524-5.292) | 0.032 | 0.415 | 1.041 (0.698-1.464) | 0.221 | 0.345 | 1.356 (1.008-4.637) | 0.031 | |||
| ASA grade, II vs I | 1.134 | 1.412 (1.053-2.637) | 0.042 | 0.643 | 1.001 (0.888-2.642) | 0.471 | 0.980 | 1.643 (0.463-1.976) | 0.318 | |||
| ASA grade, III vs I | 1.124 | 2.577 (1.656-3.487) | 0.011 | 0.214 | 1.533 (1.213-4.743) | 0.003 | 0.506 | 2.344 (1.796-4.785) | 0.022 | |||
| ASA grade, IV vs I | 1.412 | 1.456 (1.077-3.747) | 0.041 | 0.602 | 2.865 (1.092-3.853) | 0.018 | ||||||
| Gastrectomy, PG vs TG | 0.671 | 0.312 (0.111-1.764) | 0.357 | |||||||||
| mFI-11Highvs mFI-11Low | 0.931 | 4.432 (2.599-6.343) | 0.003 | 0.887 | 2.438 (1.075-5.484) | 0.033 | 1.048 | 2.852 (1.357-5.994) | 0.006 | 0.722 | 2.058 (1.188-3.563) | 0.010 |
Supplementary Tables 3-6 show univariate and multivariate logistic regression analyses of 1-year mortality, 6-mo mortality, anastomotic fistula, and admission to ICU, respectively.
To the best of our knowledge, this is the first study in China to demonstrate the relationship between preoperative frailty conditions and postoperative adverse outcomes (admission to ICU, anastomotic fistula, and 6-mo mortality) in patients over 65 years of age undergoing radical GC surgery. Similarly, for the first time, we compared the prognostic value of frailty (mFI-11), TNM stage, and PNI in postoperative outcomes in elderly GC patients. After comparing the prognostic value of mFI-11, TNM stage and PNI for the three postoperative adverse outcomes, we found that mFI-11 had the best prognostic value. It was also proved that frailty condition was an independent risk factor for the postoperative adverse outcomes, which provides some reference for clinicians to intervene in frailty condition during the perioperative period.
Radical surgery for GC is one of the best treatment methods for GC patients. However, as a kind of operation which causes great trauma to the body, radical surgery causes many adverse outcomes such as entering ICU, anastomotic fistula, and death[16]. Therefore, preoperative risk assessment is particularly important. In response to interest in accurate risk stratification, the surgical community has largely shifted from assessments based on subjective clinical judgment, such as the ASA classification, to more objective analytical approaches, including mFI-11[17]. Similarly, we sought to investigate the predictive capability of mFI-11 in a cohort of 1003 patients undergoing radical GC surgery. In our study, both the univariate and the multivariate analyses indicated that the mFI-11High was an independent risk factor for postoperative complications. Alternatively, we found that mFI-11 had a better ability to identify high-risk patients and to predict postoperative outcomes when compared to TNM stage and PNI.
In this study, TNM stage was an independent risk factor for postoperative complications of GC. However, cancer is a systemic disease whose prognosis is not only dependent on the tumor itself, but also on the underlying physical condition as well as the physiological reserve. PNI served as a representative parameter of patient nutritional status in this study, and it has been used as a surrogate indicator of nutritional status in various neoplastic diseases. Different from other tumor patients undergoing surgery, GC patients often have loss of appetite and reduced oral food intake, and even some patients need parenteral nutrition support before surgery[18]. In this study, PNI was an independent risk factor for postoperative complications of GC. The deteriorating nutritional status may lead to a poor prognosis, and improving the nutritional status of patients with low preoperative PNI improves outcomes in the perioperative treatment of GC patients[19]. However, the simple use of nutritional status indicators was not included in the physiological reserve, so as expected, this study found that mFI-11 was better and more effective than PNI in terms of predicting 6-mo postoperative mortality, 1-year mortality, postoperative ICU admission, and the incidence of anastomotic fistula.
Frailty is becoming an increasingly established risk factor for adverse postoperative outcomes. Our results are consistent with previous studies in predicting adverse outcomes with perioperative frailty assessment (mFI-11)[20]. Jung et al[21] also found that mFI-11 scores in patients with lumbar lateral fusion were associated with urinary complications. The study conducted by Harris et al[22] found that frailty risk scores predicted morbidity and mortality in patients following selective endovascular repair of a reduced thoracic aortic aneurysm. In a previous study by Joseph et al[23], they also demonstrated that frailty as measured by mFI-11 was an accurate predictor of morbidity and mortality in patients undergoing complex abdominal wall reconstruction. Shi et al[24] found that mFI-11 was linked to complications and mortality in hip replacement patients.
The mFI-11 scale might be a useful tool for evidence-based decisions, providing proper patient management, and it is a sensitive tool to stratify and predict patients’ long-term outcomes. Additionally, it provides a promising opportunity for more comprehensive and systematic preoperative risk assessment. This study should serve as a stimulus to further research in order to understand the importance and therapeutic value of frailty. Preoperative frailty condition identified by the mFI-11 scale could be used for clinical risk stratification to improve preoperative evaluation in elderly GC population. In contrast, identification of greater risks may lead to management changes, prompt consideration of close observation, and/or reducing the threshold for intervention. Radical GC surgery is a complex procedure that requires detailed preoperative risk assessment to reduce patient risk and optimize patient benefit and resource utilization[25].
This study has several important limitations. First, this study was a single-center retrospective study. The study center is conducting a large, multicenter, prospective, frailty-scale evaluation study to validate the value of frailty in predicting adverse postoperative outcomes. Second, the study population was elderly patients with elective radical GC, so the study results cannot be directly generalized to the entire surgical population. Third, despite adjustment for potential confounders, there may be other variables not considered, such as tumor size, so we must acknowledge the effect of unmeasured confounders.
In summary, high risk of frailty assessed by mFI-11 based on medical record data has been confirmed to be significantly associated with anastomotic fistula, mortality, and ICU admission after radical GC surgery in elderly patients in China. Preoperative evaluation of frailty may provide useful prognostic information for elderly patients undergoing radical GC surgery. This simple risk score tool may enable improved risk assessment and patient selection prior to elective radical GC surgery.
Preoperative evaluation of frailty is limited to a few surgical procedures. However, the evaluation in Chinese elderly gastric cancer (GC) patients remains blank.
To validate and estimate the prognostic value of the 11-index modified frailty index (mFI-11) for postoperative anastomotic fistula, intensive care unit (ICU) admission, and long-term survival in elderly patients over 65 years of age undergoing radical GC.
To explore the feasibility of mFI-11 in predicting adverse outcomes after radical GC resection in elderly patients.
A retrospective cohort study was conducted on patients over 65 years of age who received curative gastrectomy with D2 Lymph node dissection for GC. The primary outcome was 1-year all-cause mortality. The secondary outcomes were admission to ICU, anastomotic fistula, and 6-mo mortality. Survival curves between the two groups were compared, and univariate and multivariate regression analyses were performed to explore the relationship between preoperative frailty and postoperative complications in elderly patients undergoing radical GC.
A total of 1003 patients were included, of which 13.86% (139/1003) were defined as having mFI-11High and 86.14% (864/1003) as having mFI-11Low. mFI-11High patients had higher rates of 1-year mortality, 6-mo mortality, anastomotic fistula, and admission to ICU than the mFI-11Low group. Multivariate analysis revealed mFI-11 as an independent predictive indicator for 1-year postoperative mortality, 6-mo mortality, anastomotic fistula, and admission to ICU. mFI-11 showed better prognostic efficacy in predicting 1-year postoperative mortality [area under the ROC curve (AUROC): 0.731], 6-mo mortality (AUROC: 0.759), anastomotic fistula (AUROC: 0.877), and admission to ICU (AUROC: 0.776).
Frailty as measured by mFI-11 could provide prognostic information for 1-year postoperative mortality, admission to ICU, anastomotic fistula, and 6-mo mortality in patients over 65 years old undergoing radical GC.
Well-designed multi-center prospective randomized controlled studies are still needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Kawabata H, Japan; Mishra TS, India; Mynster T, Denmark; Wang SY, Taiwan S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Wu RR
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2838] [Article Influence: 567.6] [Reference Citation Analysis (5)] |
| 2. | Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw. 2010;8:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H; Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1069] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 4. | Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, Park SR, Fujii M, Kang YK, Chen LT. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535-e547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 5. | Zhang Q, Qian L, Liu T, Ding JS, Zhang X, Song MM, Wang ZW, Ge YZ, Hu CL, Li XR, Tang M, Wang KH, Barazzoni R, Song CH, Xu HX, Shi HP; Investigation on Nutrition Status and Its Clinical Outcome of Common Cancers (INSCOC) Group. Prevalence and Prognostic Value of Malnutrition Among Elderly Cancer Patients Using Three Scoring Systems. Front Nutr. 2021;8:738550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Sim JH, Kim SH, Jun IG, Kang SJ, Kim B, Kim S, Song JG. The Association between Prognostic Nutritional Index (PNI) and Intraoperative Transfusion in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma: A Retrospective Cohort Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Newman M, Dziegielewski PT, Nguyen NTA, Seikaly HS, Xie M, O'Connell DA, Harris JR, Biron VL, Gupta MK, Archibald SD, Jackson BS, Young JEM, Keyes KJ, Nichols DS, Zhang H. Relationship of depth of invasion to survival outcomes and patterns of recurrence for T3 oral tongue squamous cell carcinoma. Oral Oncol. 2021;116:105195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 1779] [Article Influence: 296.5] [Reference Citation Analysis (0)] |
| 9. | Araújo-Andrade L, Rocha-Neves JP, Duarte-Gamas L, Pereira-Neves A, Ribeiro H, Pereira-Macedo J, Dias-Neto M, Teixeira J, Andrade JP. Prognostic effect of the new 5-factor modified frailty index in patients undergoing carotid endarterectomy with regional anesthesia - A prospective cohort study. Int J Surg. 2020;80:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 967] [Article Influence: 161.2] [Reference Citation Analysis (0)] |
| 11. | Keller DS, Reif de Paula T, Kiran RP, Nemeth SK. Evaluating the association of the new National Surgical Quality Improvement Program modified 5-factor frailty index with outcomes in elective colorectal surgery. Colorectal Dis. 2020;22:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Palumbo C, Knipper S, Pecoraro A, Rosiello G, Luzzago S, Deuker M, Tian Z, Shariat SF, Simeone C, Briganti A, Saad F, Berruti A, Antonelli A, Karakiewicz PI. Patient frailty predicts worse perioperative outcomes and higher cost after radical cystectomy. Surg Oncol. 2020;32:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Tan HL, Chia STX, Nadkarni NV, Ang SY, Seow DCC, Wong TH. Frailty and functional decline after emergency abdominal surgery in the elderly: a prospective cohort study. World J Emerg Surg. 2019;14:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Rath D, Chatterjee M, Müller I, Müller K, Böckmann C, Droppa M, Stimpfle F, Karathanos A, Borst O, Seizer P, Langer H, Schwab M, Gawaz M, Geisler T. Platelet expression of transforming growth factor beta 1 is enhanced and associated with cardiovascular prognosis in patients with acute coronary syndrome. Atherosclerosis. 2014;237:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann Surg. 2020;271:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 16. | Lim L, Michael M, Mann GB, Leong T. Adjuvant therapy in gastric cancer. J Clin Oncol. 2005;23:6220-6232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Palliyaguru DL, Moats JM, Di Germanio C, Bernier M, de Cabo R. Frailty index as a biomarker of lifespan and healthspan: Focus on pharmacological interventions. Mech Ageing Dev. 2019;180:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Hyltander A, Bosaeus I, Svedlund J, Liedman B, Hugosson I, Wallengren O, Olsson U, Johnsson E, Kostic S, Henningsson A, Körner U, Lundell L, Lundholm K. Supportive nutrition on recovery of metabolism, nutritional state, health-related quality of life, and exercise capacity after major surgery: a randomized study. Clin Gastroenterol Hepatol. 2005;3:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Santos I, Mendes L, Mansinho H, Santos CA. Nutritional status and functional status of the pancreatic cancer patients and the impact of adjacent symptoms. Clin Nutr. 2021;40:5486-5493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Aceto P, Bassi P, Sollazzi L, Racioppi M, Fortunato G, Di Gianfrancesco L, Marusco I, Ragonese M, Cataldo A, Palermo G. Implementation of frailty preoperative assessment to predict outcome in patients undergoing urological surgery: a systematic review and meta-analysis. BJU Int. 2021;127:507-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Jung JM, Chung CK, Kim CH, Yang SH, Ko YS. The Modified 11-Item Frailty Index and Postoperative Outcomes in Patients Undergoing Lateral Lumbar Interbody Fusion. Spine (Phila Pa 1976). 2022;47:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Harris DG, Olson SL, Panthofer AM, Matsumura JS, DiMusto PD. A Frailty-Based Risk Score Predicts Morbidity and Mortality After Elective Endovascular Repair of Descending Thoracic Aortic Aneurysms. Ann Vasc Surg. 2020;67:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Joseph WJ, Cuccolo NG, Baron ME, Chow I, Beers EH. Frailty predicts morbidity, complications, and mortality in patients undergoing complex abdominal wall reconstruction. Hernia. 2020;24:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Shi SM, Kim DH. The challenges of using the Hospital Frailty Risk Score. Lancet. 2019;392:2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Song KY, Park YG, Jeon HM, Park CH. A nomogram for predicting individual survival of patients with gastric cancer who underwent radical surgery with extended lymph node dissection. Gastric Cancer. 2014;17:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |