Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1080
Peer-review started: January 26, 2023
First decision: March 15, 2023
Revised: April 2, 2023
Accepted: April 23, 2023
Article in press: April 23, 2023
Published online: June 27, 2023
Processing time: 139 Days and 19.7 Hours
For the management of lateral lymph node (LLN) metastasis in patients with rectal cancer, selective LLN dissection (LLND) is gradually being accepted by Chinese scholars. Theoretically, fascia-oriented LLND allows radical tumor resection and protects of organ function. However, there is a lack of studies comparing the efficacy of fascia-oriented and traditional vessel-oriented LLND. Through a preliminary study with a small sample size, we found that fascia-oriented LLND was associated with a lower incidence of postoperative urinary and male sexual dysfunction and a higher number of examined LLNs. In this study, we increased the sample size and refined the postoperative functional outcomes.
To compare the effects of fascia- and vessel-oriented LLND regarding short-term outcomes and prognosis.
We conducted a retrospective cohort study on data from 196 patients with rectal cancer who underwent total mesorectal excision and LLND from July 2014 to August 2021. The short-term outcomes included perioperative outcomes and postoperative functional outcomes. The prognosis was measured based on overall survival (OS) and progression-free survival (PFS).
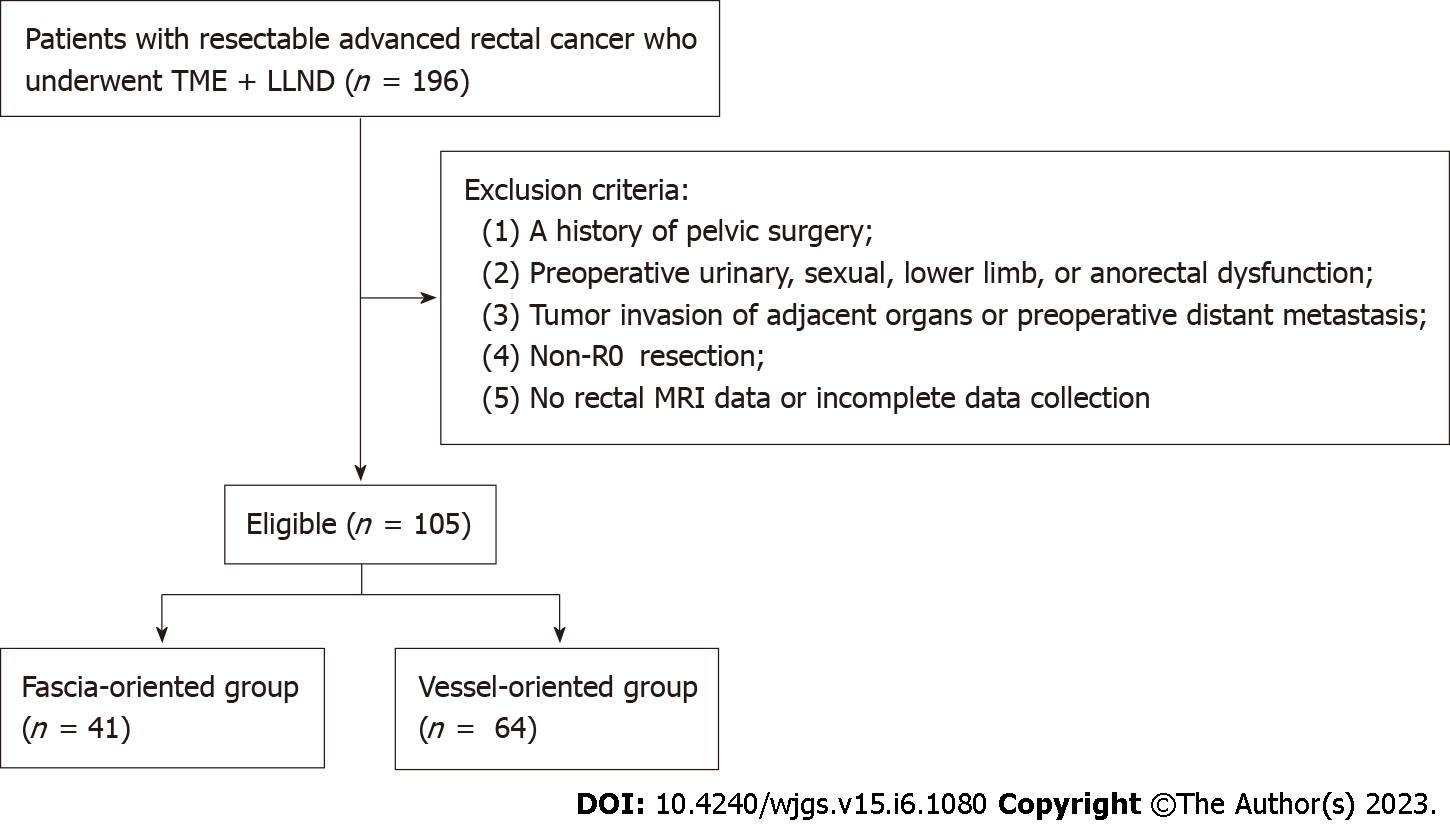
A total of 105 patients were included in the final analysis and were divided into fascia- and vessel-oriented groups that included 41 and 64 patients, respectively. Regarding the short-term outcomes, the median number of examined LLNs was significantly higher in the fascia-oriented group than in the vessel-oriented group. There were no significant differences in the other short-term outcomes. The incidence of postoperative urinary and male sexual dysfunction was significantly lower in the fascia-oriented group than in the vessel-oriented group. In addition, there was no significant difference in the incidence of postoperative lower limb dysfunction between the two groups. In terms of prognosis, there was no significant difference in PFS or OS between the two groups.
It is safe and feasible to perform fascia-oriented LLND. Compared with vessel-oriented LLND, fascia-oriented LLND allows the examination of more LLNs and may better protect postoperative urinary function and male sexual function.
Core Tip: There is a lack of studies comparing the efficacy of fascia-oriented and traditional vessel-oriented lateral lymph node dissection (LLND). To compare the effects of fascia- and vessel-oriented LLND regarding the short-term outcomes and prognosis, we conducted a retrospective cohort study based on seven years of data. We found that it is safe and feasible to perform fascia-oriented LLND. Compared with vessel-oriented LLND, fascia-oriented LLND allows the examination of more lateral lymph nodes and may better protect postoperative urinary and male sexual function.
- Citation: Zhao W, Wang ZJ, Mei SW, Chen JN, Zhou SC, Zhao FQ, Xiao TX, Huang F, Liu Q. Fascia- vs vessel-oriented lateral lymph node dissection for rectal cancer: Short-term outcomes and prognosis in a single-center experience. World J Gastrointest Surg 2023; 15(6): 1080-1092
- URL: https://www.wjgnet.com/1948-9366/full/v15/i6/1080.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i6.1080
Since Gerota first proposed the existence of lateral lymphatic drainage in the rectum in 1895, lateral lymphatic drainage has been proven to be an important lymphatic drainage pathway in the middle and lower rectum. The occurrence of lateral lymph node (LLN) metastasis in newly diagnosed rectal cancer patients ranges from 8.6% to 49%[1-3]. Neoadjuvant chemoradiotherapy (nCRT) and LLN dissection (LLND) are two strategies for the management of LLN metastasis advocated by Western and Japanese scholars, respectively. However, nCRT cannot completely eliminate metastatic tumor cells in LLNs[4]. On the other hand, LLND causes a high incidence of postoperative urinary and sexual dysfunction but has a low postoperative pathological positive LLN rate[3,5]. Therefore, depending on imaging findings in patients with enlarged LLNs, a combination of chemoradiotherapy and selective LLND is gradually being accepted by Chinese scholars[3,4,6,7].
With the expansion of fascial anatomy research, the concept of membrane anatomy-guided surgery has become accepted by surgeons. Theoretically, zoning the lateral space of the rectum and performing LLND guided by the fascia can establish a clear surgical plane and dissection boundary and prevent insufficient and excessive dissection. At the same time, dissociation along the fascial margin can prevent a breach into the lymphoid tissues, preventing the spread of metastatic cancer cells and helping to protect the pelvic autonomic nerves. Therefore, fascia-oriented LLND follows anatomical theory regarding radical tumor resection and protection of organ function and is also conducive to the popularization and quality control of lateral dissection[8]. Although several published studies have demonstrated that fascia-oriented LLND is safe in the perioperative period[9-13], these studies either did not explore the effect of fascia-oriented LLND on postoperative neurological function and prognosis or had relatively small sample sizes. In addition, there is a lack of evidence-based medical studies comparing the efficacy of fascia-oriented and traditional vessel-oriented LLND. Through a preliminary study with a small sample size[14], we found that fascia-oriented LLND was associated with a lower incidence of postoperative urinary and male sexual dysfunction and a higher number of examined LLNs. In this study, we increased the sample size, refined the postoperative functional outcomes, and further analyzed the clinical data from rectal cancer patients undergoing treatment with two different anatomical approaches for LLND at a high-volume center in China to compare their effects on short-term outcomes and prognosis.
In this retrospective cohort study, clinical data from 196 patients with rectal cancer who underwent mesorectal excision with curative intent and simultaneous LLND in the Department of Colorectal Surgery, Cancer Hospital Chinese Academy of Medical Sciences from July 2014 to August 2021 was collected. All patients in this study underwent rectal magnetic resonance imaging (MRI) before neoadjuvant therapy and before surgery. All operations were performed by experienced surgical specialists in colorectal oncology at our center. The surgical approach (fascia-oriented or vessel-oriented LLND) used was determined at the discretion of the individual surgeon.
The patient inclusion criteria were as follows: (1) Pathological diagnosis of rectal cancer; (2) Lower tumor margin below the peritoneal reflection; and (3) Preoperative clinical suspicion or clinical diagnosis of LLN metastasis.
The patient exclusion criteria were as follows: (1) A history of pelvic surgery (including rectal cancer surgery); (2) Preoperative urinary, sexual, lower limb, or anorectal dysfunction; (3) Tumor invasion of adjacent organs or preoperative distant metastasis; (4) Non-R0 resection; and (5) No rectal MRI data or incomplete data collection.
The final analysis comprised 105 patients, divided into two groups: The fascia-oriented group with 41 patients and the vessel-oriented group with 64 patients. Figure 1 shows a flowchart of patient enrollment.
Procedures for fascia-oriented LLND: During fascia-oriented LLND, dissection was performed along the fascia of the three pelvic sidewalls [ureterohypogastric nerve fascia (UNF), vesicohypogastric fascia (VF), and parietal pelvic fascia]. This technique included 4 key steps: First, the lateral side of the UNF was isolated to establish the medial border of No. 263 Lymph node dissection (Supplementary Figures 1 and 2A); second, the fascia covering the muscular surface of the pelvic wall was isolated to establish the lateral border of No. 283 Lymph node dissection (Supplementary Figures 2B and 3); third, the VF was dissociated to reveal the main branches of the internal iliac artery inside the facia according to the orientation of the VF and UNF; fourth, en bloc resection of the No. 263 Lymph node and No. 283 Lymph node was performed. Supplementary Figure 4 shows the intraoperative view after LLND.
Procedures for vessel-oriented LLND: The internal iliac artery and its main branches were exposed through intrathecal dissection. In the obturator region, the lymphatic and fatty tissue around the main internal iliac artery and its main branches were dissected. The obturator nerve was exposed throughout the whole process.
If bilateral LLND was performed, the superior or inferior bladder arteries on one side were preserved as much as possible. To prevent adverse effects from prolonged or improper patient placement in the lithotomy position on the patient's lower limb function to the greatest extent, all surgeries followed the AORN Guidelines for patient positioning[15].
The short-term outcomes included the following two aspects: (1) Perioperative outcomes, including operation time, intraoperative blood loss, incidence of perioperative surgical complications of grade II or higher[16,17], incidence of perioperative mortality, incidence of reoperation, length of postoperative hospital stay, number of examined LLNs, and LLN metastasis rate; and (2) Postoperative functional outcomes, including urinary function, defecation function, male sexual function, and lower limb motor and sensory function. The prognosis was measured based on overall survival (OS) and progression-free survival (PFS).
Postoperative urinary function, defecation function, and male sexual function were assessed according to the International Prostate Symptom Score (IPSS)[18], the low anterior resection syndrome score[19-21], and the International Index of Eerectile Function (IIEF-5)[22,23], respectively. Patients with one of the following symptoms were considered to have postoperative lower limb dysfunction: Gait disorder caused by thigh adductor weakness or movement disorders of the lower limb; loss of sensation, numbness, or radiating pain in the lower limb that was aggravated by extension and abduction or inward rotation of the thigh[24,25]. OS and PFS were defined as follows: OS referred to the duration from the date of surgery until the date of death from any cause, while PFS referred to the duration from the date of surgery until the occurrence of local or regional recurrence, distant metastases, or death from any cause.
The follow-up methods included telephone interviews and outpatient examinations. Regarding postoperative functional outcomes, follow-up regarding urinary function was performed by telephone interviews on the 14th day after the operation, follow-up on motor and sensory function of the lower limbs was performed by physical examination or telephone interviews 1 mo after the operation, and follow-up on male sexual function was performed by telephone interviews 1 year after the operation. The last follow-up date was November 31, 2021.
The median [interquartile range (IQR)] was used to present continuous variables, while numbers and proportions were used to present categorical variables. The Mann-Whitney U test was used to compare continuous variables, and the χ2 or Fisher exact test was used to compare categorical variables.
To assess risk factors for postoperative functional outcomes, univariate logistic regression was conducted on the relevant variables. The multivariate logistic regression analyses included the surgical method, potential confounding factors that could impact postoperative functional outcomes, and any baseline factors that were imbalanced between the two groups. Drawing from previous research and our clinical experience, we posited that several factors, aside from the surgical method, could potentially influence postoperative urinary, male sexual, and lower limb function. Specifically, we hypothesized that intraoperative blood loss and single/bilateral LLND may affect postoperative urinary function[5,26], while age, preoperative radiotherapy, and single/bilateral LLND may impact postoperative male sexual function[27,28]. Lastly, we also considered age and single/bilateral LLND as potential factors that could affect postoperative lower limb function, based on our clinical experience and previous studies[24,25,29].
The survival differences among groups were examined using the Kaplan-Meier method and the log-rank test. The reverse Kaplan-Meier method was used to analyze the median follow-up. Cox proportional hazards regression models were employed to select predictive factors for OS and PFS, and the multivariable Cox proportional hazards models included the surgical method, pathological LLN metastasis, and factors with a P value lower than 0.05 in the univariate analyses to identify independent risk factors for OS and PFS. IBM SPSS statistics software program, version 23 (IBM, Somers, NY, United States) was used to conduct the statistical analysis.
Table 1 presents the clinical and pathological characteristics of the patients. The two groups were comparable in terms of age, BMI, neoadjuvant and adjuvant therapy, laparoscopic surgery, bilateral LLND, and each pathological tumor stage. All clinical and pathological characteristics were well balanced between the two groups.
| Variables | Fascia-oriented group (n = 41) | Vessel-oriented group (n = 64) | P value |
| Age (yr), median (IQR) | 58.0 (48.0, 65.0) | 58.5 (47.0, 65.0) | 0.908 |
| Sex, n (%) | 0.728 | ||
| Male | 21 (51.2) | 35 (54.7) | |
| Female | 20 (48.8) | 29 (45.3) | |
| BMI (kg/m2), median (IQR) | 24.8 (21.6, 27.8) | 24.2 (21.3, 27.5) | 0.510 |
| Distance to tumour from AV (cm), median (IQR) | 4.0 (3.0, 7.0) | 4.0 (3.0, 5.0) | 0.358 |
| Pathological type, n (%) | 0.837 | ||
| Adenocarcinoma | 40 (97.6) | 62 (96.9) | |
| Non-adenocarcinoma | 1 (2.4) | 2 (3.1) | |
| Preoperative radiotherapy, n (%) | 0.356 | ||
| Yes | 10 (24.4) | 21 (32.8) | |
| No | 31 (75.6) | 43 (67.2) | |
| Preoperative chemotherapy, n (%) | 0.698 | ||
| Yes | 17 (41.5) | 29 (45.3) | |
| No | 24 (58.5) | 35 (54.7) | |
| Surgical procedure, n (%) | 0.371 | ||
| Laparoscopic surgery | 40 (97.6) | 60 (93.8) | |
| Conversion to open surgery | 1 (2.4) | 4 (6.2) | |
| Surgical approach, n (%) | 0.571 | ||
| Dixon | 23 (56.1) | 31 (48.4) | |
| Miles | 18 (43.9) | 32 (50.0) | |
| Parks | 0 (0) | 1 (1.6) | |
| LLND, n (%) | 0.137 | ||
| Unilateral dissection | 33 (80.5) | 43 (67.2) | |
| Bilateral dissection | 8 (19.5) | 21 (32.8) | |
| Pathological tumor stagea, n (%) | 0.808 | ||
| 0-I | 6 (14.6) | 10 (15.6) | |
| II | 7 (17.1) | 8 (12.5) | |
| III | 28 (68.3) | 46 (71.9) | |
| Adjuvant therapy, n (%) | 0.544 | ||
| Yes | 32 (78.0) | 53 (82.8) | |
| No | 9 (22.0) | 11 (17.2) |
Perioperative outcomes: The lack of a significant difference was found in operation time and length of postoperative hospital stay between the two groups, with respective P values of 0.908 and 0.435. The vessel-oriented group had a higher proportion of patients with intraoperative blood loss of ≥ 300 mL compared to the fascia-oriented group (9.4% vs 2.4%). Nevertheless, the observed difference was not statistically significant with a P value of 0.242. The fascia- and vessel-oriented groups had incidences of perioperative surgical complications of 9.8% and 7.8%, respectively, and the difference between the two groups was not statistically significant (P = 0.852). There were no cases of reoperation or perioperative death in either group. Table 2 shows that the fascia-oriented group had a significantly higher median number of examined LLNs than the vessel-oriented group (9.0 vs 6.5, P = 0.020). However, there was no significant difference in the positive pathological rate of LLNs between the two groups (22.0% vs 25.0%, P = 0.720).
| Variables | Fascia-oriented group (n = 41) | Vessel-oriented group (n = 64) | P value |
| Operation time (min), median (IQR) | 245.0 (220.0, 270.0) | 269.5 (210.0, 300.0) | 0.908 |
| Blood loss (mL), n (%) | 0.242 | ||
| ≥ 300 | 1 (2.4) | 6 (9.4) | |
| < 300 | 40 (97.6) | 58 (90.6) | |
| No. of examined LLN, median (IQR) | 9.0 (7.0, 13.0) | 6.5 (3.0, 10.3) | 0.020 |
| Pathological LLN, n (%) | 0.720 | ||
| Positive | 9 (22.0) | 16 (25.0) | |
| Negative | 32 (78.0) | 48 (75.0) | |
| Surgical complicationsa, n (%) | 0.852 | ||
| Yes | 4 (9.8) | 5 (7.8) | |
| No | 37 (90.2) | 59 (92.2) | |
| Urinary dysfunction, n (%) | 0.015 | ||
| Yes | 9 (22.0) | 29 (45.3) | |
| No | 32 (78.0) | 35 (54.7) | |
| Male sexual dysfunction, n (%) | 0.019 | ||
| Yes | 9 (42.9) | 26 (74.3) | |
| No | 12 (57.1) | 9 (25.7) | |
| Lower limb dysfunction, n (%) | 0.554 | ||
| Yes | 10 (24.4) | 19 (29.7) | |
| No | 31 (75.6) | 45 (70.3) | |
| Post-operative hospital stay (d), median (IQR) | 7.00 (7.00, 8.00) | 8.00 (7.00, 9.00) | 0.435 |
| Perioperative mortality, n (%) | 0 (0) | 0 (0) | 1.000 |
| Reoperation, n (%) | 0 (0) | 0 (0) | 1.000 |
Postoperative functional outcomes: (1) Urinary function: Among the 105 patients, the incidence of postoperative urinary dysfunction was 36.2%. The rate of postoperative urinary dysfunction was significantly lower in the fascia-oriented group than in the vessel-oriented group (22.0% vs 45.3%, P = 0.015), as shown in Table 2. Multivariate logistic regression analysis, after adjustment for intraoperative blood loss and single/bilateral LLND, showed that vessel-oriented LLND increased the risk of postoperative urinary dysfunction (OR = 2.897, 95%CI = 1.163–7.213, P = 0.022), as shown in Supplementary Table 1;
(2) Male sexual function: Among the patients included in the final analysis, 56 were male, including 21 in the fascia-oriented group and 35 in the vessel-oriented group. The percentage of patients who received unilateral LLND was significantly higher in the fascia-oriented group than in the vessel-oriented group (85.7% vs 65.7%, P = 0.015); other clinical and pathological characteristics were well balanced between the two groups, as shown in Supplementary Table 2.
Among male patients, the incidence of postoperative sexual dysfunction was 62.5%. The incidence of postoperative sexual dysfunction was significantly lower in the fascia-oriented group than in the vessel-oriented group (42.9% vs 74.3%, P = 0.019); additionally, the incidence of sexual dysfunction was significantly lower among patients treated with preoperative radiotherapy than patients not treated with preoperative radiotherapy (41.2% vs 71.8%, P = 0.030). Multivariate logistic regression analyses showed that vessel-oriented LLND increased the risk of postoperative male sexual dysfunction (OR = 5.109, 95%CI = 1.078–24.206, P = 0.040), while preoperative radiotherapy decreased the risk of postoperative male sexual dysfunction (OR = 0.118, 95%CI = 0.024–0.577, P = 0.008), as shown in Supplementary Table 3;
(3) Lower limb function: Among the 105 patients, the incidence of lower limb dysfunction was 27.6%. The incidence of lower limb dysfunction in the fascia- and vessel-oriented groups was 24.4% and 29.7%, respectively. The difference was not statistically significant (P = 0.554), as indicated in Table 2. Multivariate logistic regression analyses showed that vessel-oriented LLND, age ≥ 65 years, and bilateral LLND did not increase the risk of postoperative lower limb dysfunction, as shown in Supplementary Table 4;
And (4) Defecation function: As of the last follow-up, 64 (61.0%) of 105 patients had temporary or permanent enterostomies, including 20 (48.8%) in the fascia-oriented group and 44 (68.7%) in the vessel-oriented group. Since defecation function evaluations were not available for these patients, this study did not compare defecation function between the two groups.
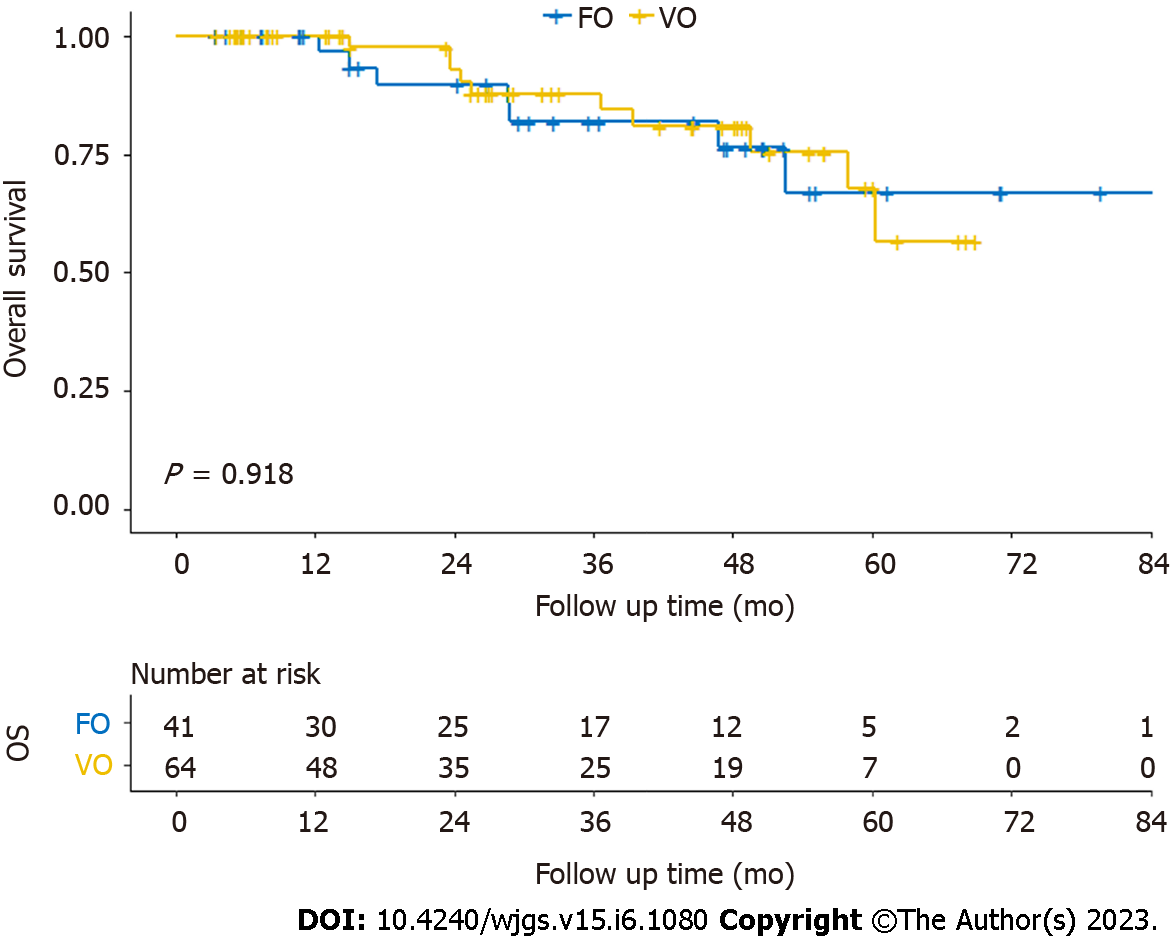
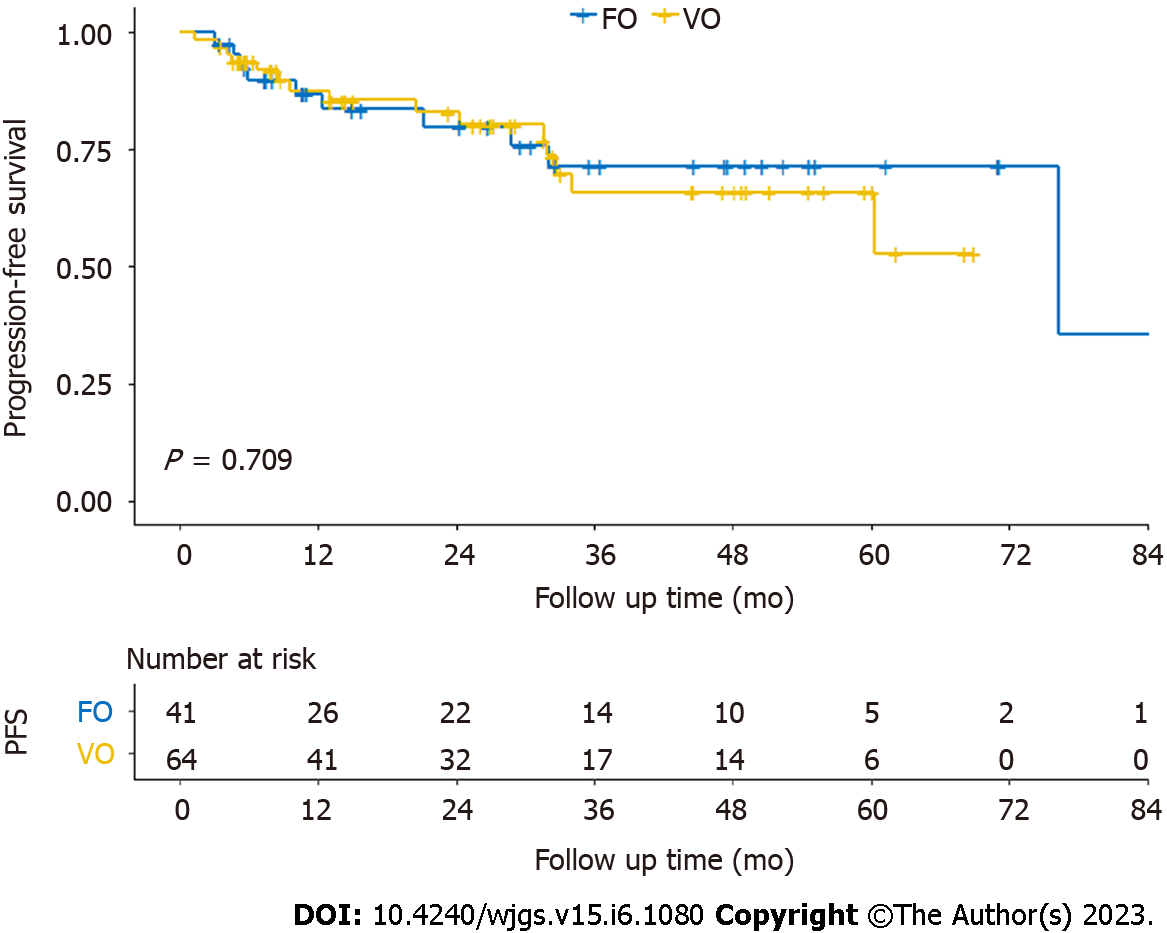
All patients were followed up. The median follow-up time was 32.6 mo. The 2-year OS rate of all 105 patients was 91.6%. The 2-year OS rates in the fascia- and vessel-oriented groups was 89.7% and 92.8%, respectively. Among all 105 patients, the 2-year PFS rate was 81.7%. In the fascia- and vessel-oriented groups, the 2-year PFS rates were 79.8% and 82.9%, respectively.
Kaplan–Meier curves for OS and PFS are shown in Figures 2 and 3. There was no significant difference in OS (log-rank P = 0.918) or PFS (log-rank P = 0.709) on the log-rank test between the fascia- and vessel-oriented groups.
The results of Cox regression analyses for univariate and multivariable are presented in Tables 3 and 4. For OS, univariate Cox regression analysis showed that vessel-oriented LLND, age ≥ 65 years, female sex, pathological LLN metastasis, and postoperative adjuvant therapy did not affect OS; however, pathological stage III disease was a risk factor for poor OS (HR = 9.98, 95%CI = 1.32–75.55, P = 0.026). After adjusting for pathological LLN metastases and pathological tumor stage, the multivariable Cox regression analyses showed that vessel-oriented LLND (HR = 0.94, 95%CI = 0.35–2.48, P = 0.893) and pathological LLN metastases (HR = 1.14, 95%CI = 0.39–3.31, P = 0.807) were not independent risk factors for poor OS, while pathological stage III disease independently increased the risk of poor OS (HR = 9.66, 95%CI = 1.25–74.66, P = 0.030).
| Univariable | Multivariable | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| LLND method | ||||
| Vessel–oriented LLND | Reference | Reference | ||
| Fascia–oriented LLND | 0.95 (0.36–2.50) | 0.918 | 0.94 (0.35–2.48) | 0.893 |
| Age | ||||
| < 65 | Reference | — | ||
| ≥ 65 | 2.56 (0.87–7.51) | 0.088 | — | |
| Sex | ||||
| Male | Reference | — | ||
| Female | 0.78 (0.30–2.06) | 0.621 | — | |
| p/yp tumor stagea | ||||
| 0–II | Reference | Reference | ||
| III | 9.98 (1.32–75.55) | 0.026 | 9.66 (1.25–74.66) | 0.030 |
| Pathological LLN | ||||
| Negative | Reference | Reference | ||
| Positive | 1.82 (0.64–5.18) | 0.264 | 1.14 (0.39–3.31) | 0.807 |
| Adjuvant therapy | ||||
| No | Reference | — | ||
| Yes | — | 0.202 | — | |
| Univariable | Multivariable | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| LLND method | ||||
| Vessel–oriented LLND | Reference | Reference | ||
| Fascia–oriented LLND | 1.17 (0.51 - 2.68) | 0.709 | 1.16 (0.51–2.66) | 0.729 |
| Age | ||||
| < 65 | Reference | — | ||
| ≥ 65 | 1.20 (0.47 - 3.07) | 0.706 | — | |
| Sex | ||||
| Male | Reference | — | ||
| Female | 0.7 (0.31-1.55) | 0.374 | — | |
| p/yp tumor stagea | ||||
| 0–II | Reference | Reference | ||
| III | 2.99 (1.02–8.76) | 0.045 | 3.16 (1.04–9.60) | 0.042 |
| Pathological LLN | ||||
| Negative | Reference | Reference | ||
| Positive | 0.83 (0.33 - 2.12) | 0.703 | 0.83 (0.31–2.22) | 0.714 |
| Adjuvant therapy | ||||
| No | Reference | — | ||
| Yes | 2.08 (0.62-7.02) | 0.239 | — | |
For PFS, univariate Cox regression analysis showed that vessel-oriented LLND, age ≥ 65 years, female sex, pathological LLN metastasis, and postoperative adjuvant therapy did not affect PFS; however, pathological stage III disease was a risk factor for poor PFS (HR = 2.99, 95%CI = 1.02–8.76, P = 0.045). After adjusting for pathological LLN metastases and pathological tumor stage, the multivariable Cox regression analyses showed that vessel-oriented LLND (HR = 1.16, 95%CI = 0.51–2.66, P = 0.729) and pathological LLN metastases (HR = 0.83, 95%CI = 0.31–2.22, P = 0.714) were not independent risk factors for poor PFS, while the presence of pathological stage III disease was associated with a significant decline in PFS (HR = 3.16, 95%CI = 1.04–9.60, P = 0.042).
In this retrospective cohort study, we compared the impact of fascia-oriented and vessel-oriented LLND on short-term outcomes and prognosis in newly diagnosed rectal cancer patients. Our results indicated that the median number of examined LLNs in the fascia-oriented group was notably higher than that in the vessel-oriented group. Simultaneously, there was no notable discrepancy in the rate of pathological LLN metastasis, operation time, intraoperative blood loss, incidence of perioperative surgical complications, or length of postoperative hospital stay. In terms of postoperative functional indicators, the incidence of postoperative urinary and male sexual dysfunction was significantly lower in the fascia-oriented group than in the vessel-oriented group. In addition, there was no significant difference in the incidence of postoperative lower limb dysfunction between the two groups. In terms of prognosis, no significant difference was observed in either PFS or OS between the two groups.
In this study, we found that compared with traditional vessel-oriented LLND, fascia-oriented LLND did not increase the operative time, length of postoperative hospital stay, or incidence of perioperative surgical complications, and there were no cases of reoperation or perioperative deaths in either group, which is consistent with previous studies[9,10,11-13]. The proportion of patients with intraoperative blood loss ≥ 300 mL was higher in the vessel-oriented group than in the fascia-oriented group (9.4% vs 2.4%). Although the observed difference did not reach statistical significance, it likely reflects the inherent advantages of the surgical procedure for fascial-oriented LLND in reducing bleeding events. Using fascia as an anatomical landmark makes it easy to identify anatomical locations and important blood vessels and perform separation on the avascular plane during LLND. The incidence of grade II or higher perioperative surgical complications in the fascia-oriented group was 9.8%, which is consistent with previous studies[13]; additionally, this rate is lower than that reported for laparoscopic LLND[30]. In this study, the 2-year OS and PFS rates were 91.6% and 81.7%, respectively, consistent with previous reports[31,32]. The above results indicate that fascia-oriented LLND is safe and feasible.
The median number of examined LLNs in the fascia-oriented group was 9.0, consistent with previous studies on laparoscopic LLND[30,33]; furthermore, this number was significantly higher than that in the vessel-oriented group (9.0 vs 6.5). In terms of the surgical method, this difference may be related to the thoroughness of lymph node dissection. Vessel-oriented LLND consists of a fragmented and sporadic dissection, which is likely to lead to the omission of lymph nodes and does not conform to the principle of en bloc tumor resection. In fascia-oriented LLND, clear boundaries of medial and lateral dissection are established when dissecting the No. 263 and No. 283 Lymph nodes, which is conducive to guiding the removal of lymphoid tissue in the lateral space and makes it easier to achieve en bloc resection and prevent the omission of lymph nodes. Previous studies have shown that increasing the number of examined lymph nodes may improve the accuracy of tumor staging[34]; therefore, fascia-oriented LLND may be beneficial for assessing of the severity of rectal cancer patients with LLN metastasis.
The incidence of postoperative urinary dysfunction and male sexual dysfunction was much lower in the fascia-oriented group than in the vessel-oriented group. Although the incidence of lower limb dysfunction was comparable between the two groups, the incidence was less than 30% in both groups. Multivariate analyses showed that vessel-oriented LLND was an independent risk factor for postoperative urinary dysfunction and male sexual dysfunction. The above results indicated that compared with vessel-oriented LLND, fascia-oriented LLND effectively prevents intraoperative pelvic nerve damage, which may be attributed to several factors.
First, since the surface of the pelvic autonomic nerve is covered with the UNF, this provides a fascial marker for autonomic nerves protection during surgery. In establishing the medial boundary of LLND, the tissue is separated along the lateral side of the UNF, which protects the integrity of the UNF and prevents injury to the autonomic nerve. Second, the obturator nerve can be exposed after dissociating along the pelvic parietal fascia to the obturator foramen. The surrounding tissue can be dissected from far to near along the obturator nerve so that the obturator nerve can be safely exposed throughout the process of LLND. Similarly, dissociating along the pelvic parietal fascia and the VF can reveal the neurovessel bundle, effectively reducing the probability of nerve damage during surgery.
This study had several limitations. First, this was a retrospective study with a small sample size. Thus, selection bias may have been a concern and prospective studies including more patients enrolled will be needed in the future to verify the conclusions drawn in this study. Second, regarding the assessment of postoperative urinary dysfunction, although the IPSS is widely used in clinical work because of its simplicity and feasibility, it is more accurate to evaluate urinary dysfunction through the residual bladder volume. Third, there is currently no uniform standard for evaluating female sexual dysfunction; therefore, this study did not perform postoperative sexual function evaluations in female patients.
In conclusion, this study demonstrated that it is safe and feasible to perform fascia-oriented LLND at experienced high-volume centers. Compared with vessel-oriented LLND, fascia-oriented LLND allows the examination of more LLNs and may better protect postoperative urinary function and postoperative male sexual function. The conclusions drawn need to be verified in future prospective studies including more patients.
There is a lack of studies comparing the efficacy of fascia-oriented and traditional vessel-oriented lateral lymph node dissection (LLND). Through a preliminary study with a small sample size, we found that fascia-oriented LLND was associated with a lower incidence of postoperative urinary and male sexual dysfunction and a higher number of examined lateral lymph nodes (LLNs). In this study, we increased the sample size and refined the postoperative functional outcomes.
For the management of LLN metastasis in patients with rectal cancer, selective LLND is gradually being accepted by Chinese scholars. Theoretically, fascia-oriented LLND both allows radical tumor resection and protects organ function. However, there is a lack of evidence-based medical studies comparing the efficacy of fascia-oriented and traditional vessel-oriented LLND. The present study will provide information for surgeons regarding the selection of the optimal surgical procedure for LLND.
This study aimed to compare the effects of fascia- and vessel-oriented LLND regarding the short-term outcomes and prognosis.
We conducted a retrospective cohort study on data from 196 patients with rectal cancer who underwent total mesorectal excision and LLND from July 2014 to August 2021. The short-term outcomes included perioperative outcomes and postoperative functional outcomes. The prognosis was measured based on overall survival (OS) and progression-free survival (PFS).
Regarding short-term outcomes, the fascia-oriented group had a higher median number of examined LLNs compared to the vessel-oriented group. However, there were no notable differences in other short-term outcomes. The fascia-oriented group had significantly lower rates of postoperative urinary and male sexual dysfunction compared to the vessel-oriented group, and there were no significant differences in postoperative lower limb dysfunction between the two groups. As for prognosis, there was no significant disparity in PFS or OS between the two groups.
Our study suggests that fascia-oriented LLND is a safe and feasible option for patients with rectal cancer. Although no significant difference was observed in prognosis compared to vessel-oriented LLND, fascia-oriented LLND may allow for the examination of more LLNs and potentially offer benefits in preserving postoperative urinary and sexual function.
While our study supports the use of fascia-oriented LLND for rectal cancer, it is important to verify our conclusions with larger prospective studies. Further research is needed to confirm the potential benefits of fascia-oriented LLND, including preserving postoperative urinary and sexual function.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Preziosi F, Italy; Sano W, Japan S-Editor: Li L L-Editor: A P-Editor: Wu RR
| 1. | Takahashi T, Ueno M, Azekura K, Ohta H. Lateral node dissection and total mesorectal excision for rectal cancer. Dis Colon Rectum. 2000;43:S59-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 188] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Kanemitsu Y, Komori K, Shida D, Ochiai H, Tsukamoto S, Kinoshita T, Moriya Y. Potential impact of lateral lymph node dissection (LLND) for low rectal cancer on prognoses and local control: A comparison of 2 high-volume centers in Japan that employ different policies concerning LLND. Surgery. 2017;162:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Yano H, Moran BJ. The incidence of lateral pelvic side-wall nodal involvement in low rectal cancer may be similar in Japan and the West. Br J Surg. 2008;95:33-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, van de Velde CJH, Beets GL, Rutten HJT, Kusters M; Lateral Node Study Consortium. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol. 2019;37:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 5. | Ito M, Kobayashi A, Fujita S, Mizusawa J, Kanemitsu Y, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Akasu T, Moriya Y; Colorectal Cancer Study Group of Japan Clinical Oncology Group. Urinary dysfunction after rectal cancer surgery: Results from a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for clinical stage II or III lower rectal cancer (Japan Clinical Oncology Group Study, JCOG0212). Eur J Surg Oncol. 2018;44:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Kusters M, Slater A, Muirhead R, Hompes R, Guy RJ, Jones OM, George BD, Lindsey I, Mortensen NJ, Cunningham C. What To Do With Lateral Nodal Disease in Low Locally Advanced Rectal Cancer? Dis Colon Rectum. 2017;60:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Akiyoshi T, Ueno M, Matsueda K, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, Unno T, Kano A, Kuroyanagi H, Oya M, Yamaguchi T, Watanabe T, Muto T. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol. 2014;21:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 8. | Beppu N, Ikeda M, Kimura K, Kataoka K, Yamano T, Uchino M, Ikeuchi H, Tomita N. Extended Total Mesorectal Excision Based on the Avascular Planes of the Retroperitoneum for Locally Advanced Rectal Cancer with Lateral Pelvic Sidewall Invasion. Dis Colon Rectum. 2020;63:1475-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Matsumoto A, Arita K. A technique of laparoscopic lateral pelvic lymph node dissection based on vesicohypogastric fascia and ureterohypogastric nerve fascia for advanced low rectal cancer. Surg Endosc. 2017;31:945-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Zhang X, Deng X, Wei M, Zhang H, Yang Y, Wu Q, Gu C, Meng W, Wang Z. A Modified Technique of Laparoscopic Lateral Lymph Node Dissection Combining Fascia-Oriented Dissection and Routine Upfront Distal Visceral Vessels Ligation for Mid- to Low-Lying Rectal Cancer. Dis Colon Rectum. 2021;64:e67-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Beppu N, Jihyung S, Takenaka Y, Kimura K, Babaya A, Yasuhara M, Kataoka K, Uchino M, Ikeuchi H, Ikeda M. Laparoscopic lateral pelvic lymph node dissection combined with removal of the internal iliac vessels in rectal cancer: how to standardize this surgical procedure. Tech Coloproctol. 2021;25:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Sun Y, Lian L, Zhang H, Bai X, Xie Z, Ouyang J, Wang K, Yuan H, Xu C, Luo H, Deng H, Li J, Yang H, Zhang Z, Li P, Zhang X. The feasibility and technical strategy of a fascia space priority approach in laparoscopic lateral lymph node dissection for advanced middle and low rectal cancer: a retrospective multicentre study. Wideochir Inne Tech Maloinwazyjne. 2021;16:312-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Jiang HH, Liu HL, Li AJ, Wang WC, Lv L, Peng J, Pan ZH, Chang Y, Lin MB. Laparoscopic lateral lymph node dissection in two fascial spaces for locally advanced lower rectal cancer. World J Gastroenterol. 2021;27:3654-3667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 14. | Wang ZJ, Liu Z, Liang JW, Zhang MG, Mei SW, Shen HY, Chen JN, Li J, Zhao FQ, Wei FZ, Xiao TX, Liu Q. [Comparison on efficacy between fascia-oriented vs vascular-oriented lateral lymph node dissection in patients with rectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:611-618. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Stanton C. Guideline for Positioning the Patient. AORN J. 2022;115:P5-P7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Kehlet H, Jørgensen CC. Advancing Surgical Outcomes Research and Quality Improvement Within an Enhanced Recovery Program Framework. Ann Surg. 2016;264:237-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24776] [Article Influence: 1179.8] [Reference Citation Analysis (0)] |
| 18. | Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549-57; discussion 1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2337] [Cited by in RCA: 2509] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 19. | Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 745] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 20. | Foo CC, Kin Ng K, Tsang JS, Siu-Hung Lo O, Wei R, Yip J, Lun Law W. Low Anterior Resection Syndrome After Transanal Total Mesorectal Excision: A Comparison With the Conventional Top-to-Bottom Approach. Dis Colon Rectum. 2020;63:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Mori R, Uemura M, Tsuboyama T, Fujino S, Hata T, Ogino T, Takahashi H, Miyoshi N, Mizushima T, Doki Y, Eguchi H. The prediction of postoperative anorectal dysfunction after low anterior resection for lower rectal cancer by measuring the volume of defecation-related muscles. Surg Today. 2022;52:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 2174] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 23. | Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 24. | Nezhat FR, Chang-Jackson SR, Acholonu UC Jr, Vetere PF. Robotic-assisted laparoscopic transection and repair of an obturator nerve during pelvic lymphadenectomy for endometrial cancer. Obstet Gynecol. 2012;119:462-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Göçmen A, Şanlıkan F. Immediate repair of an incompletely transected obturator nerve during robotic-assisted pelvic lymphadenectomy. J Minim Invasive Gynecol. 2015;22:302-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Lange MM, Maas CP, Marijnen CA, Wiggers T, Rutten HJ, Kranenbarg EK, van de Velde CJ; Cooperative Clinical Investigators of the Dutch Total Mesorectal Excision Trial. Urinary dysfunction after rectal cancer treatment is mainly caused by surgery. Br J Surg. 2008;95:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 27. | Huang M, Lin J, Yu X, Chen S, Kang L, Deng Y, Zheng J, Luo Y, Wang L, Lan P, Wang J. Erectile and urinary function in men with rectal cancer treated by neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy alone: a randomized trial report. Int J Colorectal Dis. 2016;31:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Saito S, Fujita S, Mizusawa J, Kanemitsu Y, Saito N, Kinugasa Y, Akazai Y, Ota M, Ohue M, Komori K, Shiozawa M, Yamaguchi T, Akasu T, Moriya Y; Colorectal Cancer Study Group of Japan Clinical Oncology Group. Male sexual dysfunction after rectal cancer surgery: Results of a randomized trial comparing mesorectal excision with and without lateral lymph node dissection for patients with lower rectal cancer: Japan Clinical Oncology Group Study JCOG0212. Eur J Surg Oncol. 2016;42:1851-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 29. | Kitagawa R, Kim D, Reid N, Kline D. Surgical management of obturator nerve lesions. Neurosurgery. 2009;65:A24-A28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Yamaguchi T, Konishi T, Kinugasa Y, Yamamoto S, Akiyoshi T, Okamura R, Ito M, Nishimura Y, Shiozawa M, Yamaguchi S, Hida K, Sakai Y, Watanabe M. Laparoscopic Versus Open Lateral Lymph Node Dissection for Locally Advanced Low Rectal Cancer: A Subgroup Analysis of a Large Multicenter Cohort Study in Japan. Dis Colon Rectum. 2017;60:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Yang X, Gu C, Hu T, Bi L, Wei M, Deng X, Wang Z, Zhou Z. Is laparoscopic selective lateral lymph node dissection for locally advanced rectal cancer after neoadjuvant chemoradiotherapy safe? ANZ J Surg. 2019;89:E492-E497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Chen JN, Liu Z, Wang ZJ, Mei SW, Shen HY, Li J, Pei W, Wang Z, Wang XS, Yu J, Liu Q. Selective lateral lymph node dissection after neoadjuvant chemoradiotherapy in rectal cancer. World J Gastroenterol. 2020;26:2877-2888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Ohya H, Watanabe J, Suwa H, Suwa Y, Ozawa M, Ishibe A, Kunisaki C, Endo I. Near-Infrared Imaging Using Indocyanine Green for Laparoscopic Lateral Pelvic Lymph Node Dissection for Clinical Stage II/III Middle-Lower Rectal Cancer: A Propensity Score-Matched Cohort Study. Dis Colon Rectum. 2022;65:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Zhang C, Zhao S, Wang X. Analysis of the risk factor of insufficient examined lymph nodes in stage II colon cancer from the perspective of stage migration: A retrospective study combined with external validation. Int J Surg. 2022;101:106628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |