Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.992
Peer-review started: December 28, 2022
First decision: January 10, 2023
Revised: January 23, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 27, 2023
Processing time: 148 Days and 21.7 Hours
Leiomyosarcoma (LMS) has a poor prognosis and rarely originates from the colon. If resection is possible, surgery is the first treatment most commonly considered. Unfortunately, no standard treatment exists for hepatic metastasis of LMS; although, several treatments, such as chemotherapy, radiotherapy, and surgery, have been used. Subsequently, the management of liver metastases remains controversial.
We present a rare case of metachronous liver metastasis in a patient with LMS originating from the descending colon. A 38-year-old man initially reported abdominal pain and diarrhea over the previous two months. Colonoscopy revealed a 4-cm diameter mass in the descending colon, 40 cm from the anal verge. Computed tomography revealed intussusception of the descending colon due to the 4-cm mass. The patient underwent a left hemicolectomy. Immunohistochemical analysis of the tumor revealed that it was positive for smooth muscle actin and desmin, and negative for cluster of differentiation 34 (CD34), CD117, and discovered on gastrointestinal stromal tumor (GIST)-1, which are characteristic of gastrointestinal LMS. A single liver metastasis developed 11 mo post-operatively; the patient subsequently underwent curative resection thereof. The patient remained disease-free after six cycles of adjuvant chemotherapy (doxorubicin and ifosfamide), and 40 and 52 mo after liver resection and primary surgery, respectively. Similar cases were obtained from a search of Embase, PubMed, MEDLINE, and Google Scholar.
Early diagnosis and surgical resection may be the only potential curative options for liver metastasis of gastrointestinal LMS.
Core Tip: Leiomyosarcoma (LMS) rarely originates from the colon; hepatic metastasis of LMS lacks standard treatment. We present a case report of a 38-year-old man who was found to have intussusception in the descending colon due to a 4-cm LMS. The patient underwent a left hemicolectomy, however, a single liver metastasis developed 11 mo after the primary surgery. He underwent curative resection of the metastatic lesion and six cycles of adjuvant chemotherapy. The patient remained disease-free for 52 mo after the primary surgery. An early diagnosis and R0 resection may be the only potential curative approach to liver metastasis of gastrointestinal LMS.
- Citation: Lee SH, Bae SH, Lee SC, Ahn TS, Kim Z, Jung HI. Curative resection of leiomyosarcoma of the descending colon with metachronous liver metastasis: A case report. World J Gastrointest Surg 2023; 15(5): 992-999
- URL: https://www.wjgnet.com/1948-9366/full/v15/i5/992.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i5.992
Leiomyosarcoma (LMS) is a rare cancer that accounts for approximately 14% of soft tissue sarcomas (STSs), with an incidence of < 1/100000/year in Europe[1]. LMS is primarily observed in middle-aged patients; it is equally prevalent in men and women. Approximately 20% of LMSs are found in the gastrointestinal tract, of which the small intestine is the most common site. LMS within the colorectum is extremely rare, accounting for < 1% of all malignancies of the colon and rectum[2]; however, colonic LMSs appear to be highly aggressive tumors. In 62% of all visceral sarcomas, hepatic metastases occur frequently owing to hematogenous spread via the portal vein[3]. Unfortunately, there is currently no standard treatment for LMS with metachronous liver metastasis. Therefore, a database search of Embase, PubMed, MEDLINE, and Google Scholar was performed to identify similar case reports using the following terms: LMS, hepatic metastases, and treatment. Through a literature review and case presentation of metachronous liver metastasis in a patient with LMS originating from the descending colon, we discuss which potential treatment options may be used in such cases.
A 38-year-old man was admitted to our hospital with an initial presentation of abdominal pain and diarrhea over the preceding 2 mo.
The patient visited a local hospital with abdominal pain and diarrhea over the previous 2 mo. Colonoscopy performed at a local hospital revealed a 4-cm diameter mass in the descending colon, 40 cm from the anal verge.
The patient had no remarkable past medical history.
There were no significant findings in the patient’s personal and family history.
The patient complained of tenderness in the left lower abdomen upon palpation, but no rebound tenderness. The abdomen was otherwise soft, undistended, and revealed no palpable mass. There were no evidence of a mass or hematochezia on digital rectal examination.
White blood cell count was mildly increased to 13960 cells/μL; however, laboratory investigations, including markers of liver function and renal function, were normal. The patient’s carcinoembryonic antigen (CEA; 3.04 ng/mL) and carbohydrate antigen 19-9 (CA 19-9; 7.44 U/mL) levels were normal.
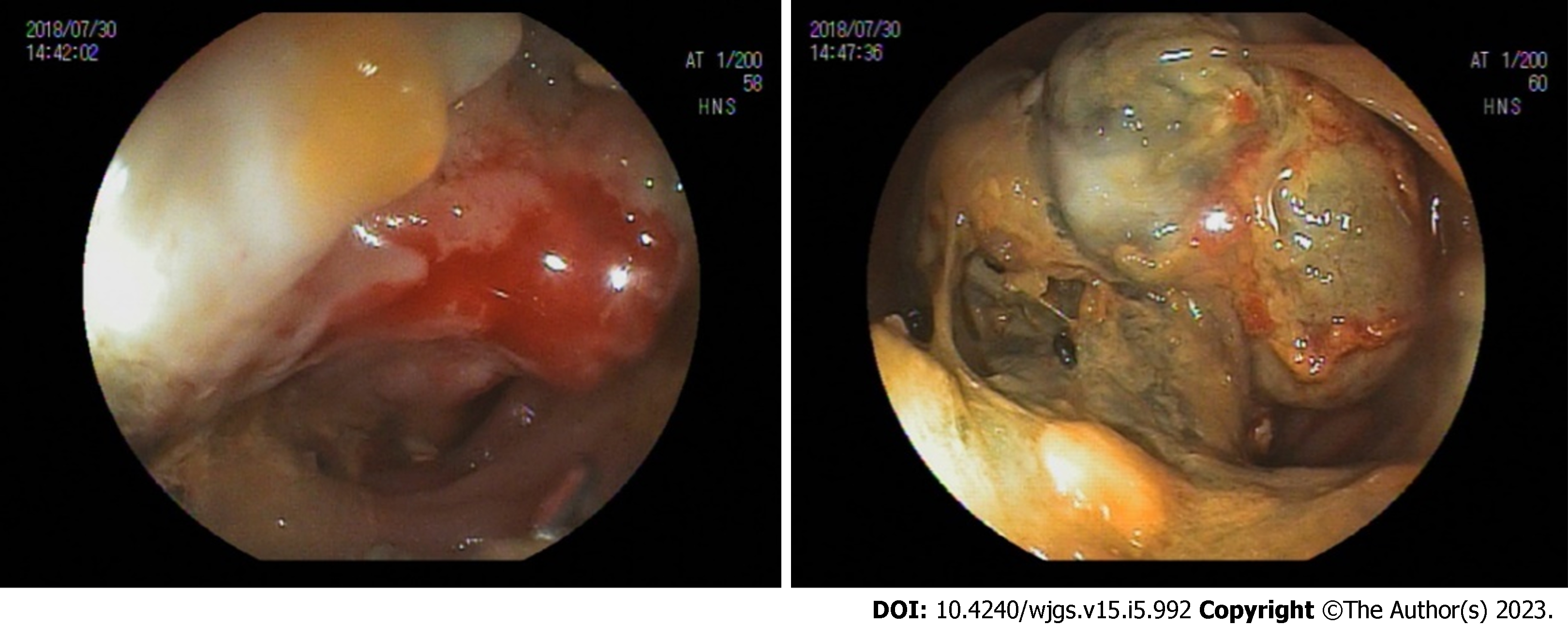
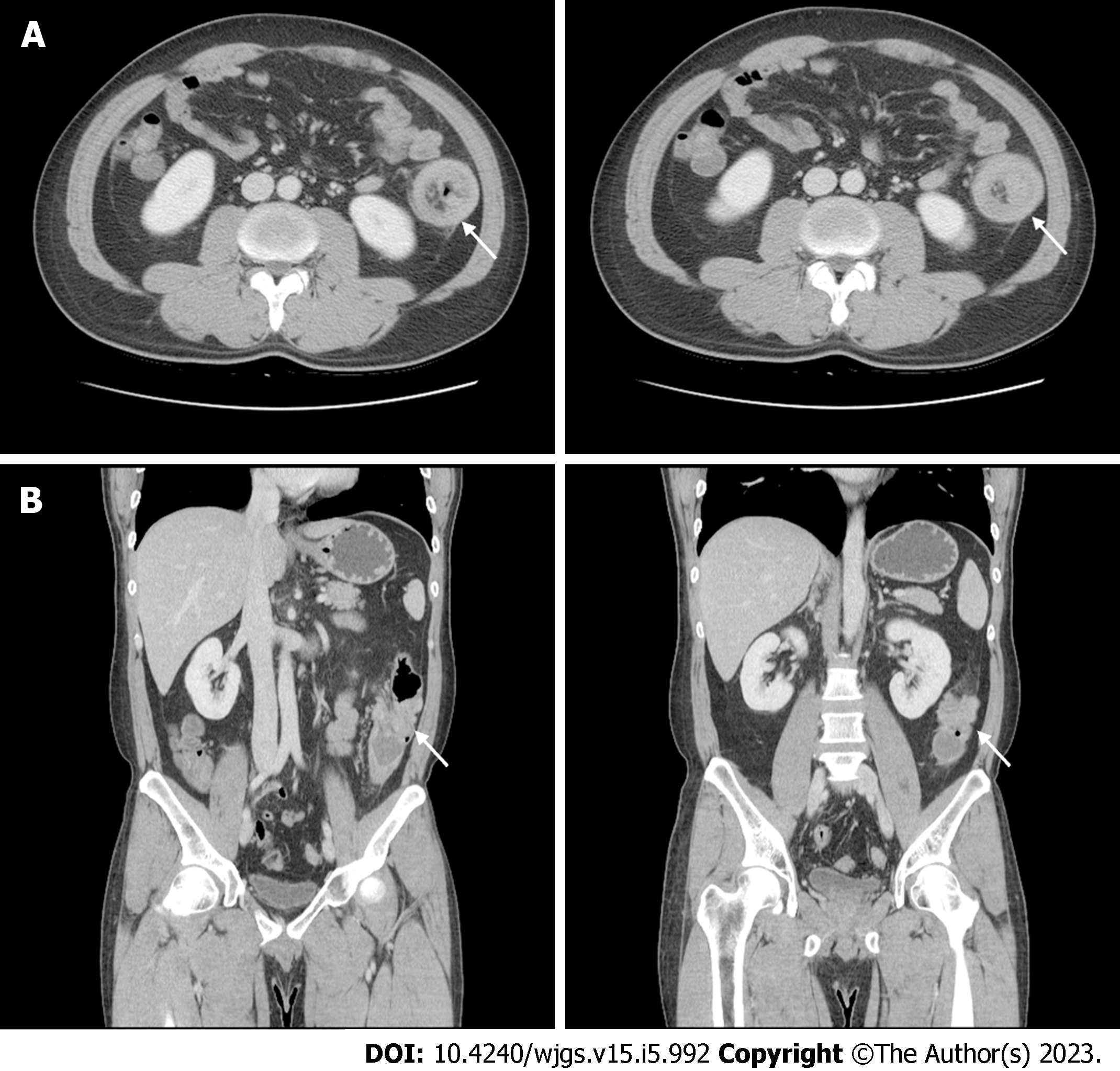
Colonoscopy showed a 4-cm diameter mass in the descending colon, and a biopsy was performed (Figure 1). The colonoscopy biopsy specimen result showed an atypical spindle cell lesion, suggestive of a malignant mesenchymal tumor. Immunohistochemically, the tumor was positive for smooth muscle actin (SMA) and desmin, and negative for cluster of differentiation 34 (CD34), CD117, and discovered on gastrointestinal stromal tumor (GIST)-1 (DOG-1). Abdomino-pelvic computed tomography (CT) revealed intussusception in the descending colon due to a mass of approximately 4 cm with liquefaction, a small amount of ascites, and no distant metastasis in other solid organs (Figure 2). There was no abnormal finding on chest CT.
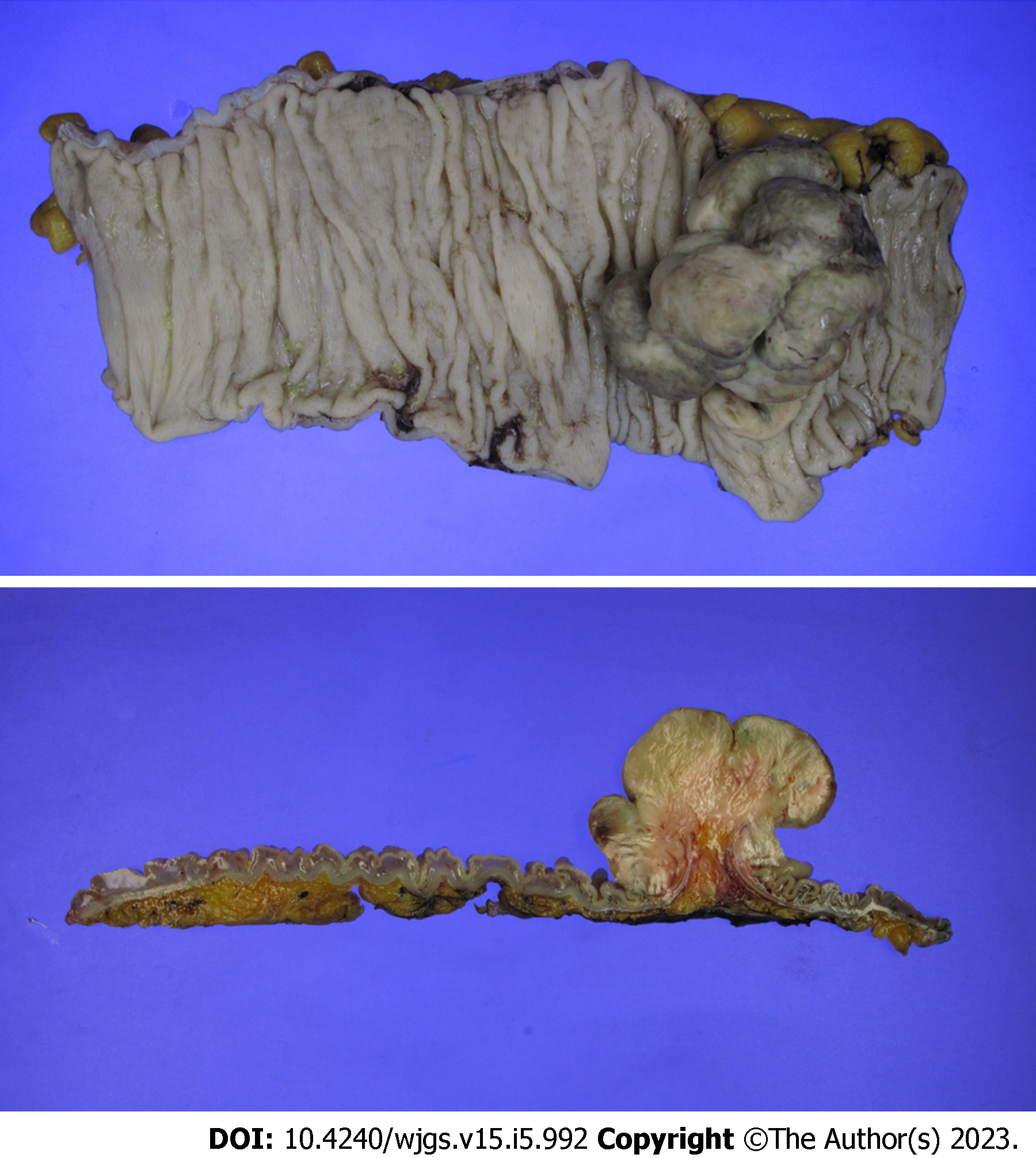
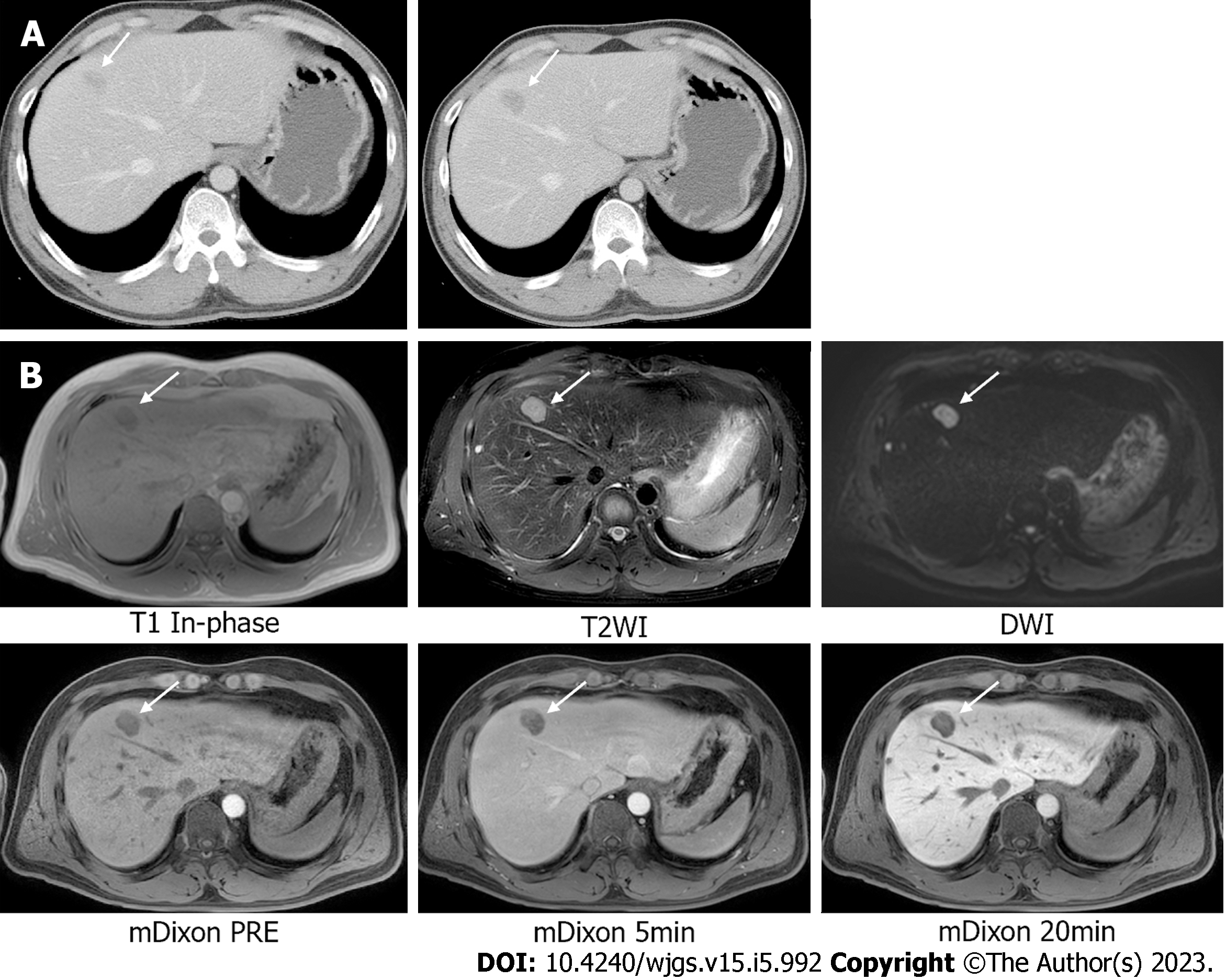
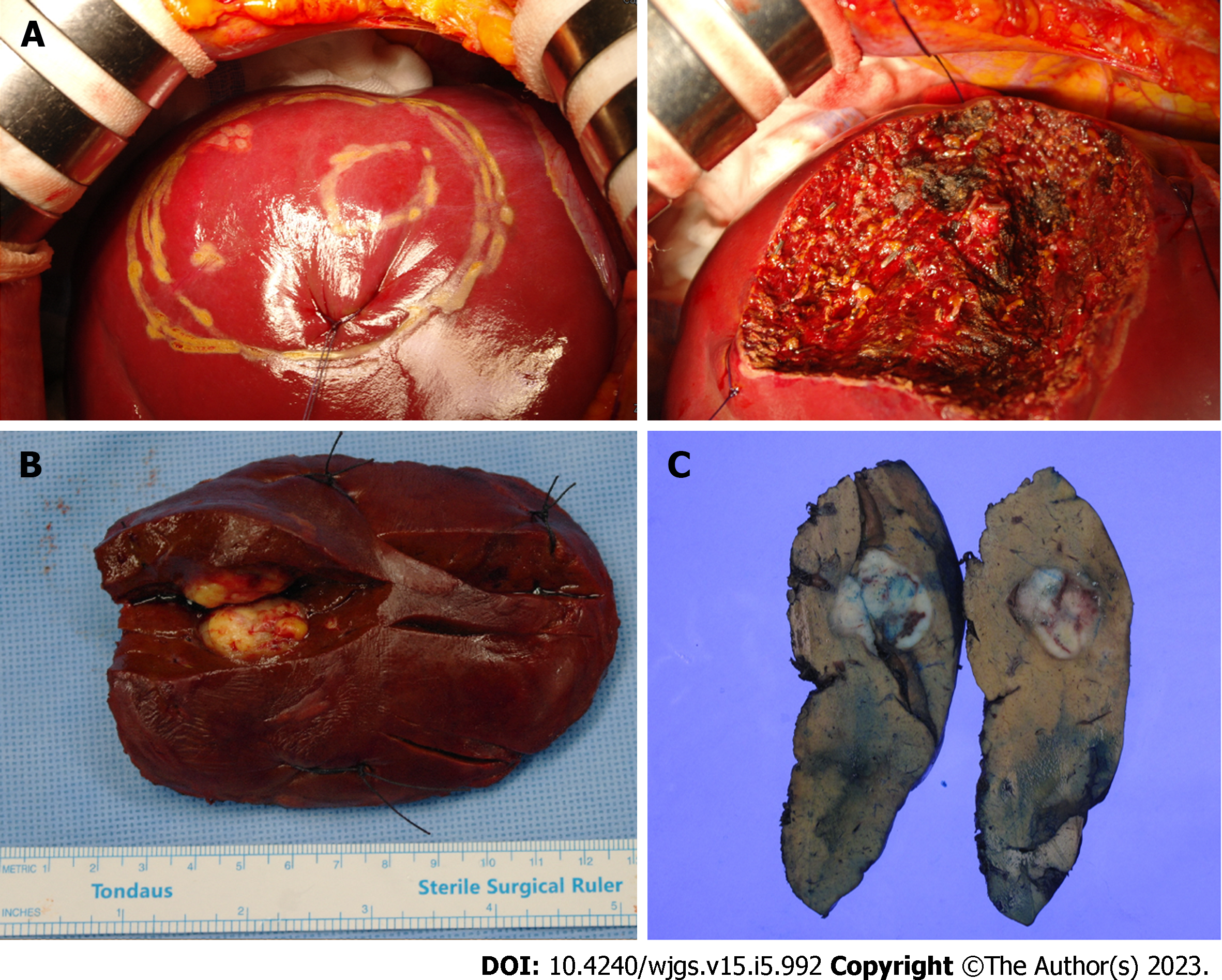
Based on the results of the biopsy and CT, a diagnosis of LMS arising from descending colon was suspected. The patient underwent a left hemicolectomy; no intraoperative or postoperative complications were noted. The pathology report showed a 7.5 cm × 5.5 cm × 4.0 cm LMS without necrosis in the descending colon with a clear resection margin and no metastasis in all 27 lymph nodes (Figure 3). Mitotic counts were as high as 32/10 high-power field, and immunohistochemical analysis revealed SMA and focal desmin positivity, and CD34, CD117, DOG-1, and S-100 protein negativity. Without adjuvant treatment, the patient underwent a checkup every 3 mo using a routine blood test and CT. The patient was found to have a possibly newly developed liver metastasis in segment 8 on an abdomino-pelvic CT performed 11 mo after the primary surgery; however, there were no evidence of local recurrence at the anastomosis site (Figure 4A). In addition, magnetic resonance imaging of the liver identified a suspected 2.3-cm-sized liver metastasis in the same region (Figure 4B). The patient’s CEA level had also increased to 10.23 ng/mL. The patient’s Child-Pugh score was 5 points.
Metachronous liver metastasis in segment 8 originating from the LMS in the descending colon.
Intraoperative ultrasonography revealed a single metastatic lesion of the liver. A segmentectomy of segment 8 was performed to resect the tumor.
The pathology report showed a metastatic LMS measuring 2.4 cm × 2.2 cm × 2.0 cm with no hepatic capsular invasion; the safety margin was 1.1 cm (Figure 5). After the second operation, the patient was administered six cycles of doxorubicin and ifosfamide combination chemotherapy (doxorubicin 60 mg/m2 iv on Day 1 and ifosfamide 2500 mg/m2 iv on Days 1-3). On the last day of each cycle, tripegfilgrastim 6 mg iv was administered 24 h after the end of administration of the last treatment. Allergic dermatitis and myalgia appeared as side effects of chemotherapy; however, chemotherapy dose reduction was not performed. After the end of adjuvant chemotherapy, we performed a blood test, CT, and bone scans for the patient’s follow-up. Abdomino-pelvic CT, chest CT, and bone scans were performed every 3 mo; there was no tumor recurrence or distant metastasis. In addition, the levels of CEA and CA 19-9 were maintained within the normal range without significant changes. There was no evidence of recurrence or metastasis after 52 and 40 mo after the first and second surgeries, respectively.
GISTs have a good prognosis for targeted therapy with tyrosine kinase inhibitors and their treatment guidelines have been established. However, other STSs, including LMSs, lack effective standardized treatment and thus have a poor prognosis. Factors affecting the prognosis of STS include malignancy grade, tumor size, primary tumor location, tumor resectability, surgical margin quality, and preoperative/intraoperative tumor rupture[4]. However, the most important prognosticator is the presence of metastases. For this reason, studies on the standard treatment for STSs (excluding GISTs), especially metastatic STSs, have been conducted. However, there are limitations, particularly the remarkably small number of patients that were included. Based on a search of the main online databases (Embase, PubMed, MEDLINE, and Google Scholar), 37 cases of colonic LMS have been published. Among them, liver metastases were found in eight patients, and two patients were referred for resection for liver metastases, all of which were synchronous metastases[5,6]. One patient was a 74-year-old woman who was diagnosed with a descending colon cancer with liver metastasis in segment 5/6 of the liver. Unfortunately, she died 10 mo after surgery due to multiple lung metastases[6]. In another case, a 66-year-old woman underwent surgery and received adjuvant chemotherapy for gastric cancer with liver metastases. She was subsequently diagnosed with LMS of the sigmoid colon with multiple liver metastases and underwent resection of four liver tumors. However, she died 7 mo later because of multiple liver and lung metastases[7]. Thus, reports of colon LMS with liver metastasis are significantly rare, making it difficult to establish treatment guidelines.
According to the clinical practice guidelines recently published by the European Society for Medical Oncology, surgery is considered the standard treatment for locoregional soft tissue and visceral sarcomas, and en bloc excision with R0 resection is required. Except for patients with a high risk of death from surgery, adjuvant and neoadjuvant chemotherapies are not standard treatments. Radiotherapy can be added to surgery as part of the standard treatment for high-grade (Grade 2–3) lesions; however, local control and overall survival (OS) are not influenced by the timing of radiotherapy. The standard treatment for advanced STS is surgery for metachronous and resectable lung metastases without extrapulmonary disease, and chemotherapy for synchronous lung metastases without extrapulmonary disease. Anthracycline-based chemotherapy is recommended for the treatment of unresectable STS[1]. However, even in these guidelines, the treatment of the liver metastases is insufficient, and most of the patients documented in the guideline had STSs arising from the extremities and trunk walls.
Several reports have confirmed that LMS is relatively resistant to chemotherapy and radiotherapy; therefore, it is difficult to expect favorable effects through these treatment strategies[6,8]. Thus, a greater emphasis has been placed on the importance of surgical resection. The 5-year survival rate of patients with LMS who did not undergo surgical resection is only 4%, which is lower compared to that of patients undergoing resection (20%–30%)[9]. Liver resection for metastatic STS has a median OS of 46 mo and a median progression-free survival (PFS) after liver resection of 16 mo. Even after R2 resection, the median survival period is 20 mo, which is longer than that of patients treated with chemotherapy (10 mo), as mentioned in the European Organisation for Research and Treatment of Cancer trial[3]. In addition, when liver resection is performed for metachronous metastases, the median PFS is better than that for synchronous metastases[3,10]. However, in some studies, synchronous disease showed a lower median OS than metachronous disease, although the difference was not statistically significant. Therefore, it is difficult to consider it a prognostic factor of OS[11]. The most important point to improve OS mentioned in most reports is R0 resection; the number and size of liver metastases or the extent of liver resection does not affect survival[10]. Lymph node metastases are remarkably rare and lymph node dissection is unnecessary[12]. In our case, all 27 lymph nodes were nonmetastatic, although the primary LMS in the descending colon was > 7 cm in diameter and had a high mitotic rate.
LMS may remain asymptomatic for a long time; therefore, there may be no operability at the time of discovery. In the case of liver-dominant metastatic LMS which cannot be surgically resected, chemotherapy is provided; unfortunately, the patients’ response is poor. The median OS period is up to 21.9 mo, and the PFS period is 6.9 mo. In such cases, liver-directed treatment can be provided instead of chemotherapy. When comparing transarterial chemoembolization with doxorubicin-eluting beads, yttrium-90 radioembolization, and percutaneous microwave ablation, the median OS period was 27 mo from the development of liver metastases and the median liver PFS period increased by 9 mo, similar to patients who underwent surgical resection[13]. However, there is a disadvantage in that Grade 1 or 2 clinical toxicities due to liver-directed treatment appear in 96% of patients during the first 3 mo. In addition, the effect of extrahepatic metastases on liver resection in patients who have undergone liver metastasectomy is controversial. Extrahepatic metastases in patients undergoing liver resection are a negative prognostic factor[14]; however, the presence of resectable extrahepatic disease does not interfere with liver resection[11,15]. For this reason, there remains controversy about performing liver resection for liver metastases accompanied by extrahepatic metastases; therefore, additional studies are required.
Existing studies recommend surgery for resectable metastases in advanced STS arising from the extremities. Furthermore, in gastrointestinal LMS with liver metastases, if there is no other organ metastasis and resection is possible, regardless of the number of metastases, synchronous or metachronous, surgical resection is helpful for OS and PFS. Therefore, aggressive surgical interventions, rather than chemotherapy or radiotherapy, should be considered, including R0 resection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Korean Society of Surgical Oncology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Badheeb A, Saudi Arabia; Yu K, China S-Editor: Hu YR L-Editor: A P-Editor: Cai YX
| 1. | Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee and EURACAN. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv268-iv269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 2. | Faraj W, El-Kehdy J, Nounou GE, Deeba S, Fakih H, Jabbour M, Haydar A, El Naaj AA, Abou-Alfa GK, O'Reilly EM, Shamseddine A, Khalife M, Mukherji D. Liver resection for metastatic colorectal leiomyosarcoma: a single center experience. J Gastrointest Oncol. 2015;6:E70-E76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 3. | Grimme FAB, Seesing MFJ, van Hillegersberg R, van Coevorden F, de Jong KP, Nagtegaal ID, Verhoef C, de Wilt JHW; On behalf of the Dutch Liver Surgery Working Group. Liver Resection for Hepatic Metastases from Soft Tissue Sarcoma: A Nationwide Study. Dig Surg. 2019;36:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Tanaka K, Ozaki T. New TNM classification (AJCC eighth edition) of bone and soft tissue sarcomas: JCOG Bone and Soft Tissue Tumor Study Group. Jpn J Clin Oncol. 2019;49:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Bananzadeh A, Mokhtari M, Sohooli M, Shekouhi R. Two cases of primary leiomyosarcoma of sigmoid colon treated with laparoscopic surgery: A case report and a review of literature. Int J Surg Case Rep. 2021;87:106420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Massaras D, Kontis E, Stamatis K, Zampeli E, Myoteri D, Primetis E, Pantiora E, Fragulidis G. Primary leiomyosarcoma of the colon with synchronous liver metastasis. Rare Tumors. 2022;14:20363613221080549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Hamai Y, Hihara J, Emi M, Aoki Y, Kushitani K, Tanabe K, Okada M. Leiomyosarcoma of the sigmoid colon with multiple liver metastases and gastric cancer: a case report. BMC Gastroenterol. 2012;12:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kim YW, Lee JH, Kim JE, Kang J. Surgical resection of liver metastasis of leiomyosarcoma. Korean J Clin Oncol. 2017;13:143-146. [DOI] [Full Text] |
| 9. | Mizoshiri N, Shirai T, Terauchi R, Tsuchida S, Mori Y, Katsuyama Y, Hayashi D, Konishi E, Kubo T. Hepatic metastases from primary extremity leiomyosarcomas: Two case reports. Medicine (Baltimore). 2018;97:e0598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Delisle M, Alshamsan B, Nagaratnam K, Smith D, Wang Y, Srikanthan A. Metastasectomy in Leiomyosarcoma: A Systematic Review and Pooled Survival Analysis. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Lang H, Nussbaum KT, Kaudel P, Frühauf N, Flemming P, Raab R. Hepatic metastases from leiomyosarcoma: A single-center experience with 34 liver resections during a 15-year period. Ann Surg. 2000;231:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Devriendt S, Leman G, Vanrykel F. Primary leiomyosarcoma of the colon: a case report and review of the literature. Acta Chir Belg. 2020;120:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Krzyston H, Morse B, Deperalta D, Rishi A, Kayaleh R, El-Haddad G, Smith J, Druta M, Kis B. Liver-directed treatments of liver-dominant metastatic leiomyosarcoma. Diagn Interv Radiol. 2020;26:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Tirotta F, Hodson J, Parente A, Pasquali S, Sutcliffe R, Desai A, Muiesan P, Ford SJ, Fiore M, Gronchi A, Almond LM. Liver resection for sarcoma metastases: A systematic review and experience from two European centres. Eur J Surg Oncol. 2020;46:1807-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Marudanayagam R, Sandhu B, Perera MT, Bramhall SR, Mayer D, Buckels JA, Mirza DF. Liver resection for metastatic soft tissue sarcoma: an analysis of prognostic factors. Eur J Surg Oncol. 2011;37:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |