Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.953
Peer-review started: January 12, 2023
First decision: February 10, 2023
Revised: February 20, 2023
Accepted: March 31, 2023
Article in press: March 31, 2023
Published online: May 27, 2023
Processing time: 134 Days and 0.3 Hours
Gastrointestinal surgery is a complicated process used to treat many gastro
To assess whether early postoperative nutritional support can improve the nutritional status of patients based on literature search and meta-analysis.
Articles comparing the effect of early nutritional support and delayed nutritional support were retrieved from PubMed, EMBASE, Springer Link, Ovid, China National Knowledge Infrastructure, China Biology Medicine databases. Notably, only randomized controlled trial articles were retrieved from the databases (from establishment date to October 2022). The risk of bias of the included articles was determined using Cochrane Risk of Bias V2.0. The outcome indicators, such as albumin, prealbumin, and total protein, after statistical intervention were combined.
Fourteen literatures with 2145 adult patients undergoing gastrointestinal surgery (1138 patients (53.1%) receiving early postoperative nutritional support and 1007 patients (46.9%) receiving traditional nutritional support or delayed nutritional support) were included in this study. Seven of the 14 studies assessed early enteral nutrition while the other seven studies assessed early oral feeding. Furthermore, six literatures had "some risk of bias," and eight literatures had "low risk". The overall quality of the included studies was good. Meta-analysis showed that patients receiving early nutritional support had slightly higher serum albumin levels, than patients receiving delayed nutritional support [MD (mean difference) = 3.51, 95%CI: -0.05 to 7.07, Z = 1.93, P = 0.05]. Also, patients receiving early nutritional support had shorter hospital stay (MD = -2.29, 95%CI: -2.89 to -1.69), Z = -7.46, P < 0.0001) shorter first defecation time (MD = -1.00, 95%CI: -1.37 to -0.64), Z = -5.42, P < 0.0001), and fewer complications (Odd ratio = 0.61, 95%CI: 0.50 to 0.76, Z = -4.52, P < 0.0001) than patients receiving delayed nutritional support.
Early enteral nutritional support can slightly shorten the defecation time and overall hospital stay, reduce complication incidence, and accelerate the rehabilitation process of patients undergoing gastrointestinal surgery.
Core Tip: Gastrointestinal tract surgery is a complex process, with a wide range of operations and large trauma. It is easy to have various infectious complications in postoperative recovery, which affects the efficacy of surgical treatment. Early postoperative nutritional support can provide necessary nutrition, restore intestinal barrier, and reduce complications. However, whether early postoperative nutritional support can significantly improve the nutritional status of patients, different studies have reached different conclusions. This study used literature retrieval and Meta analysis to conduct quantitative analysis. It was found that early enteral nutrition support could shorten the defecation time after gastrointestinal surgery, the overall hospital stay, reduce the incidence of complications, and speed up the rehabilitation process. However, the improvement of nutritional status was not significant.
- Citation: He LB, Liu MY, He Y, Guo AL. Nutritional status efficacy of early nutritional support in gastrointestinal care: A systematic review and meta-analysis. World J Gastrointest Surg 2023; 15(5): 953-964
- URL: https://www.wjgnet.com/1948-9366/full/v15/i5/953.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i5.953
Gastrointestinal diseases (especially tumors) are becoming common yearly, thus seriously threatening the health and quality of life of many patients and burdening families and the whole society[1,2]. Gastrointestinal surgery is a complicated process used to treat many gastrointestinal diseases and is associated with large trauma. Patients have different degrees of malnutrition and immune dysfunction before surgery and are prone to various infectious complications during postoperative recovery, affecting the efficacy of surgical treatment[3]. However, nutritional support therapy can improve the above problems. Perioperative enteral or parenteral nutritional support provides the necessary nutritional supply and energy demand, thus improving the nutritional status of the patients and promoting early recovery of normal physiological function, especially gastrointestinal function. As a result, nutritional support therapy has received great clinical attention in recent years[4]. Parenteral nutrition (PN) and enteral nutrition (EN) are the most commonly used nutritional support methods. PN is mostly used in the early stage after gastrointestinal surgery in clinical practice[5]. However, PN can cause metabolic and functional complications by affecting intestinal mucosal metabolism and function, leading to impairment of the intestinal mucosal barrier, bacterial and endotoxin translocation, and increasing the incidence of enterogenous infections[6]. The rapid development of fast-track surgical nutrition in recent years can improve postoperative small intestinal peristalsis, digestion, and absorption function after a few hours of abdominal surgery, thus promoting the rapid development of early postoperative EN and early EN support[7]. Jordan et al[8] indicated that early EN can improve the reconstruction of the immune barrier, accelerate postoperative recovery, reduce complication incidence, and shorten the length of hospital stay. Besides, early EN is simpler, economical, and free of serious complications. However, no meta-analysis has studied the effect of early EN on nutritional status. Das et al[9] showed that early EN support cannot significantly improve the nutritional status of patients compared with traditional nutritional support. This study aimed to quantitatively investigate the effect of early nutritional support on nutritional status of patients undergoing gastrointestinal surgery based on meta-analysis.
All articles published before October 2022 were retrieved from PubMed, EMBASE, Scopus, Web of Science, China National Knowledge Infrastructure, and Chinese BioMedical Literature Database, regardless of the language. The clinical study registration website (Clinicaltrials.org) was also checked to avoid missing unpublished literature.
The following keywords were used for literature search: ("early"[All Fields] AND ("nutritional support"[MeSH Terms] OR ("nutritional"[All Fields] AND "support"[All Fields]) OR "nutritional support"[All Fields]) AND ("digestive system surgical procedures"[MeSH Terms] OR ("digestive"[All Fields] AND "system"[All Fields] AND "surgical"[All Fields] AND "procedures"[All Fields]) OR "digestive system surgical procedures"[All Fields] OR ("gastrointestinal"[All Fields] AND "surgery"[All Fields]) OR "gastrointestinal surgery"[All Fields])) AND (randomized controlled trial[Filter]).
Inclusion criteria: (1) Only single or multi-center randomized controlled trials (RCTs); (2) Patients undergoing gastrointestinal surgery, including esophageal cancer resection, gastric cancer resection, pancreatic cancer resection, acute pancreatitis, colorectal cancer resection and other types of surgery, excluding patients intolerant to early EN support; (3) Good quality studies based on implementation process (randomization process, data deviation, and data measurement). The patients were divided into the experimental group (observation group) and the control group. The possibility of deviation from the established intervention in the study quality was evacuated if there were differences in the basic data, such as age, type, tumor grade, and surgical classification between the two groups. Patients in the two groups underwent surgery via the same surgical methods, preoperative preparation, and infection control. However, patients in the experimental group began to receive nutritional support in the early postoperative period, while those in the control group received traditional nutritional support or delayed nutritional support. Early nutritional support was performed 1-3 d after surgery (enteral nutritional support, oral feeding of liquid diet, PN, or a mixture of multiple nutritional support methods), while conventional nutritional support was given using indwelling intestinal nasal tube, conventional intravenous infusion. The patients were gradually given clear water, liquid food, semi-liquid food after the first defecation; and (4) The primary outcome indicators included nutritional status indicators, serum albumin indicators, serum prealbumin indicators, and serum total protein indicators after the intervention, while the secondary outcome indicators included length of hospital stay, first defecation time, and incidence of postoperative complications.
Exclusion criteria: (1) Non-RCT studies (descriptive literature, observational studies, meeting minutes, review studies); (2) Studies with stroke patients, joint replacement patients, and other patients undergoing non-gastrointestinal surgery; (3) Studies with no nutritional status outcome indicators, or where data on outcome indicators could not be obtained; and (4) Studies comparing different nutritional formulations, or studies comparing EN with PN.
The quality of the included RCTs was conducted using Cochrane Risk of Bias V2.0[10]. This process involved five domains (randomization process, implementation bias, data bias, data measurement bias, and selection bias) and 1 overall bias assessment. Three evaluations ("low risk", "some concerns of risk" and "high risk") were used for each domain (or overall bias).
No other nutritional indicators, such as postoperative weight loss, muscle loss, hemoglobin, serum sodium, and potassium, were included in this study according to the actual retrieved literature.
Two researchers screened the retrieved literatures, read the abstract, obtained the remaining literatures after preliminary screening according to the inclusion and exclusion criteria, read the full text and further screened the RCTs, and removed the studies with serious bias and low quality after quality evaluation.
Data, such as interventions, total number of people, grouping, characteristics of study subjects, and outcome indicators, were extracted and entered Excel sheets. A uniform unit was used to represent the data. For example, g/dL was converted to g/L, 1 g/dL = 10 g/L and hour (h) was converted to day (d).
Continuous data (serum albumin, serum prealbumin, serum total protein, length of hospital stay, first defecation time after intervention) were expressed using combined mean difference (MD) and 95%CI as effect size, while discrete data (complication rate) were expressed using odd ratio (OR) as effect size. The combined results were presented as a forest plot using random effects model with P < 0.05 considered statistically significant. Tau values were calculated using Q test to ensure literature heterogeneity (P < 0.05 indicated heterogeneity). Subgroup analysis and one-by-one exclusion were used to calculate the contribution of each study to the results in case of heterogeneity between the articles. Publication bias was quantified using Egger' test and presented using trim-filled funnel plots.
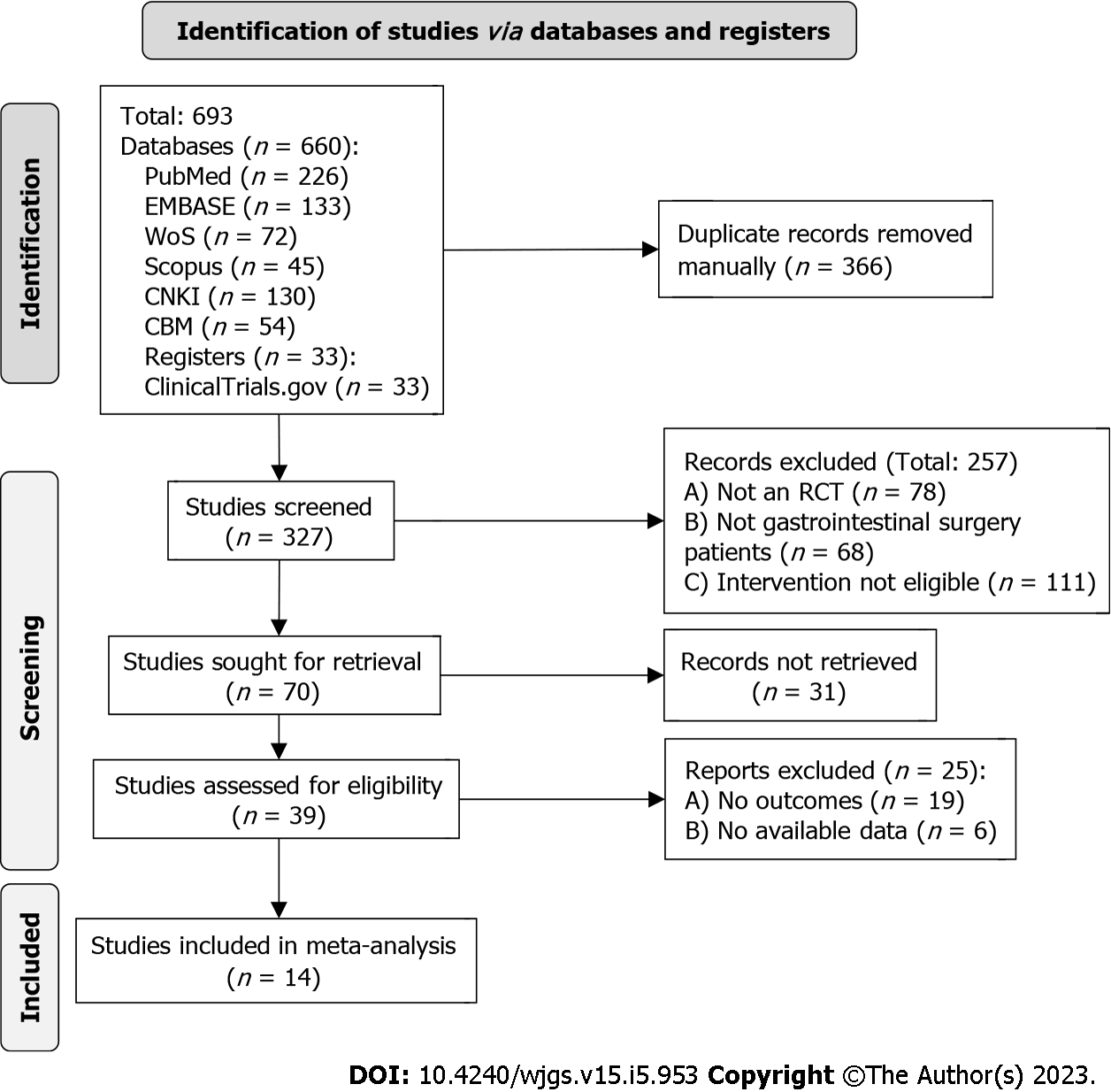
Literature search and screening (identification, screening involving the three main processes) followed The Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendation. The flow chart is shown in Figure 1. A total of 693 literatures were initially retrieved, and only 14 literatures were included in the final study after de-duplication and screening (Table 1).
| Ref. | Year | Design | Intention-to-treat total | Sample (E/D) | Surgery type | Age (yr) | Nutrition support mode | Outcomes |
| Sun et al[11] | 2017 | A prospective, randomized, single-blinded, controlled study | 107 | 53/54 | Major abdominal surgery | 56 ± 10 | Oral feeding | e, f |
| Pragatheeswarane et al[12] | 2014 | A randomized controlled study | 120 | 60/60 | Elective open bowel surgeries | 46.5 ± 17.2 | Oral feeding | d, e, f |
| Dag et al[13] | 2011 | A randomized controlled study | 199 | 99/100 | Elective open colorectal cancer surgery | 62 (35-85) | Oral feeding | d, e, f |
| Fujii et al[14] | 2014 | A controlled study | 120 | 62/58 | Elective colorectal resection surgery | 67.4 ± 11.7 | Oral feeding | a, d, e, f |
| Liao et al[15] | 2020 | A randomized controlled study | 41 | 21/20 | Esophageal carcinoma surgery | 57.2 ± 8.2 | Enteral nutrition | d, f |
| Mi et al[16] | 2012 | A randomized controlled study | 60 | 30/30 | Gastrectomy | 57.2 ± 9.5 | Oral feeding | a, b, d, f |
| Mahmoodzadeh et al[17] | 2015 | A randomized controlled study | 109 | 54/55 | Gastrointestinal surgeries | 64.2 ± 8.2 | Oral feeding | d, f |
| Wang et al[18] | 2005 | A retrospective comparative study | 454 | 227/227 | Colorectal cancer resection surgery | 63.5 ± 11.3 | Enteral nutrition | d, e, f |
| Qiu et al[19] | 2020 | A retrospective comparative study | 26 | 13/13 | Severe acute pancreatitis treatment | 33.4 ± 5.7 | Enteral nutrition | a, c, d |
| Wang et al[20] | 2015 | A randomized controlled study | 188 | 101/87 | Esophagectomy | 59.5 ± 8.4 | Enteral nutrition | a, c, d, e, f |
| Klappenbach et al[21] | 2013 | A randomized controlled study | 295 | 148/147 | Abdominal elective surgery | 37.3 ± 18.1 | Oral feeding | d, e, f |
| Li et al[22] | 2015 | A randomized controlled study | 300 | 150/150 | Gastric cancer surgery | 59.2 ± 9.7 | Enteral nutrition | a, b, d, f |
| Zou et al[23] | 2014 | A retrospective comparative study | 93 | 46/47 | Severe acute pancreatitis treatment | 46.5 (34.6-59.3) | Enteral nutrition | a, d, f |
| Barlow et al[24] | 2011 | A randomized controlled study | 121 | 64/57 | Upper gastrointestinal cancer surgery | 64.0 ± 15.0 | Eternal feeding | f |
Fourteen studies with 2145 adult patients (1138 patients (53.1%) who received early postoperative nutritional support and 1007 patients (46.9%) who received traditional nutritional support or delayed nutritional support) were included in this analysis. Seven of the 14 studies adopted early EN, while the other seven studies adopted early oral feeding (Table 1).
Three of the 14 articles (21.4%)[18-19,23] were retrospective controlled studies and had "some risk of bias" in terms of deviations from established interventions, data measurement bias, while four articles (28.6%)[11,16,18,19] had "some risk of bias" in terms of data measurement. Six articles had "some risk of bias" overall while eight articles had "low risk". All articles had good overall quality. The details of the assessment using Cochrane Risk of Bias V2.0 are shown in Figure 2A and Table 2.
| Ref. | Randomization Process | Bias from defined interventions | Data missing bias | Data measurement offset | Optional reporting | Overall bias | Weight (%) |
| Sun et al[11] | Low | Low | Low | Some concerns | Low | Some concerns | 8 |
| Sun et al[11] | Low | Low | Low | Low | Low | Low | 8 |
| Pragatheeswarane et al[12] | Low | Low | Low | Low | Low | Low | 8 |
| Dag et al[13] | Low | Low | Low | Low | Low | Low | 8 |
| Fujii et al[14] | Low | Low | Low | Low | Low | Low | 8 |
| Liao et al[15] | Low | Low | Low | Some concerns | Low | Some concerns | 8 |
| Mi et al[16] | Low | Low | Low | Low | Low | Some concerns | 8 |
| Mahmoodzadeh et al[17] | Low | Some concerns | Low | Some concerns | Low | Some concerns | 8 |
| Wang et al[18] | Low | Some concerns | Low | Some concerns | Low | Some concerns | 8 |
| Qiu et al[19] | Low | Low | Low | Low | Low | Low | 8 |
| Wang et al[20] | Low | Low | Low | Low | Low | Low | 8 |
| Klappenbach et al[21] | Low | Low | Low | Low | Low | Low | 8 |
| Li et al[22] | Low | Some concerns | Low | Low | Low | Some concerns | 8 |
| Zou et al[23] | Low | Low | Low | Low | Low | Low | 8 |
| Barlow et al[24] | Low | Some concerns | Low | Some concerns | Low | Some concerns | 8 |
| Klappenbach et al[21] | Low | Low | Low | Low | Low | Low | 8 |
| Li et al[22] | Low | Low | Low | Low | Low | Low | 8 |
| Zou et al[23] | Low | Some concerns | Low | Low | Low | Some concerns | 8 |
| Barlow et al[24] | Low | Low | Low | Low | Low | Low | 8 |
| Klappenbach et al[21] | Low | Low | Low | Low | Low | Low | 8 |
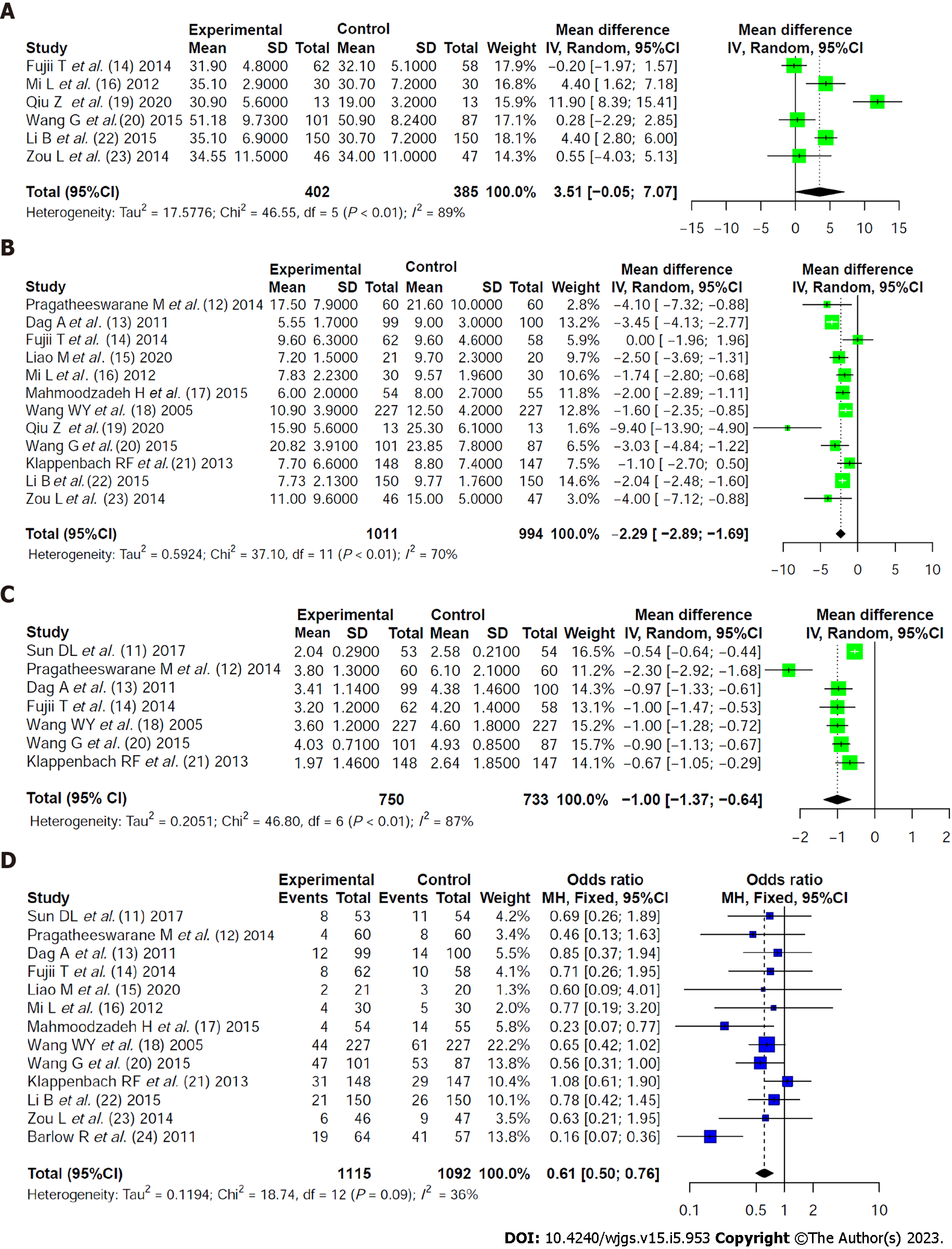
Albumin (g/L): Six literatures reported albumin levels after nutritional support intervention in the two groups. The heterogeneity among the literatures was statistically significant (χ2 = 46.55, I2 = 89%, P < 0.01), including 402 patients who received early nutritional support and 385 patients who received traditional nutritional support. A random-effects model showed that serum albumin levels were slightly higher in patients receiving early nutritional support than in patients receiving traditional nutritional support (MD = 3.51, 95%CI: -0.05 to 7.07, Z = 1.93, P = 0.05, Figure 2A).
Prealbumin and total serum protein (g/L): Only two literatures reported prealbumin and serum total protein levels (Table 3).
| Outcomes | Literature number | Analysis mode | P value | Effect size | Pooling value | Z, P value |
| Prealbumin | 2 | Fixed effect mode | 0.22 | mean difference | 12.4776 (9.1231, 15.8320) | 7.29, < 0.0001 |
| Serum total protein | 2 | Random effect mode | 0.0002 | mean difference | 5.2401 (-5.1833, 15.6635) | 0.99, 0.3245 |
Length of stay (d): Twelve literatures compared the length of hospital stay between the two groups. The heterogeneity among the literatures was statistically significant (χ2 = 37.10, I2 = 70%, P < 0.01) (1011 patients who received early nutritional support and 994 patients who received traditional nutritional support). A random-effects model showed that patients receiving early nutritional support spent significantly less time in the hospital than patients receiving traditional nutritional support (MD = -2.29, 95%CI: -2.89 to -1.69, Z = -7.46, P < 0.0001, Figure 2B).
Time to first defecation: Sevan literatures compared the first defecation time between the two groups. The heterogeneity among the literatures was statistically significant (Chi2=46.80, I2=87%, P < 0.01) (750 patients receiving early nutritional support and 733 patients receiving traditional nutritional support). A random-effects model showed that patients receiving early nutritional support took a significantly shorter time to first defecation than patients receiving traditional nutritional support (MD = -1.00, 95%CI: -1.37 to -0.64, Z = -5.42, P < 0.0001, Figure 2C).
Complication rate: Thirteen literatures compared the incidence of complications between the two groups. There was no statistically significant heterogeneity among the literatures (χ2 = 18.74, I2 = 36%, P = 0.09). A fixed effect model showed that the incidence rate of complications was significantly lower in patients receiving early nutritional support than in patients receiving traditional nutritional support (OR = 0.61, 95%CI: 0.50 to 0.76, Z = -4.52, P < 0.0001, Figure 2D).
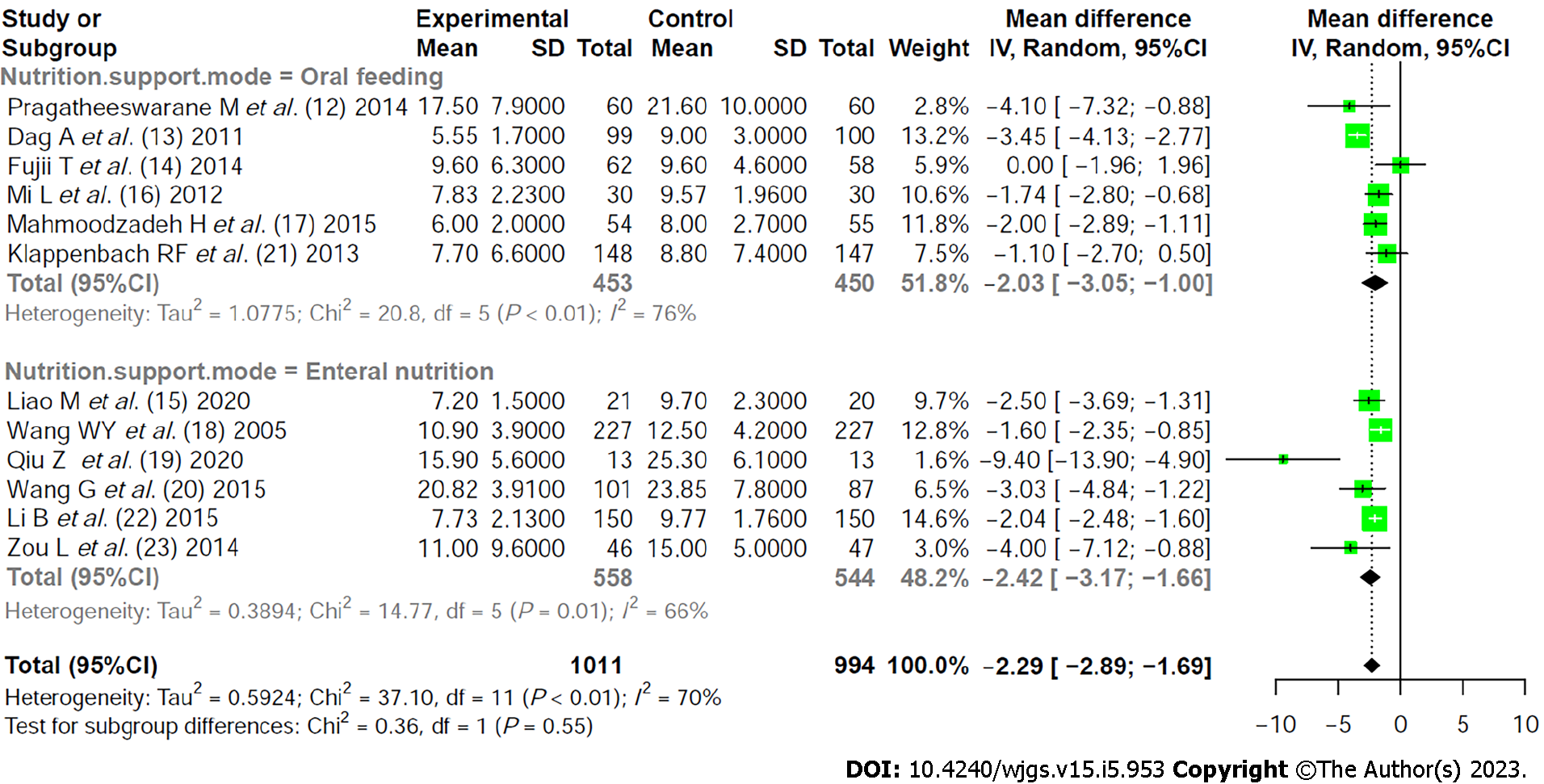
Heterogeneity investigation: Twelve literatures were divided into two subgroups based on different methods of early nutritional support to analyze the source of literature heterogeneity. Subgroup analysis showed that there was no significant difference between the two groups (P = 0.55), indicating that early nutritional support method was not the source of literature heterogeneity (Figure 3).
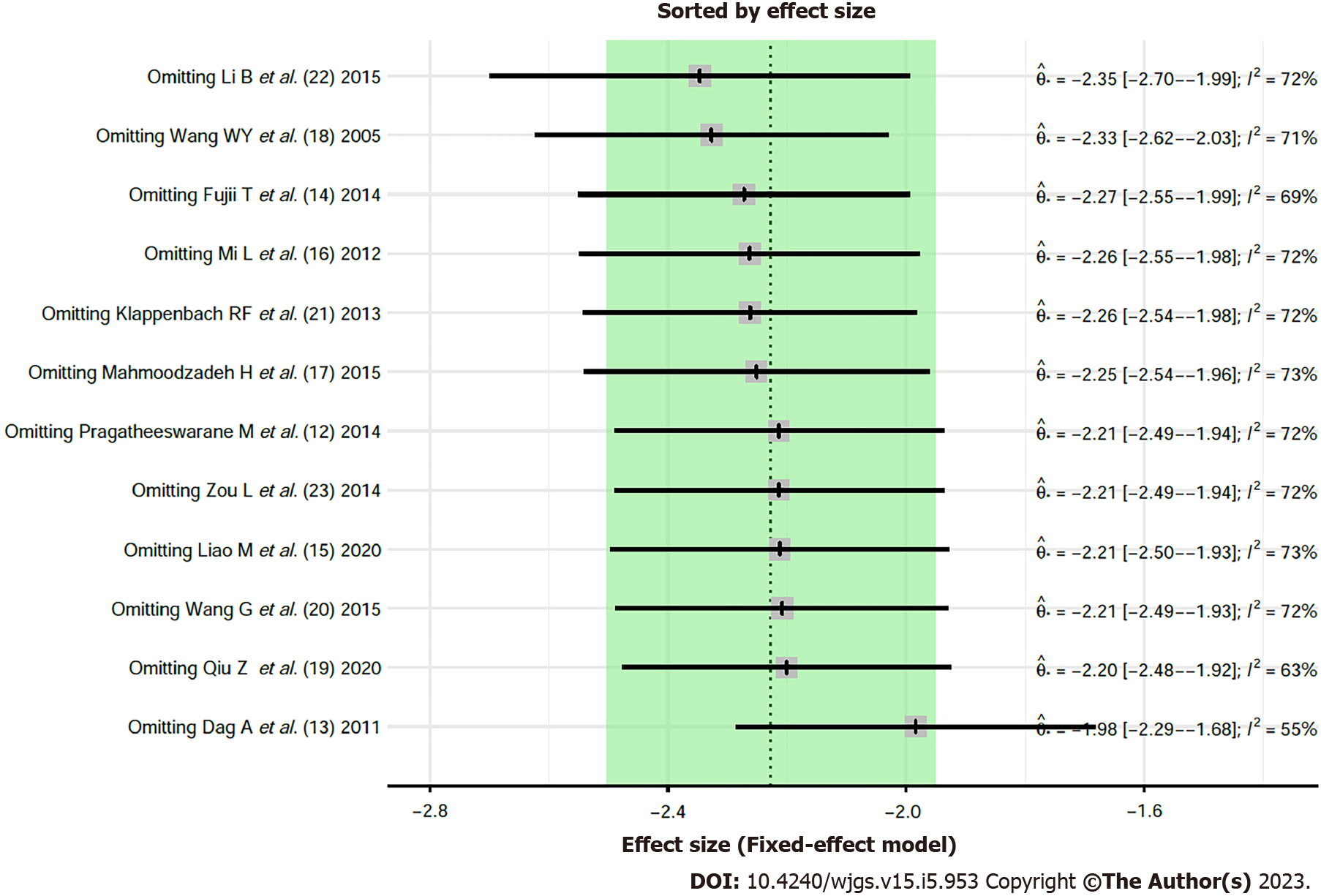
Influence analysis: The influence analysis on the outcome indicators of postoperative hospital stay was performed by removing the literatures one by one. The results did not find any significant differences, indicating that the overall results were stable and there was no variability in the study results (Figure 4).
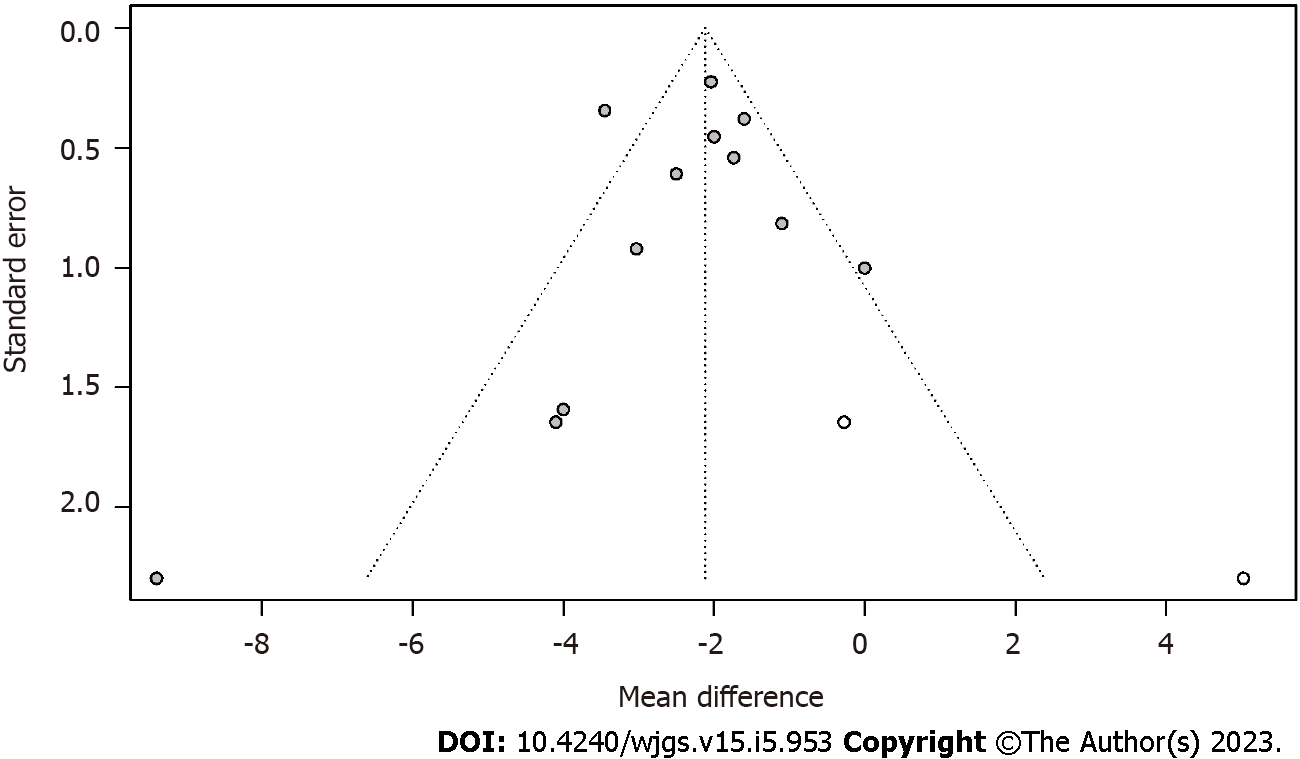
Publication bias analysis: Publication bias in the combined results of postoperative hospital stay outcome indicators was measured using Egger' test (t = -0.78, P = 0.4551). The P value was > 0.05, indicating that there was no publication bias. The funnel plot after trim-filled is shown in Figure 5.
Gastrointestinal surgery can lead to many pathophysiological changes in the human body (acute phase reactions), especially after larger operations, causing significant and persistent metabolic alterations characterized by hypercatabolism and declining total somatic cell counts[25]. Yuan et al[26] suggested that early EN may mitigate this endocrine and metabolic response. The recovery of intestinal function takes about three days after abdominal surgery due to anesthesia and surgical trauma, and most recovery markers are anal excretions. However, postoperative gastrointestinal paralysis mainly occurs in the stomach and colon. Besides, most small intestines with normal preoperative function recover from peristalsis a few hours after surgery and thus can absorb nutrients for about 12 h, thus providing a theoretical basis for the implementation of EN in the early postoperative period[27].
In this study, serum prealbumin levels were significantly higher in the early nutritional support group than in the traditional nutritional support. However, albumin level and serum total protein levels were not significantly different between the two groups, suggesting that early nutritional support does not significantly improve nutritional status of patients. Also, the combined results showed that early nutritional support shortened the first defecation time and hospital stay, reduced complications (infection), and accelerated postoperative rehabilitation of patients. Postoperative gastrointestinal paralysis only occurs in the stomach and colon. The small intestine can quickly restore peristalsis and absorption function. The intestinal mucosa with intraluminal nutrition is the main way to obtain energy when the body is hungry, fasting, disease process, surgical trauma, and other circumstances. However, the intestinal mucosa cannot obtain the nutritional substrates required for its energy supply from the intestinal lumen. Intestinal mucosal barrier and immune barrier damage may lead to intestinal flora imbalance, intestinal failure, resulting in poor prognosis. Partial nutrient supplementation can promote early recovery of intestinal physiological function after surgery, protect the barrier function of intestinal mucosa, and prevent postoperative infectious complications[28]. In addition, early EN support ensures the energy supply of immune cells and normal operation of immune cell function while providing nutrients for the intestinal mucosa, thereby promoting the recovery of immune function after surgery and effectively inhibiting the inflammatory response[29]. In this study, early nutritional support was consistent with the nutritional formula adopted for delayed nutritional support. The effect of the two nutritional support regimens on patient nutrition was not significantly different. Besides, no theoretical support has indicated whether early nutritional intervention after surgery can improve the nutritional status of patients. The improvement of the nutritional status of patients is mainly determined by the patient's physical condition and the formulation of nutritional preparations. Nonetheless, the clinical value of early nutritional intervention for a better prognosis should not be ignored.
In this meta-analysis, Boscarino et al[30] concluded that EN can improve intestinal mucosal circulation, facilitate epithelial cells to take energy directly from the intestine and improve microecological environment, prevent translocation of intestinal flora, protect intestinal mucosal barrier, reduce bacterial infection, and promote intestinal peristalsis in postoperative patients compared with PN. However, this meta-analysis did not focus on the type of nutritional support.
Early postoperative nutritional support cannot be as early as possible. Notably, EN may only increase the burden of body metabolism when the respiratory, circulatory, water electrolyte, and acid-base balance of critically ill patients are not stable. In addition, EN may cause diarrhea, abdominal distension, vomiting, and other symptoms when intestinal function has not been resuscitated, thus aggravating the physiological dysfunction. Therefore, special attention should be paid to indications when early postoperative enteral nutritional support is applied. Early nutritional support should be discontinued and changed to PN once a patient develops intolerance[31].
Furthermore, although heterogeneity was significant among literatures, heterogeneity was not detected within subgroups after subgroup analysis according to factors (nutritional support route) that can cause heterogeneity among literatures, suggesting that the source of heterogeneity was independent of nutritional support route. Therefore, the heterogeneity could have been caused by sample characteristics of subjects in different studies.
Although seven literatures had "some concerns of risk", the overall quality of the literatures was good, the results were stable, and there was no publication bias. However, only six literatures reported albumin of nutritional indicators, while only two literatures reported preprotein and total protein indicators, indicating that the effect of early nutritional support on the improvement of nutritional status should be further studied. Furthermore, very few reports had analyzed the key nutritional indicators, such as potassium, sodium, hemoglobin, and weight loss in such RCT studies, and thus a meta-analysis synthesis could not be performed. Therefore, more studies of better quality are needed for in-depth analysis of different indicators from different perspectives.
Although this study showed that early EN support can shorten the postoperative defecation time, overall hospital stay, reduce the incidence of complications, and accelerate the rehabilitation process in patients undergoing gastrointestinal surgery, the improvement of nutritional status was not significant. Also, this study included a few articles and thus lacked an in-depth analysis for some important nutritional indicators. Therefore, more clinical multicenter, large-sample, high-quality studies are needed to further evaluate the effect of early EN support on patient's nutritional status.
Gastrointestinal tract surgery is a complex process, with a wide range of operations and large trauma. It is easy to have various infectious complications in postoperative recovery, which affects the efficacy of surgical treatment.
Early postoperative nutritional support can provide necessary nutrition, restore intestinal barrier, and reduce complications.
This study aimed to assess whether early postoperative nutritional support can improve the nutritional status of patients based on literature search and meta-analysis.
This study used literature retrieval and meta-analysis to conduct quantitative analysis.
It was found that early enteral nutrition (EN) support could shorten the defecation time after gastrointestinal surgery, the overall hospital stay, reduce the incidence of complications, and speed up the rehabilitation process.
Early enteral nutritional support can slightly shorten the defecation time and overall hospital stay, reduce complication incidence, and accelerate the rehabilitation process of patients undergoing gastrointestinal surgery.
More clinical multicenter, large-sample, high-quality studies are needed to further evaluate the effect of early EN support on patient's nutritional status.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Nutrition and dietetics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haghpanah A, Iran; Silva LD, Brazil; Tan RJDD, Philippines S-Editor: Wang JL L-Editor: A P-Editor: Wu RR
| 1. | Wobith M, Weimann A. Oral Nutritional Supplements and Enteral Nutrition in Patients with Gastrointestinal Surgery. Nutrients. 2021;13. [PubMed] [DOI] [Full Text] |
| 2. | Vining CC, Skowron KB, Hogg ME. Robotic gastrointestinal surgery: learning curve, educational programs and outcomes. Updates Surg. 2021;73:799-814. [PubMed] [DOI] [Full Text] |
| 3. | He FJ, Wang MJ, Yang K, Chen XL, Jin T, Zhu LL, Zhuang W. Effects of Preoperative Oral Nutritional Supplements on Improving Postoperative Early Enteral Feeding Intolerance and Short-Term Prognosis for Gastric Cancer: A Prospective, Single-Center, Single-Blind, Randomized Controlled Trial. Nutrients. 2022;14. [PubMed] [DOI] [Full Text] |
| 4. | Ogbadua AO, Agida TE, Akaba GO, Akitoye OA, Ekele BA. Early Versus Delayed Oral Feeding after Uncomplicated Cesarean Section under Spinal Anesthesia: A Randomized Controlled Trial. Niger J Surg. 2018;24:6-11. [PubMed] [DOI] [Full Text] |
| 5. | Allen K, Hoffman L. Enteral Nutrition in the Mechanically Ventilated Patient. Nutr Clin Pract. 2019;34:540-557. [PubMed] [DOI] [Full Text] |
| 6. | Tian F, Heighes PT, Allingstrup MJ, Doig GS. Early Enteral Nutrition Provided Within 24 Hours of ICU Admission: A Meta-Analysis of Randomized Controlled Trials. Crit Care Med. 2018;46:1049-1056. [PubMed] [DOI] [Full Text] |
| 7. | Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ. Impact of early enteral and parenteral nutrition on prealbumin and high-sensitivity C-reactive protein after gastric surgery. Genet Mol Res. 2015;14:7130-7135. [PubMed] [DOI] [Full Text] |
| 8. | Jordan EA, Moore SC. Enteral nutrition in critically ill adults: Literature review of protocols. Nurs Crit Care. 2020;25:24-30. [PubMed] [DOI] [Full Text] |
| 9. | Das BC, Haque M, Uddin MS, Nur-E-Elahi M, Khan ZR. Effect of early and delay starting of enteral feeding in post-pancreaticoduodenectomy patients. Ann Hepatobiliary Pancreat Surg. 2019;23:56-60. [PubMed] [DOI] [Full Text] |
| 10. | Minozzi S, Dwan K, Borrelli F, Filippini G. Reliability of the revised Cochrane risk-of-bias tool for randomised trials (RoB2) improved with the use of implementation instruction. J Clin Epidemiol. 2022;141:99-105. [PubMed] [DOI] [Full Text] |
| 11. | Sun DL, Li WM, Li SM, Cen YY, Xu QW, Li YJ, Sun YB, Qi YX, Lin YY, Yang T, Lu QP, Xu PY. Comparison of multi-modal early oral nutrition for the tolerance of oral nutrition with conventional care after major abdominal surgery: a prospective, randomized, single-blind trial. Nutr J. 2017;16:11. [PubMed] [DOI] [Full Text] |
| 12. | Pragatheeswarane M, Muthukumarassamy R, Kadambari D, Kate V. Early oral feeding vs. traditional feeding in patients undergoing elective open bowel surgery-a randomized controlled trial. J Gastrointest Surg. 2014;18:1017-1023. [PubMed] [DOI] [Full Text] |
| 13. | Dag A, Colak T, Turkmenoglu O, Gundogdu R, Aydin S. A randomized controlled trial evaluating early versus traditional oral feeding after colorectal surgery. Clinics (Sao Paulo). 2011;66:2001-2005. [PubMed] [DOI] [Full Text] |
| 14. | Fujii T, Morita H, Sutoh T, Yajima R, Yamaguchi S, Tsutsumi S, Asao T, Kuwano H. Benefit of oral feeding as early as one day after elective surgery for colorectal cancer: oral feeding on first versus second postoperative day. Int Surg. 2014;99:211-215. [PubMed] [DOI] [Full Text] |
| 15. | Liao M, Xia Z, Huang P, Shi Q, Li H, He R, Bao M, Qiao K. Early enteral feeding on esophageal cancer patients after esophageal resection and reconstruction. Ann Palliat Med. 2020;9:816-823. [PubMed] [DOI] [Full Text] |
| 16. | Mi L, Zhong B, Zhang DL, Zhou YB, Wang DS. [Effect of early oral enteral nutrition on clinical outcomes after gastric cancer surgery]. Zhonghua Waike Weichang Zazhi. 2012;15:464-467. [DOI] [Full Text] |
| 17. | Mahmoodzadeh H, Shoar S, Sirati F, Khorgami Z. Early initiation of oral feeding following upper gastrointestinal tumor surgery: a randomized controlled trial. Surg Today. 2015;45:203-208. [PubMed] [DOI] [Full Text] |
| 18. | Wang WY, Chen CW, Wang TJ, Lin KL, Liu CY. Outcomes of early enteral feeding in patients after curative colorectal cancer surgery: A retrospective comparative study. Eur J Oncol Nurs. 2021;54:101970. [PubMed] [DOI] [Full Text] |
| 19. | Qiu Z, Cheng F, Jiang H, Li L, Zheng C, Du Z, Wang Z. Efficacy of Microecopharmaceutics Combined with Early Enteral Nutrition Support in the Treatment of Severe Acute Pancreatitis. J Coll Physicians Surg Pak. 2020;30:96-98. [PubMed] [DOI] [Full Text] |
| 20. | Wang G, Chen H, Liu J, Ma Y, Jia H. A comparison of postoperative early enteral nutrition with delayed enteral nutrition in patients with esophageal cancer. Nutrients. 2015;7:4308-4317. [PubMed] [DOI] [Full Text] |
| 21. | Klappenbach RF, Yazyi FJ, Alonso Quintas F, Horna ME, Alvarez Rodríguez J, Oría A. Early oral feeding versus traditional postoperative care after abdominal emergency surgery: a randomized controlled trial. World J Surg. 2013;37:2293-2299. [PubMed] [DOI] [Full Text] |
| 22. | Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ. Impact of early postoperative enteral nutrition on clinical outcomes in patients with gastric cancer. Genet Mol Res. 2015;14:7136-7141. [PubMed] [DOI] [Full Text] |
| 23. | Zou L, Ke L, Li W, Tong Z, Wu C, Chen Y, Li G, Li N, Li J. Enteral nutrition within 72 h after onset of acute pancreatitis vs delayed initiation. Eur J Clin Nutr. 2014;68:1288-1293. [PubMed] [DOI] [Full Text] |
| 24. | Barlow R, Price P, Reid TD, Hunt S, Clark GW, Havard TJ, Puntis MC, Lewis WG. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clin Nutr. 2011;30:560-566. [PubMed] [DOI] [Full Text] |
| 25. | Patel JJ, Rice T, Heyland DK. Safety and Outcomes of Early Enteral Nutrition in Circulatory Shock. JPEN J Parenter Enteral Nutr. 2020;44:779-784. [PubMed] [DOI] [Full Text] |
| 26. | Yuan F, Yang F, Zhang W, Jia Y, Ma Y, Qu Y, Wang X, Huo K, Wang C, Yuan X, Song C, Zhang B, Jiang W; OPENS study group. Optimizing early enteral nutrition in severe stroke (OPENS): protocol for a multicentre randomized controlled trial. BMC Neurol. 2019;19:24. [PubMed] [DOI] [Full Text] |
| 27. | Srinivasan V, Hasbani NR, Mehta NM, Irving SY, Kandil SB, Allen HC, Typpo KV, Cvijanovich NZ, Faustino EVS, Wypij D, Agus MSD, Nadkarni VM; Heart and Lung Failure-Pediatric Insulin Titration (HALF-PINT) Study Investigators. Early Enteral Nutrition Is Associated With Improved Clinical Outcomes in Critically Ill Children: A Secondary Analysis of Nutrition Support in the Heart and Lung Failure-Pediatric Insulin Titration Trial. Pediatr Crit Care Med. 2020;21:213-221. [PubMed] [DOI] [Full Text] |
| 28. | Gao X, Liu Y, Zhang L, Zhou D, Tian F, Gao T, Tian H, Hu H, Gong F, Guo D, Zhou J, Gu Y, Lian B, Xue Z, Jia Z, Chen Z, Wang Y, Jin G, Wang K, Zhou Y, Chi Q, Yang H, Li M, Yu J, Qin H, Tang Y, Wu X, Li G, Li N, Li J, Pichard C, Wang X. Effect of Early vs Late Supplemental Parenteral Nutrition in Patients Undergoing Abdominal Surgery: A Randomized Clinical Trial. JAMA Surg. 2022;157:384-393. [PubMed] [DOI] [Full Text] |
| 29. | Sun HB, Li Y, Liu XB, Wang ZF, Zhang RX, Lerut T, Zheng Y, Liu SL, Chen XK. Impact of an Early Oral Feeding Protocol on Inflammatory Cytokine Changes After Esophagectomy. Ann Thorac Surg. 2019;107:912-920. [PubMed] [DOI] [Full Text] |
| 30. | Boscarino G, Conti MG, Di Chiara M, Bianchi M, Onestà E, Faccioli F, Deli G, Repole P, Oliva S, Cresi F, Terrin G. Early Enteral Feeding Improves Tolerance of Parenteral Nutrition in Preterm Newborns. Nutrients. 2021;13. [PubMed] [DOI] [Full Text] |
| 31. | Sun YB, Li YL, Li WM, Sun DL, Li SM, Xu QW, Li YJ, Lin YY, Cen YY, Xu PY. Effect of appetite-conditioned reflex stimulation on early enteral nutrition tolerance after surgery. Acta Gastroenterol Belg. 2020;83:527-531. [PubMed] |