Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.906
Peer-review started: February 19, 2023
First decision: March 1, 2023
Revised: March 11, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 27, 2023
Processing time: 95 Days and 23.3 Hours
Colorectal cancer (CRC) is a highly prevalent malignancy of the digestive tract worldwide, characterized by a significant morbidity and mortality rate and subtle initial symptoms. Diarrhea, local abdominal pain, and hematochezia occur with the development of cancer, while systemic symptoms such as anemia and weight loss occur in patients with advanced CRC. Without timely interventions, the disease can have fatal consequences within a short span. The current therapeutic options for colon cancer include olaparib and bevacizumab, which are widely utilized. This study intends to evaluate the clinical efficacy of olaparib combined with bevacizumab in the treatment of advanced CRC, hoping to provide insights into advanced CRC treatment.
To investigate the retrospective efficacy of olaparib combined with bevacizumab in the treatment of advanced CRC.
A retrospective analysis was conducted on a cohort of 82 patients with advanced colon cancer who were admitted to the First Affiliated Hospital of the University of South China between January 2018 and October 2019. Among them, 43 patients subjected to the classical FOLFOX chemotherapy regimen were selected as the control group, and 39 patients undergoing treatment with olaparib combined with bevacizumab were selected as the observation group. Subsequent to different treatment regimens, the short-term efficacy, time to progression (TTP), and incidence rate of adverse reactions between the two groups were compared. Changes in serum-related indicators [vascular endothelial growth factor (VEGF), matrix metalloprotein-9 (MMP-9), cyclooxygenase-2 (COX-2)] and tumor markers [human epididymis protein 4 (HE4), carbohydrate antigen 125 (CA125), carbohydrate antigen 199 (CA199)] levels before and after treatment were compared between the two groups at the same time.
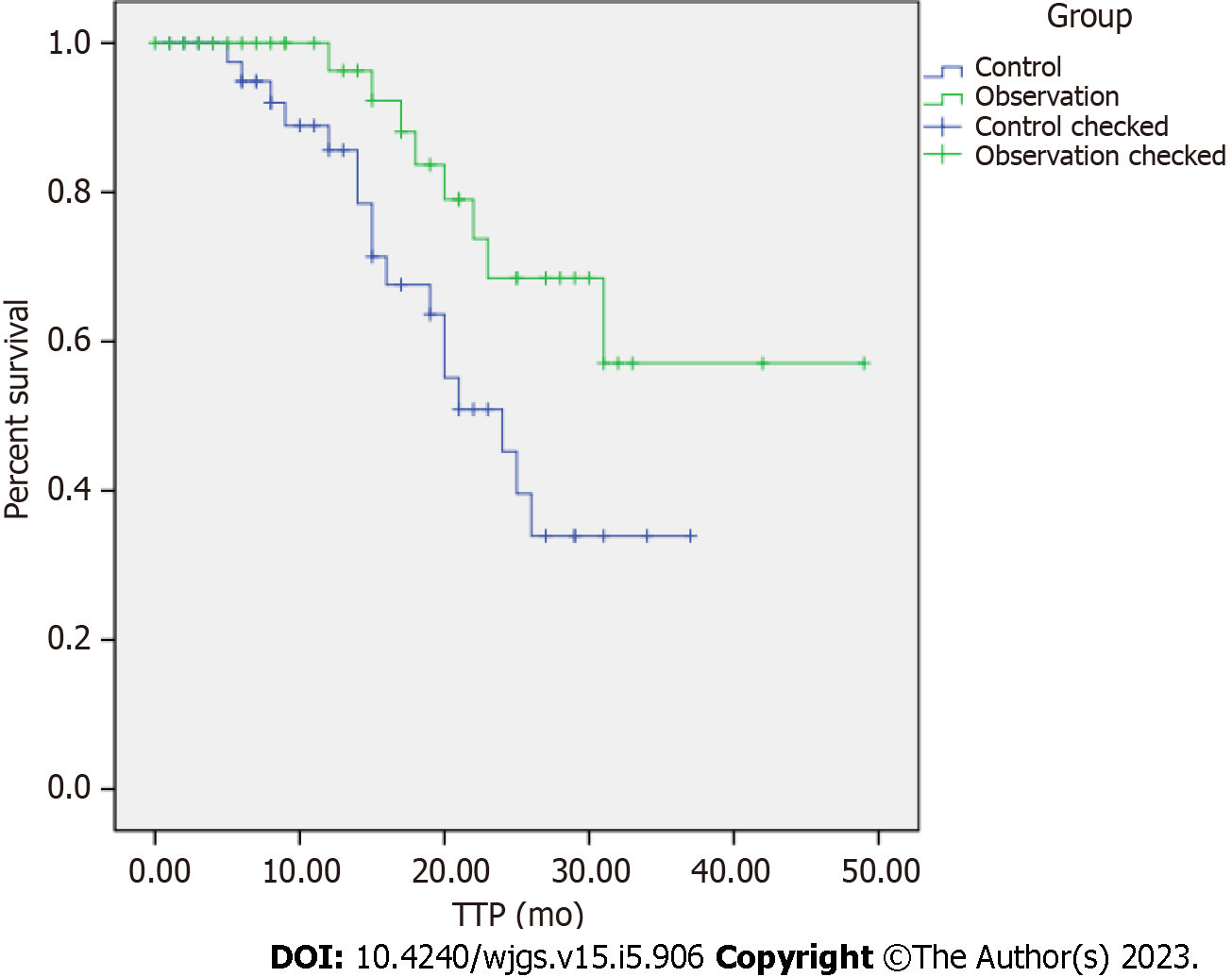
The objective response rate was discovered to be 82.05%, and the disease control rate was 97.44% in the observation group, which were significantly higher than the respective rates of 58.14% and 83.72% in the control group (P < 0.05). The median TTP was 24 mo (95%CI: 19.987-28.005) in the control group and 37 mo (95%CI: 30.854-43.870) in the observation group. The TTP in the observation group was significantly better than that in the control group, and the difference held statistical significance (log-rank test value = 5.009, P = 0.025). Before treatment, no substantial difference was detected in serum VEGF, MMP-9, and COX-2 levels and tumor markers HE4, CA125, and CA199 levels between the two groups (P > 0.05). Following treatment with different regimens, the above indicators in the two groups were remarkably promoted (P < 0.05), VEGF, MMP-9, and COX-2 in the observation group were lower than those in the control group (P < 0.05), and HE4, CA125, and CA199 levels were also lower than those in the control group (P < 0.05). Vis-à-vis the control group, the total incidence of gastrointestinal reactions, thrombosis, bone marrow suppression, liver and kidney function injury, and other adverse reactions in the observation group was notably lowered, with the difference considered statistically significant (P < 0.05).
Olaparib combined with bevacizumab in the treatment of advanced CRC demonstrates a strong clinical effect of delaying disease progression and reducing the serum levels of VEGF, MMP-9, COX-2 and tumor markers HE4, CA125 and CA199. Moreover, given its fewer adverse reactions, it can be regarded as a safe and reliable treatment option.
Core Tip: Colorectal cancer (CRC) presents insignificant symptoms in the early stage and is commonly diagnosed in the middle and advanced stages. Therefore, surgery is usually not viable because the best timing is missed, and chemotherapy, targeted therapies and other regimens are often utilized as interventions. Olaparib and bevacizumab are common targeted therapies with excellent therapeutic effects in a variety of solid tumors. This research collected the clinical data of 82 patients with advanced CRC, retrospectively investigated the clinical efficacy and safety of olaparib combined with bevacizumab in advanced CRC treatment, and analyzed the effect of this treatment regimen on the serum levels of vascular endothelial growth factor, matrix metalloprotein-9, cyclooxygenase-2, and related tumor markers.
- Citation: Jiang YL, Fu XY, Yin ZH. Retrospective efficacy analysis of olaparib combined with bevacizumab in the treatment of advanced colorectal cancer. World J Gastrointest Surg 2023; 15(5): 906-916
- URL: https://www.wjgnet.com/1948-9366/full/v15/i5/906.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i5.906
Colorectal cancer (CRC), a malignant tumor disease of the digestive tract originating from the epithelial tissues of the colon or rectal mucosa, is the third most prevalent cancer worldwide and the fourth leading contributor to cancer-related death[1-3]. Epidemiological statistics show that as of 2018, approximately 1.8 million new CRC pathologies were reported worldwide, resulting in 881000 deaths, underscoring the considerable harmful consequences of this disease[4]. Over the past five decades, the incidence and mortality of colon cancer have increased year by year in young and middle-aged people (< 50 years old), with an annual increase of 2% in individuals < 50 years old since 1994, leading to a surge in CRC incidence in younger age groups[5]. According to different histological types, colon cancer can be classified into three categories: adenocarcinoma, mucinous adenocarcinoma, and undifferentiated carcinoma[6,7]. Early-stage colon cancer often lacks obvious clinical symptoms and is easily neglected. Progressing to the middle and later stages, the disease often demonstrates abdominal pain, abdominal distension, diarrhea, hematochezia, and other symptoms. Moreover, about 20%-50% of patients have developed distant organ metastasis to varying degrees at the time of diagnosis[8], which seriously impacts patient survival and prognosis.
At present, colon cancer is predominantly treated with surgical resection, but this approach is not suitable for advanced metastatic colon cancer. Chemotherapeutic drugs such as 5-fluorouracil, oxaliplatin, and olaparib are used clinically for first-line targeted chemotherapy[9,10]. In addition, with the continuous improvement of targeted molecular therapies, treatment strategies targeting vascular endothelial growth factor (VEGF) as well as epidermal growth factor (EGF) receptors have been demonstrated to heighten the survival rate of patients with advanced colon cancer[11,12]. Bevacizumab, an anti-angiogenic targeted therapeutic agent with strong anti-tumor activities, has been applied in the clinical treatment of malignant solid tumors such as breast cancer, non-small cell lung cancer, and CRC, and has achieved certain results[13,14]. Nevertheless, the efficacy of olaparib combined with beva
Eighty-two patients with advanced colon cancer admitted to the First Affiliated Hospital of the University of South China from January 2018 to October 2019 were enrolled as the subjects of this retrospective study. The control group consisted of 43 patients who received the classical FOLFOX chemotherapy regimen, while the observation group included 39 patients who received olaparib combined with bevacizumab. The control group was composed of 24 males and 19 females, with age ranging from 22 to 71 years (average age: 46.02 ± 7.28 years), while the observation group encompassed 19 males and 20 females aged between 21-73 years (mean age: 48.37 ± 6.41 years). There was no remarkable statistical difference in gender, age, body mass index, histological type, and tumor-node-metastasis stage between the two groups (P > 0.05), as shown in Table 1, indicating the comparability of the two groups.
| General information | Control group (n = 43) | Observation group (n = 39) | t/χ2 | P value |
| Gender (n) | 0.413 | 0.521 | ||
| Male | 24 | 19 | ||
| Female | 19 | 20 | ||
| Age (yr), mean ± SD | 46.02 ± 7.28 | 48.37 ± 6.41 | 1.545 | 0.126 |
| BMI (kg/m2), mean ± SD | 22.45 ± 3.46 | 23.01 ± 2.98 | 0.781 | 0.437 |
| Histological classification (n) | 0.427 | 0.808 | ||
| Adenocarcinoma | 27 | 22 | ||
| Mucinous cancer | 12 | 12 | ||
| Undifferentiated carcinoma | 4 | 5 | ||
| TNM stage (n) | 2.201 | 0.138 | ||
| III | 12 | 17 | ||
| IV | 31 | 22 |
Inclusion criteria: Participants were aged 18 years or older; diagnosed with advanced colon cancer by magnetic resonance imaging, computed tomography, and other imaging examinations combined with pathological section, cytological examination, and clinical diagnosis; and were at stage III-IV according to the eighth edition of the American Joint Committee on Cancer Staging Manual[15]. Additionally, patients were required to have at least one objective measurable tumor lesion, blood routine, electrocardiogram, and other biochemical tests before treatment. Participants with no history of drug allergy and complete clinical data were included in the study.
Exclusion criteria: Patients with liver cancer, gastric cancer, and other malignant tumor diseases; patients with blood diseases or autoimmune diseases; patients with heart, liver and kidney, and other vital organ dysfunction; patients who received radiotherapy or other regimens of chemotherapy before enrollment; patients with hypertension, diabetes, heart disease, and other underlying diseases; patients with mental disorders, Alzheimer’s disease or other cognitive impairment; patients during pregnancy and lactation; an expected survival of fewer than 3 mo.
The control group (43 patients) received classical FOLFOX chemotherapy, which consisted of oxaliplatin (Zhejiang Hisun Pharmaceutical Co., Ltd.; approval number: GYZZ H20093487) administered intravenously at a dose of 135 mg/m2 for 2 h on day 1, calcium folinate (Jiangsu Hengrui Medicine Co., Ltd.; approval number: GYZZ H20000418) administered at 200 mg/m2 for 2 h on days 1-3, and 5-fluorouracil (Hainan Zhuotai Pharmaceutical Co., Ltd.; approval number: GYZZ H20051626) given continuously by an intravenous pump at a dose of 2600 mg/m2 for 46-48 h, every 3 weeks for a total of 6 cycles of chemotherapy.
The observation group (39 patients) underwent the treatment of olaparib combined with bevacizumab. To wit, olaparib (AstraZeneca; registration certificate number: H20180049) was taken orally at a dose of 200 mg twice daily- once in the morning and once in the evening; bevacizumab injection (Shanghai Roche Pharmaceutical Co., Ltd.; approval No.: S20120068) was intravenously injected at 15 mg/kg for 0.5-1 h on day 1, every three weeks for a total of six cycles of chemotherapy.
Short-term efficacy: As per the iRECIST response evaluation criteria for cancer immunotherapy [16], the efficacy of both patient groups was assessed at the conclusion of treatment. Short-term efficacy was categorized into immune complete response (iCR), immune partial response (iPR), immune stable disease (iSD) and immune progressive disease (iPD). Among them, iCR was identified as tumor changes disappeared and clinical symptoms improved; iPR was recognized upon lessened tumor volume and no detection of new tumor metastasis or lesion; iSD was identified when the tumor volume was reduced (degree of reduction less than 25%), and no new metastasis or lesion occurred; iPD was recognized when the tumor volume barely changed, and the number of distant metastasis and lesion increased instead. Objective response rate (ORR) = (iCR + iPR)/total number of patients > 100%, disease control rate (DCR) = (iCR + iPR + iSD)/total number of patients > 100%.
Time to progression (TTP): The time required from grouping to objective tumor progression was observed and counted for both groups during the follow-up period. Non-progression and survivors were considered censored data following the conclusion of the follow-up.
Serum-related parameters: Before treatment and after six treatment cycles, 5 mL fasting venous blood was harvested from each patient in both groups. The serum was separated after high-speed centrifugation and stored in an ultra-low temperature freezer at -80 ℃ for later use. Serum levels of VEGF, MMP-9, and COX-2 were determined using an enzyme-linked immunosorbent assay[17]. VEGF kits were purchased from Beijing Zhongshan Biotechnology Co., Ltd., MMP-9 kits were bought from Anhui Anke Biological Co., Ltd., and COX-2 kits were purchased from Shanghai Kaibo Biochemical Reagent Co., Ltd. All procedures were performed in strict accordance with the kit instructions.
Tumor markers: Prior to treatment and subsequent to six treatment cycles, 5 mL fasting venous blood was collected from each patient in the two groups. The serum was separated after high-speed centrifugation and stored in an ultra-low temperature freezer at -80 ℃ for later use. The levels of tumor markers HE4, CA125, and CA199 in serum samples were confirmed through enzyme-linked immunosorbent assay [18] with the assistance of an ST-360 automatic enzyme-linked immunosorbent assay analyzer (Shanghai Kehua Experimental Instrument Co., Ltd.). The kits were purchased from Shanghai Youkewei Biotechnology Co., Ltd. All operations were conducted in strict accordance with the kit instructions.
Adverse reactions: Gastrointestinal reactions, thrombosis, bone marrow suppression, and liver and kidney function injury, and other adverse reactions and symptoms were closely observed, recorded, and analyzed in both groups, and the incidence was calculated.
All data in this study were processed and analyzed using SPSS 22.0 software. Measurement data were presented as mean ± SD, and t-test was applied for analysis. Enumeration data were represented as percentages, and χ2 test was used for analysis. The Kaplan-Meier method was utilized to calculate the TTP. When P < 0.05, statistical significance was determined.
Following distinct treatment strategies, a comparative evaluation of short-term efficacy was conducted between the two groups. The results showed that ORR attained 82.05%, while DCR was 97.44% in the observation group, which were higher than the respective rates of 58.14% and 83.72% in the control group. As highlighted in Table 2, there was a notable difference in the short-term efficacy between the two groups (P < 0.05).
| Group | iCR | iPR | iSD | iPD | ORR | DCR |
| Control group (n = 43) | 9 (20.93) | 16 (37.21) | 11 (25.58) | 7 (16.28) | 25 (58.14) | 36 (83.72) |
| Observation group (n = 39) | 20 (51.28) | 12 (30.77) | 6 (15.38) | 1 (2.56) | 32 (82.05) | 38 (97.44) |
| χ2 | 5.518 | 4.369 | ||||
| P value | 0.019 | 0.037 |
The Kaplan-Meier analysis displayed that the median TTP was 24 mo (95%CI: 19.987-28.005) in the control group and 37 mo (95%CI: 30.854-43.870) in the observation group, which was better than that in the control group (Figure 1). The difference was statistically meaningful (log-rank test value = 5.009, P = 0.025).
Prior to treatment, no substantial difference was observed in serum VEGF, MMP-9, and COX-2 levels between the two groups (P > 0.05). After the adoption of different treatment methods, the above indicators were improved in both groups (P < 0.05). VEGF, MMP-9, and COX-2 in the observation group were 294.81 ± 20.63 pg/mL, 200.43 ± 15.02 mg/L, and 311.36 ± 22.14 ng/L, respectively. In contrast, the control group exhibited higher levels of these indicators (375.60 ± 22.05 pg/mL, 256.78 ± 17.62 mg/L, and 523.41 ± 27.48 ng/L, respectively), as presented in Table 3. The difference held statistical significance (P < 0.05).
| Group | VEGF (pg/mL) | MMP-9 (mg/L) | COX-2 (ng/L) | |||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Control group (n = 43), mean ± SD | 421.38 ± 36.41 | 375.60 ± 22.05a | 301.25 ± 26.73 | 256.78 ± 17.62a | 711.39 ± 54.43 | 523.41 ± 27.48a |
| Observation group (n = 39), mean ± SD | 427.91 ± 37.23 | 294.81 ± 20.63a | 308.43 ± 22.08 | 200.43 ± 15.02a | 718.43 ± 49.26 | 311.36 ± 22.14a |
| t | 0.802 | 17.083 | 1.318 | 15.504 | 0.612 | 38.227 |
| P value | 0.425 | < 0.001 | 0.191 | < 0.001 | 0.542 | < 0.001 |
Prior to treatment, there was no discernible difference in the levels of tumor markers HE4, CA125, and CA199 between the two groups (P > 0.05). After distinct treatment strategies, the above indicators in the two groups were vigorously bolstered (P < 0.05). HE4, CA125 and CA199 in the observation group were 121.36 ± 19.48 pmol/L, 35.61 ± 4.25 ng/mL and 56.37 ± 7.41 U/mL, respectively. On the contrary, the control group had higher levels of the above indicators (184.65 ± 22.34 pmol/L, 58.56 ± 6.08 ng/mL, and 82.84 ± 9.28 U/mL, respectively), as displayed in Table 4. The difference held statistical significance (P < 0.05).
| Group | HE4 (pmol/L) | CA125 (ng/mL) | CA199 (U/mL) | |||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Control group (n = 43), mean ± SD | 343.75 ± 51.26 | 184.65 ± 22.34a | 82.43 ± 11.27 | 58.56 ± 6.08a | 119.60 ± 15.62 | 82.84 ± 9.28a |
| Observation group (n = 39), mean ± SD | 349.81 ± 45.79 | 121.36 ± 19.48a | 83.26 ± 10.33 | 35.61 ± 4.25a | 121.03 ± 19.85 | 56.37 ± 7.41a |
| t | 0.562 | 13.610 | 0.346 | 19.618 | 0.364 | 14.177 |
| P value | 0.576 | < 0.001 | 0.730 | < 0.001 | 0.717 | < 0.001 |
The total incidence rate of adverse reactions, including gastrointestinal reactions, thrombosis, bone marrow suppression, and liver and kidney function injury, was significantly lower in the observation group (5.12%) compared to the control group (20.94%), as indicated in Table 5. The difference was statistically significant (P < 0.05). Timely symptomatic treatment ameliorated the adverse reactions for patients in both groups and had no substantial impact on the implementation of this treatment plan.
| Group | Gastrointestinal reactions | Thrombus | Myelosuppression | Hepatic and renal impairment | Total incidence |
| Control group (n = 43) | 4 (9.30) | 1 (2.33) | 3 (6.98) | 1 (2.33) | 9 (20.94) |
| Observation group (n = 39) | 1 (2.56) | 0 (0) | 0 (0) | 1 (2.56) | 2 (5.12) |
| χ2 | 4.397 | ||||
| P value | 0.036 |
According to global cancer incidence and mortality statistics, colon cancer has emerged as the second most common cancer worldwide, surpassed only by lung cancer, with an incidence rate as high as 10.2% and a mortality rate of 9.2%[19]. CRC exhibits no evident specific symptoms in the early stage and is mostly screened out during routine health examinations. However, patients are typically diagnosed when they seek medical treatment for hematochezia, diarrhea, abdominal pain, weight loss, anemia, and weight loss, at which point they are usually in the middle or advanced stage. Tumor lesions and metastases can severely impair the patient’s physical condition, and invasive surgical resection or treatment methods with greater toxicity and side effects may not be suitable for advanced patients[20,21]. Therefore, it is of paramount significance to explore effective treatment plans for advanced CRC that prolong the patient’s life cycle and improve their quality of life.
The primary treatment for early-stage colon cancer is surgical resection of the tumor lesion. However, for advanced colon cancer, the high degree of malignancy and strong metastasis pose significant clinical challenges[22,23]. In a survey of 7786 patients who underwent resection of colon cancer, Moghadamyeghaneh et al[24] reported that approximately 10.8% of patients developed metastases at the time of surgery, and patients with metastatic colon cancer displayed higher postoperative morbidity and mortality than those with localized colon cancer. At present, for the treatment of advanced colon cancer, cytotoxic drugs such as 5-fluorouracil, oxaliplatin, capecitabine, and irinotecan combined with biological agents such as cetuximab, panitumumab, and bevacizumab are mainly used for chemotherapy, with good clinical efficacy in improving progression-free survival and overall survival rates[25-27]. Based on previous studies, this study compared the short-term efficacy, time to progression, incidence of adverse reactions, and changes in serum VEGF, MMP-9, COX-2 levels and tumor markers HE4, CA125, and CA199 levels before and after treatment in advanced colon cancer patients treated with the standard FOLFOX chemotherapy of 5-fluorouracil, oxaliplatin, and Calcium Folinate combination, as well as the chemotherapy using olaparib combined with bevacizumab.
In this study, 43 patients in the control group treated with standard FOLFOX chemotherapy and 39 patients in the observation group treated with olaparib combined with bevacizumab chemotherapy were retrospectively analyzed to compare the clinical efficacy, disease progression time and adverse reactions between the two strategies during advanced CRC treatment. The outcomes demonstrated that ORR and DCR in the observation group were higher than those in the control group, the disease progression time was longer than that in the control group, while the total incidence of adverse reactions was lower than that in the control group, indicating that olaparib combined with bevacizumab in the treatment of advanced CRC not only had excellent short-term efficacy, but also prolonged the disease progression of patients, and had smaller toxic and side effects. As compared with the classical FOLFOX chemotherapy, it was safer and more reliable, with better clinical efficacy. FOLFOX chemotherapy is a widely used approach for the clinical management of CRC. Nonetheless, its clinical application may result in bone marrow suppression, digestive system disorders, and nervous system reactions, resulting in significant adverse effects on the patients [28]. Furthermore, studies have reported that approximately 60% of patients with CRC do not respond adequately to FOLFOX chemotherapy[29]. Kim et al[30] have pointed out that in patients with unresectable or metastatic CRC, the use of drugs such as olaparib or bevacizumab specifically targets proteins that promote cancer cell proliferation, with fewer toxic effects than FOLFOX chemotherapy. This study’s results were consistent with previous reports, demonstrating that olaparib combined with bevacizumab was more effective than oxaliplatin, calcium folinate, and 5-fluorouracil chemotherapy for treating advanced CRC. Specifically, this therapy suppressed the homologous recombination defect of tumor genes by repressing PARP protein and tumor angiogenesis, effectively adopted cytotoxic therapy to increase cell killing, improved the efficiency of killing mutant cancer cells[31], and also effectively delayed tumor angiogenesis. For patients with small tumor masses requiring fewer chemotherapy cycles, it dampened the chance of inducing drug resistance, lowered the possibility of CRC cells becoming resistant to olaparib and bevacizumab, enhanced the immune activity after the resection of large tumors, bettered the clinical efficacy from multiple aspects[32], prolonged the patient’s life cycle, and also lessened the damage done to the patient’s body by the toxic and side effects of chemotherapeutic drugs[33,34]. This treatment method is relatively safer and more efficient.
Furthermore, this study compared the serum-associated indicators and tumor markers between the two groups subjected to different treatment regimens. The findings revealed no remarkable difference in various indicators between the two groups before treatment, while all indicators were effectively boosted subsequent to distinct treatment regimens. Serum VEGF, MMP-9, and COX-2 levels were significantly reduced in the observation group receiving olaparib combined with bevacizumab compared to the control group. Moreover, a more substantial decrease was observed in tumor markers, including HE4, CA125, and CA199 levels, in the observation group compared to the control group. These results provided evidence that olaparib combined with bevacizumab could effectively dampen tumor neovascularization, kill tumor cells, and assist in reducing tumor burden in patients diagnosed with advanced CRC. VEGF, MMP-9, and COX-2 are critical regulators of tumor angiogenesis, cell migration, as well as extracellular matrix degradation, and are typically present at high levels in advanced CRC[35,36]. HE4, an acidic protein tumor marker mainly expressed in epididymis and vas deferens epithelial cells, has been extensively applied in the diagnosis and prognostic evaluation of cancers such as endometrial cancer, ovarian cancer and lung cancer. The latest studies have also found it abnormally elevated in digestive system tumors [37]. CA125 is a heterogeneous mucin-like glycoprotein widely distributed in the mesothelial cell group and is dramatically heightened in patients with ovarian, cervical, liver, lung, as well as CRC progression[38]. CA199, a glycolipid substance on the cell membrane, belongs to oligosaccharide tumor-correlated antigens and is widely believed to be highly expressed in patients with cholangiocarcinoma, gastric cancer, liver cancer, as well as colon cancer[39]. Here, bevacizumab was harnessed to block VEGF binding to its receptor to impede tumor neovascularization and suppress the formation of type IV collagen and integral membrane-bound protease[40]. Meanwhile, olaparib functioned in blocking base excision repair, specifically killing cancer cells while also repairing DNA damage after chemotherapy to some extent[41], hence impeding serum VEGF, MMP-9, and COX-2 levels and effectively attenuating the profiles of tumor-related markers HE4, CA125 and CA199 in serum[42].
Nonetheless, this study has some limitations that should be acknowledged. Firstly, the retrospective design of the study may have introduced inherent biases. Additionally, all enrolled cases were from the same hospital, and the research outcomes may be affected by the unique practice of the unit. As such, further prospective studies are recommended to validate these findings and fill the gaps.
In summary, the combination of olaparib and bevacizumab has superior clinical efficacy compared to the conventional FOLFOX chemotherapy regimen for treating patients with advanced CRC. Specifically, the combination therapy was found to significantly delay disease progression and reduce serum VEGF, MMP-9, and COX-2 levels, as well as tumor marker HE4, CA125, and CA199 levels, while also exhibiting fewer adverse reactions and a high level of safety and reliability. These findings provide valuable insights for targeted therapies in the context of advanced rectal cancer and have significant clinical implications.
Olaparib and bevacizumab are well-established targeted drugs utilized in the treatment of solid tumors in clinical settings. They can exert anti-tumor effects by inhibiting PARP and tumor neovascularization. Advanced colorectal cancer (CRC) has a high degree of malignancy, and conventional surgical treatment and chemotherapy are effective. However, there is a pressing need to identify safe and effective treatments for patients with advanced CRC.
Olaparib combined with bevacizumab in the treatment of advanced CRC has an ideal clinical efficacy.
This study aims to investigate the short-term efficacy, time to progression, safety, and their effects on the serum parameters of olaparib combined with bevacizumab in advanced CRC treatment.
Comparisons were made for the assessment of the short-term efficacy, time to progression (TTP), the incidence of adverse reactions, serum-related parameters [vascular endothelial growth factor (VEGF), matrix metalloprotein-9 (MMP-9), cyclooxygenase-2 (COX-2)], and tumor markers [human epididymis protein 4 (HE4), carbohydrate antigen 125 (CA125), carbohydrate antigen 199 (CA199)] levels in patients with advanced CRC treated with classical FOLFOX chemotherapy and olaparib combined with bevacizumab chemotherapy.
The objective response rate and disease control rate in the observation group were significantly higher than those in the control group, and the median TTP in the observation group was better than that in the control group. After treatment, the serum levels of VEGF, MMP-9, COX-2, HE4, CA125, and CA199 in the observation group were lower than those in the control group, and the total incidence of adverse reactions in the observation group was also lower than that in the control group.
Olaparib combined with bevacizumab in the treatment of advanced CRC has a remarkable clinical effect. Specifically, the combination can delay the disease and reduce serum VEGF, MMP-9, and COX-2 levels and tumor markers HE4, CA125, and CA199 levels, with a high degree of safety and reliability.
The mechanism of olaparib combined with bevacizumab in the treatment of advanced CRC can be further investigated so as to enable a better understanding of its target and provide a comprehensive theoretical basis and data support for the clinical application of this treatment modality in patients with advanced CRC.
Provenance and peer review: Unsolicited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Celik GK, Turkey; Flynn DE, Australia; Rumpold H, Austria S-Editor: Gong ZM L-Editor: A P-Editor: Zhao S
| 1. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 383] [Article Influence: 127.7] [Reference Citation Analysis (6)] |
| 2. | Kumar R, Harilal S, Carradori S, Mathew B. A Comprehensive Overview of Colon Cancer- A Grim Reaper of the 21st Century. Curr Med Chem. 2021;28:2657-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3004] [Article Influence: 500.7] [Reference Citation Analysis (3)] |
| 4. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (2)] |
| 5. | Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13:109-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 407] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 6. | Khaliq AM, Erdogan C, Kurt Z, Turgut SS, Grunvald MW, Rand T, Khare S, Borgia JA, Hayden DM, Pappas SG, Govekar HR, Kam AE, Reiser J, Turaga K, Radovich M, Zang Y, Qiu Y, Liu Y, Fishel ML, Turk A, Gupta V, Al-Sabti R, Subramanian J, Kuzel TM, Sadanandam A, Waldron L, Hussain A, Saleem M, El-Rayes B, Salahudeen AA, Masood A. Refining colorectal cancer classification and clinical stratification through a single-cell atlas. Genome Biol. 2022;23:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 7. | Chen K, Collins G, Wang H, Toh JWT. Pathological Features and Prognostication in Colorectal Cancer. Curr Oncol. 2021;28:5356-5383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 8. | Kim K, Kim YW, Shim H, Kim BR, Kwon HY. Differences in clinical features and oncologic outcomes between metastatic right and left colon cancer. J BUON. 2018;23:11-18. [PubMed] |
| 9. | Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol Ther. 2020;206:107447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 597] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 10. | Limagne E, Thibaudin M, Nuttin L, Spill A, Derangère V, Fumet JD, Amellal N, Peranzoni E, Cattan V, Ghiringhelli F. Trifluridine/Tipiracil plus Oxaliplatin Improves PD-1 Blockade in Colorectal Cancer by Inducing Immunogenic Cell Death and Depleting Macrophages. Cancer Immunol Res. 2019;7:1958-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Nappi A, Berretta M, Romano C, Tafuto S, Cassata A, Casaretti R, Silvestro L, Divitiis C, Alessandrini L, Fiorica F, Ottaiano A, Nasti G. Metastatic Colorectal Cancer: Role of Target Therapies and Future Perspectives. Curr Cancer Drug Targets. 2018;18:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer. 2019;109:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 13. | Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 722] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 14. | Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, Bordonaro R, Latiano TP, Tamburini E, Santini D, Passardi A, Marmorino F, Grande R, Aprile G, Zaniboni A, Murgioni S, Granetto C, Buonadonna A, Moretto R, Corallo S, Cordio S, Antonuzzo L, Tomasello G, Masi G, Ronzoni M, Di Donato S, Carlomagno C, Clavarezza M, Ritorto G, Mambrini A, Roselli M, Cupini S, Mammoliti S, Fenocchio E, Corgna E, Zagonel V, Fontanini G, Ugolini C, Boni L, Falcone A; GONO Foundation Investigators. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 15. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4392] [Article Influence: 549.0] [Reference Citation Analysis (4)] |
| 16. | Inno A, Lo Russo G, Salgarello M, Corrao G, Casolino R, Galli G, Modena A, Romano L, Pusceddu S, Greco FG, Garassino MC, Gori S. The evolving landscape of criteria for evaluating tumor response in the era of cancer immunotherapy: From Karnofsky to iRECIST. Tumori. 2018;104:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Karsten MM, Beck MH, Rademacher A, Knabl J, Blohmer JU, Jückstock J, Radosa JC, Jank P, Rack B, Janni W. VEGF-A165b levels are reduced in breast cancer patients at primary diagnosis but increase after completion of cancer treatment. Sci Rep. 2020;10:3635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Kristjansdottir B, Levan K, Partheen K, Sundfeldt K. Diagnostic performance of the biomarkers HE4 and CA125 in type I and type II epithelial ovarian cancer. Gynecol Oncol. 2013;131:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55765] [Article Influence: 7966.4] [Reference Citation Analysis (132)] |
| 20. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1795] [Article Influence: 359.0] [Reference Citation Analysis (0)] |
| 21. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 950] [Article Influence: 237.5] [Reference Citation Analysis (0)] |
| 22. | Wrobel P, Ahmed S. Current status of immunotherapy in metastatic colorectal cancer. Int J Colorectal Dis. 2019;34:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 23. | Li S, Ma J, Hong X, Zheng M, Goto S, Takimoto R, Kamigaki T, Zang L. Significant clinical response of advanced colorectal cancer to combination therapy involving capecitabine and adoptive cell transfer therapy: a case report. Transl Cancer Res. 2019;8:693-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Moghadamyeghaneh Z, Hanna MH, Hwang G, Mills S, Pigazzi A, Stamos MJ, Carmichael JC. Outcomes of colon resection in patients with metastatic colon cancer. Am J Surg. 2016;212:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Sherman SK, Lange JJ, Dahdaleh FS, Rajeev R, Gamblin TC, Polite BN, Turaga KK. Cost-effectiveness of Maintenance Capecitabine and Bevacizumab for Metastatic Colorectal Cancer. JAMA Oncol. 2019;5:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Zhang PF, Wen F, Zhou J, Huang JX, Zhou KX, Wu QJ, Wang XY, Zhang MX, Liao WT, Li Q. Cost-effectiveness analysis of capecitabine plus bevacizumab versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer from Chinese societal perspective. Clin Transl Oncol. 2020;22:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Modest DP, Rivera F, Bachet JB, de Braud F, Pietrantonio F, Koukakis R, Demonty G, Douillard JY. Panitumumab-based maintenance after oxaliplatin discontinuation in metastatic colorectal cancer: A retrospective analysis of two randomised trials. Int J Cancer. 2019;145:576-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Negarandeh R, Salehifar E, Saghafi F, Jalali H, Janbabaei G, Abdhaghighi MJ, Nosrati A. Evaluation of adverse effects of chemotherapy regimens of 5-fluoropyrimidines derivatives and their association with DPYD polymorphisms in colorectal cancer patients. BMC Cancer. 2020;20:560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Kennedy SA, Morrissey ME, Dunne MR, O’Connell F, Butler CT, Cathcart MC, Buckley AM, Mehigan BJ, Larkin JO, McCormick P, Kennedy BN, O’Sullivan J. Combining 1,4-dihydroxy quininib with Bevacizumab/FOLFOX alters angiogenic and inflammatory secretions in ex vivo colorectal tumors. BMC Cancer. 2020;20:952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Kim TW, Taieb J, Gurary EB, Lerman N, Cui K, Yoshino T. Olaparib with or without bevacizumab or bevacizumab and 5-fluorouracil in advanced colorectal cancer: Phase III LYNK-003. Future Oncol. 2021;17:5013-5022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 31. | Qin C, Ji Z, Zhai E, Xu K, Zhang Y, Li Q, Jing H, Wang X, Song X. PARP inhibitor olaparib enhances the efficacy of radiotherapy on XRCC2-deficient colorectal cancer cells. Cell Death Dis. 2022;13:505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Covens AL. A critique of surgical cytoreduction in advanced ovarian cancer. Gynecol Oncol. 2000;78:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to Anti-Angiogenic Therapy in Cancer-Alterations to Anti-VEGF Pathway. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 248] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 34. | Qin S, Li J, Bai Y, Shu Y, Li W, Yin X, Cheng Y, Sun G, Deng Y, Zhong H, Li Y, Qian X, Zhang L, Zhang J, Chen K, Kang W; HLX04-mCRC03 Investigators. Efficacy, Safety, and Immunogenicity of HLX04 Versus Reference Bevacizumab in Combination with XELOX or mFOLFOX6 as First-Line Treatment for Metastatic Colorectal Cancer: Results of a Randomized, Double-Blind Phase III Study. BioDrugs. 2021;35:445-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Yue YC, Yang BY, Lu J, Zhang SW, Liu L, Nassar K, Xu XX, Pang XY, Lv JP. Metabolite secretions of Lactobacillus plantarum YYC-3 may inhibit colon cancer cell metastasis by suppressing the VEGF-MMP2/9 signaling pathway. Microb Cell Fact. 2020;19:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Zhang Z, Ghosh A, Connolly PJ, King P, Wilde T, Wang J, Dong Y, Li X, Liao D, Chen H, Tian G, Suarez J, Bonnette WG, Pande V, Diloreto KA, Shi Y, Patel S, Pietrak B, Szewczuk L, Sensenhauser C, Dallas S, Edwards JP, Bachman KE, Evans DC. Gut-Restricted Selective Cyclooxygenase-2 (COX-2) Inhibitors for Chemoprevention of Colorectal Cancer. J Med Chem. 2021;64:11570-11596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Kleif J, Jørgensen LN, Hendel JW, Madsen MR, Vilandt J, Brandsborg S, Andersen LM, Khalid A, Ingeholm P, Ferm L, Davis GJ, Gawel SH, Martens F, Andersen B, Rasmussen M, Christensen IJ, Nielsen HJ. Early detection of colorectal neoplasia: application of a blood-based serological protein test on subjects undergoing population-based screening. Br J Cancer. 2022;126:1387-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Gao Y, Wang J, Zhou Y, Sheng S, Qian SY, Huo X. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer. Sci Rep. 2018;8:2732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 39. | Lin S, Wang Y, Peng Z, Chen Z, Hu F. Detection of cancer biomarkers CA125 and CA199 via terahertz metasurface immunosensor. Talanta. 2022;248:123628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 40. | Schiffmann LM, Brunold M, Liwschitz M, Goede V, Loges S, Wroblewski M, Quaas A, Alakus H, Stippel D, Bruns CJ, Hallek M, Kashkar H, Hacker UT, Coutelle O. A combination of low-dose bevacizumab and imatinib enhances vascular normalisation without inducing extracellular matrix deposition. Br J Cancer. 2017;116:600-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020;34:360-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 439] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 42. | Polena H, Creuzet J, Dufies M, Sidibé A, Khalil-Mgharbel A, Salomon A, Deroux A, Quesada JL, Roelants C, Filhol O, Cochet C, Blanc E, Ferlay-Segura C, Borchiellini D, Ferrero JM, Escudier B, Négrier S, Pages G, Vilgrain I. The tyrosine-kinase inhibitor sunitinib targets vascular endothelial (VE)-cadherin: a marker of response to antitumoural treatment in metastatic renal cell carcinoma. Br J Cancer. 2018;118:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |