Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.871
Peer-review started: November 15, 2022
First decision: February 15, 2023
Revised: February 22, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 27, 2023
Processing time: 191 Days and 23.1 Hours
Rikkunshito (TJ-43) relieves gastrointestinal disturbance by increases in the levels of acylated ghrelin.
To investigate the effects of TJ-43 in patients undergoing pancreatic surgery.
Forty-one patients undergoing pylorus-preserving pancreaticoduodenectomy (PpPD) were divided into two groups; patients took daily doses of TJ-43 after surgery or after postoperative day (POD) 21. The plasma levels of acylated and desacylated ghrelin, cholecystokinin (CCK), peptide YY (PYY), gastric inhibitory peptide (GIP), and active glucagon-like peptide (GLP)-1 were evaluated. Oral calorie intake was assessed at POD 21 in both groups. The primary endpoint of this study was the total food intake after PpPD.
The levels of acylated ghrelin were significantly greater in patients treated with TJ-43 than those in patients without TJ-43 administration at POD 21, and oral intake was significantly increased in patients treated with TJ-43. The CCK and PYY levels were significantly greater in patients treated with TJ-43 than those in patients without TJ-43 treatment. Furthermore, the GIP and active GLP-1 levels increased and values at POD 21 were significantly greater in patients treated with TJ-43 than those in patients without TJ-43 administration. Insulin secretion tended to increase in patients treated with TJ-43.
TJ-43 may have advantages for oral food intake in patients in the early phase after pancreatic surgery. Further investigation is needed to clarify the effects of TJ-43 on incretin hormones.
Core Tip: This study investigated the effects of a Japanese herbal medicine, namely rikkunshito (TJ-43), on patients who underwent pancreatic surgery. TJ-43 may promote oral food intake in patients in the early phase after pancreatic surgery because TJ-43 increases appetite by enhancing gastrointestinal and incretin hormone levels.
- Citation: Kono H, Hosomura N, Amemiya H, Shoda K, Furuya S, Akaike H, Kawaguchi Y, Kawaida H, Ichikawa D. Rikkunshito increases appetite by enhancing gastrointestinal and incretin hormone levels in patients who underwent pylorus-preserving pancreaticoduodenectomy: A retrospective study. World J Gastrointest Surg 2023; 15(5): 871-881
- URL: https://www.wjgnet.com/1948-9366/full/v15/i5/871.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i5.871
The Japanese traditional herbal medicine known as rikkunshito (TJ-43) is an extract of eight crude herbal medicines. 10-dehydrogingerdin, glycycoumarin, 10-gingerdion, 8-gingerol, hesperidin, hesperetin, heptamethoxyflavone, isoliquiritigenin, liquiritigenin, naringenin, nobiletin, tangeretin, 8-shogaol, and 10-shogaol, are typical components of TJ-43.
TJ-43 increases peripheral acylated-ghrelin levels secreted from the stomach[1]. It is well known that acylated ghrelin has an appetite-enhancing effect, in addition to a growth hormone secretion-promoting effect[2,3]. Furthermore, acylated ghrelin is the only hormone that exhibits an appetite-promoting effect following intravenous administration[4]. Moreover, it has various actions, including gastric acid secretion[5], growth hormone secretion, and a positive energy balance induction[6]. The blood levels of acylated ghrelin correlated with gastrointestinal disturbances. Hence, ghrelin is used to treat gastrointestinal disturbance due to anorexia[7-9]; however, the repeated intravenous treatment of ghrelin has shown a considerable burden.
TJ-43 is often used to treat upper gastrointestinal disturbance[10-12]. Rats treated with TJ-43 exhibited enhanced gastric emptying[13]. In addition, the combined administration of TJ-43 and an anti-emetic drug for breast cancer patients alleviated emesis and anorexia that occur as an adverse complication to chemotherapy[14]. Thus, TJ-43 promotes mobility and motility of the stomach. It was previously reported that decreases in plasma acylated-ghrelin levels induced by cisplatin administration and oral food intake is mediated by 5-HT2B/2C receptors and suppressed by flavonoids in TJ-43 in animal models[15,16]. Hence, an important effect of TJ-43 is an increase in acylated ghrelin.
To improve delayed gastric emptying (DGE) after pylorus-preserving pancreaticoduodenectomy (PpPD), we previously treated patients with TJ-43 from the 21st postoperative day (POD) after beginning meal consumption as a conventional method in our hospital; however, this treatment did not allow for adequate food intake in the early postoperative phase, which is important for recovery from surgical stress. Therefore, to resolve the previous results of TJ-43 treatment for oral intake, the treatment protocol was changed to the new method. In this study, the TJ-43 treatment was started in the early post
This study aimed to investigate a new clinical treatment protocol for TJ-43 and evaluate its effects on acylated-ghrelin levels and appetite in patients after pancreatic surgery. In addition to acylated-ghrelin levels, we investigated the effects of TJ-43 on other gastrointestinal hormones that affect gastrointestinal physiology and incretin hormones, including gastric inhibitory peptide (GIP, also known as a glucose-dependent insulinotropic polypeptide) and active glucagon-like peptide (GLP)-1.
This was a retrospective observational study. Blood samples were obtained from 41 patients who underwent PpPD at the University Hospital between 2015 and 2018 because of pancreato-biliary malignant tumors (Table 1). The Ethics Committee (Chief, Prof. Zentaro Yamagata; No. 820) approved this study. The study was conducted following the ethical standards outlined in the Declaration of Helsinki. On admission, informed consent was obtained from all patients. Furthermore, the tumor stage was evaluated according to the Union for International Cancer Control classification[17]. Moreover, the histological and pathological diagnoses of the specimens were confirmed using the World Health Organization classification criteria.
| Variable | TJ-43(−), n = 20 | TJ-43(+), n = 21 | Pvalue | |
| Age in yr | 67 ± 7.0 | 66 ± 7.7 | 0.962 | |
| Sex, n (%) | Male | 10 (50) | 14 (67) | 0.199 |
| Female | 10 (50) | 7 (33) | ||
| Disease, n (%) | Pancreas Ca. (Ph) | 7 (35) | 8 (38) | |
| IPMC | 2 (10) | 2 (10) | ||
| IPMA | 2 (10) | 1 (5) | ||
| CBD Ca. | 5 (25) | 5 (24) | ||
| Vater Ca. | 3 (15) | 4 (19) | ||
| Panaceas-NET | 1 (5) | 0 (0) | ||
| GB Ca. | 0 (0) | 1 (5) | ||
| UICC Tumor stage: Pancreas Ca. (0/I/IIA/IIB); Other Ca. (0/I/II/III) | Pancreas Ca. (Ph) | (0/0/1/6) | (0/0/3/5) | |
| IPMC | (1/0/1/0) | (0/0/1/1) | ||
| IPMA | N/A | N/A | ||
| CBD Ca. | (0/1/4/0) | (0/3/2/0) | ||
| Vater Ca. | (0/1/2/0) | (0/2/2/0) | ||
| P-NET | (0/1/0/0) | N/A | ||
| GB Ca. | N/A | (0/0/1/0) | ||
| Tumor differentiation (well/mod/poor) | Pancreas Ca. (Ph) | (3/2/2) | (1/6/1) | |
| IPMC | (0/2/0) | (1/0/1) | ||
| IPMA | N/A | N/A | ||
| CBD Ca. | (0/5/0) | (2/2/1) | ||
| Vater Ca. | (2/1/0) | (1/3/0) | ||
| P-NET | N/A | N/A | ||
| GB Ca. | N/A | (0/1/0) | ||
| Time of operation in min | 500 ± 56 | 509 ± 0.72 | 0.299 | |
| Blood loss in mL | 692 ± 0.54 | 959 ± 0.66 | 0.182 | |
| HbA1c, % | 5.6 ± 2.2 | 5.7 ± 2.3 | 0.892 | |
| Tumor markers | CEA in ng/mL | 3.1 ± 1.3 | 3.7 ± 1.1 | 0.872 |
| CA19-9 in U/mL | 455 ± 23 | 451 ± 29 | ||
| DGE, % | 25 | 19 | 0.773 | |
| POPF, n (%) | Grade A | 15 (75) | 16 (76) | |
| Grades B and C | 5 (25) | 5 (24) | 0.886 | |
| Post-operative pneumonia, n (%) | 1 (5) | 1 (4.8) | 0.889 |
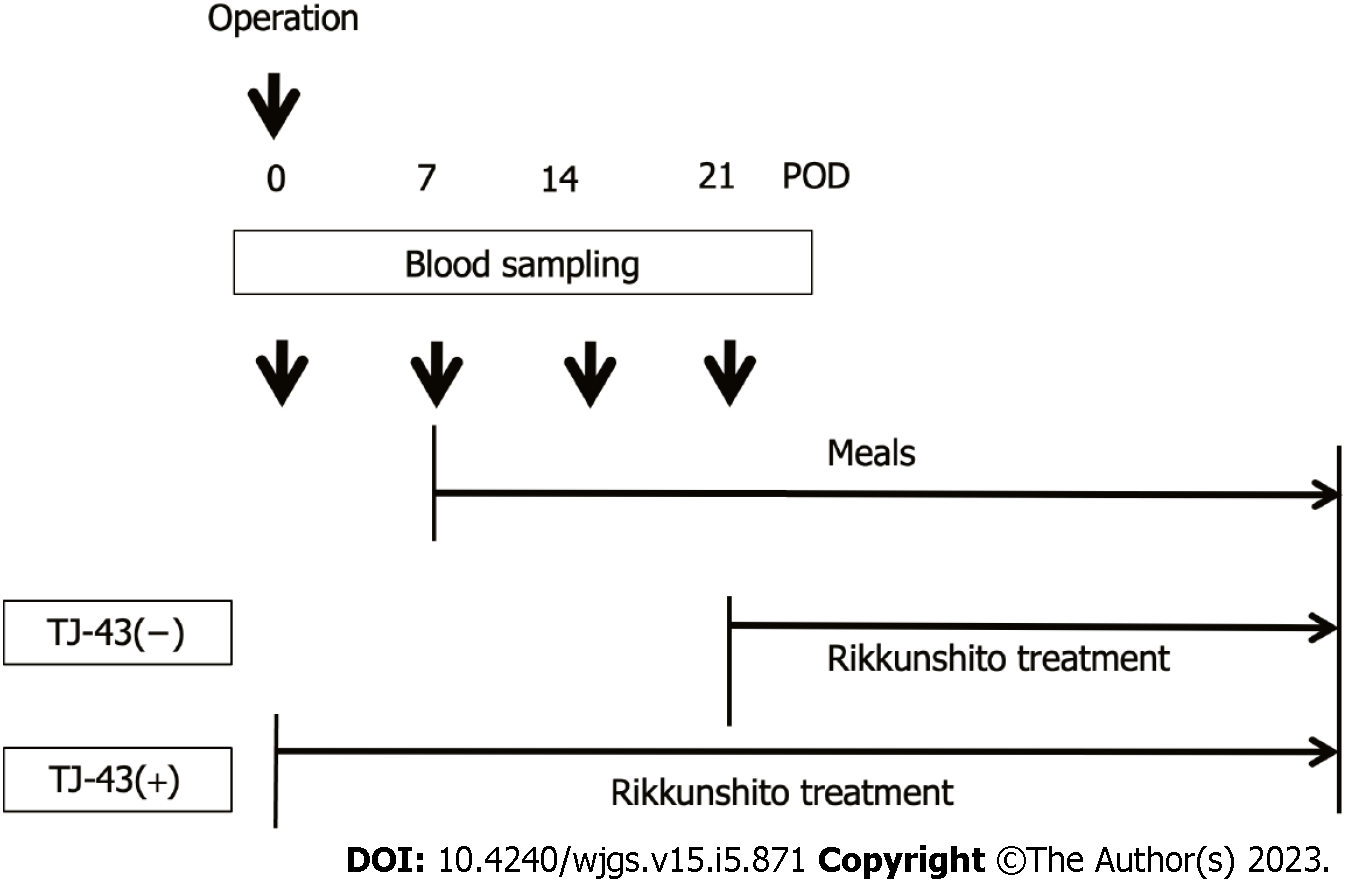
TJ-43 is a mixture of eight herbal ingredients[18] and is often used in the treatment of patients undergoing gastrointestinal surgery, including pancreatic surgery[18]. The patients were preoperatively enrolled into the two groups based on treatments of TJ-43; the TJ-43(-) group (n = 20) was treated from POD 21 with TJ-43 (7.5 g/d) using an enteral feeding catheter or by oral administration, which was a conventional treatment, and the TJ-43(+) group (n = 21) was treated daily with TJ-43 (7.5 g/d) from the 1st POD, which was the modified new treatment (Figure 1). Enteral feeding of 900 kilocalories per day was started from the 1st POD and continued throughout this study period. The postoperative diet was started at POD 7 in all cases. Furthermore, the total oral calorie intake was evaluated in both groups at 3 wk after surgery. The primary endpoint of this study was the total calorie intake.
For the definition of complications, DGE was defined based on international criteria[19]. Postoperative pancreatic fistula (POPF) was classified into grades based on the guidelines established by the International Study Group on Pancreatic Fistula in 2005[20] and revised in 2016[21]. Grade A POPF is called a biochemical fistula and has no clinical impact on the normal postoperative pathway. Clinically significant POPFs are classified as grades B and C. Grade B POPF requires one of the following conditions: An endoscopic or radiological intervention, a drain in situ for > 3 wk, clinical symptoms without organ failure, or a clinically relevant change in POPF management. Whenever a major change in clinical management or deviation from the normal clinical pathway is required or organ failure occurs, the fistula shifts to grade C POPF[21].
Blood samples were obtained before surgery (as the control samples before TJ-43 treatment in each case) and on POD 7, 14, and 21 at the time of the standard postoperative clinical blood examination, in the hospital. All samples were collected before breakfast (from 6 to 7 a.m.) and kept on ice. Immediately after blood collection, centrifugation was performed. Furthermore, EDTA-2Na and aprotinin were added to centrifuge blood samples at final concentrations of 1 mg/mL and 500 KIU, respectively. Then, DPP-IV inhibitor was added at 20 μl/mL to 1 mL of plasma, and the samples were stored at −80 °C until further analysis.
For the determination of peripheral acylated and desacylated ghrelin levels, the plasma samples were promptly centrifuged at 4 °C, and the supernatant was acidified with 1 mol/L HCl (1/10 volume)[22].
Immunoreactive insulin (IRI) was assessed by clinical laboratory analysis. IRI levels were measured using the Lumipulse G1200 immunoassay instrument (FUJIREBIO Inc., Tokyo, Japan). Lumipulse® G Insulin-N Immunoreaction cartridges for in vitro diagnostic were used in the Lumipulse G system for the quantitative measurement of insulin in serum or plasma. The assay results were available in less than 35 min.
Plasma levels of acylated and desacylated ghrelin were evaluated by enzyme-linked immunosorbent assay (ELISA) kits (Iwai Chemicals Co., Tokyo, Japan).
Plasma levels of cholecystokinin (CCK) (LifeSpan BioSciences), peptide YY (PYY) (LifeSpan BioSciences), GIP (RayBiotech Life Inc., Peachtree Corners, GA), and active GLP-1 (Invitrogen, Waltham, MA) were evaluated by ELISA.
Statistical analyses were performed using EZR software (Saitama Medical Center, Saitama, Japan), which is using the R programming language (The R Foundation for Statistical Computing, Vienna, Austria)[23].
The power calculation was performed as follows: The number of samples required for statistical analysis was 20 in each group. Data are expressed as the mean ± standard error of the mean. The paired t-test or ANOVA with Bonferroni’s post-hoc test was used for comparisons between the two groups. P < 0.05 was considered significantly different.
Adverse events were not observed in patients treated with TJ-43. Furthermore, no significant differences in the clinical features were observed in both groups (Table 1).
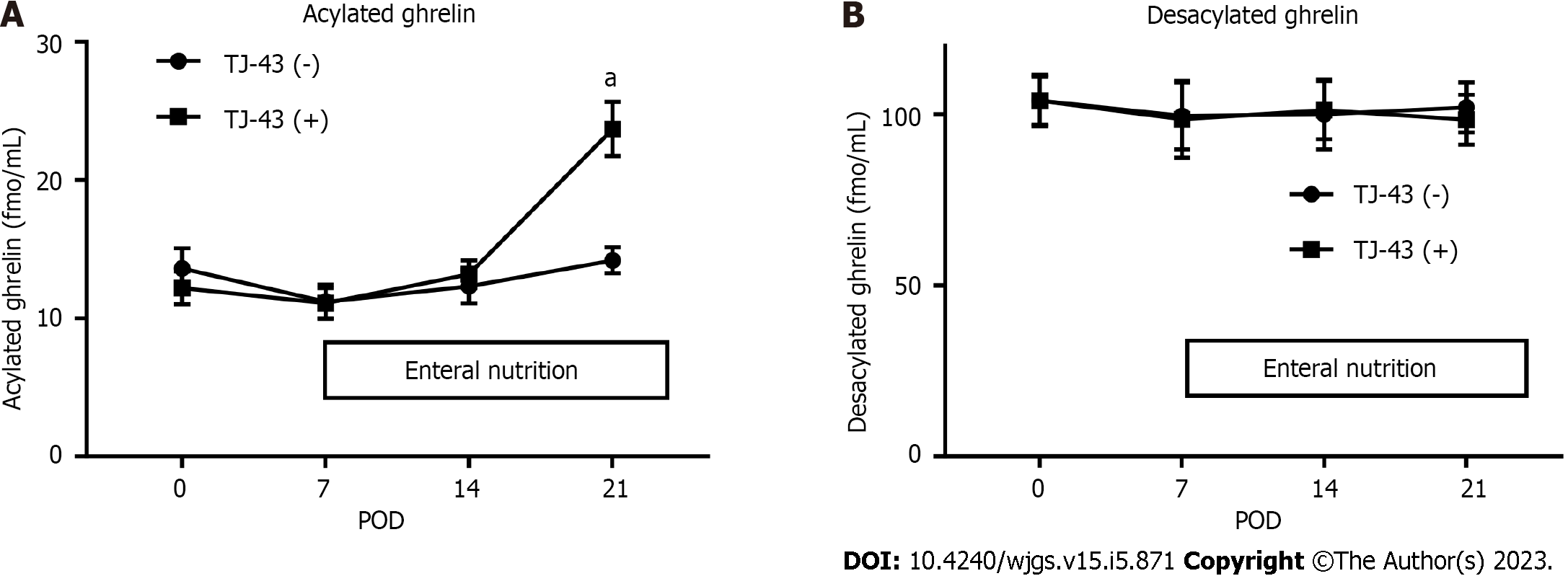
Acylated ghrelin was detected in plasma collected from patients before the operation (Figure 2A). The levels of acylated ghrelin did not significantly change after the operation in patients without TJ-43 treatment; however, the levels gradually increased after the operation in patients treated with TJ-43, compared with their levels before the operation. There were significant differences in levels of acylated ghrelin at POD 21 between the TJ-43(-) and TJ-43(+) groups (P < 0.05).
The plasma desacylated-ghrelin levels were not markedly changed in either group after the operation, compared with their levels before the operation (Figure 2B).
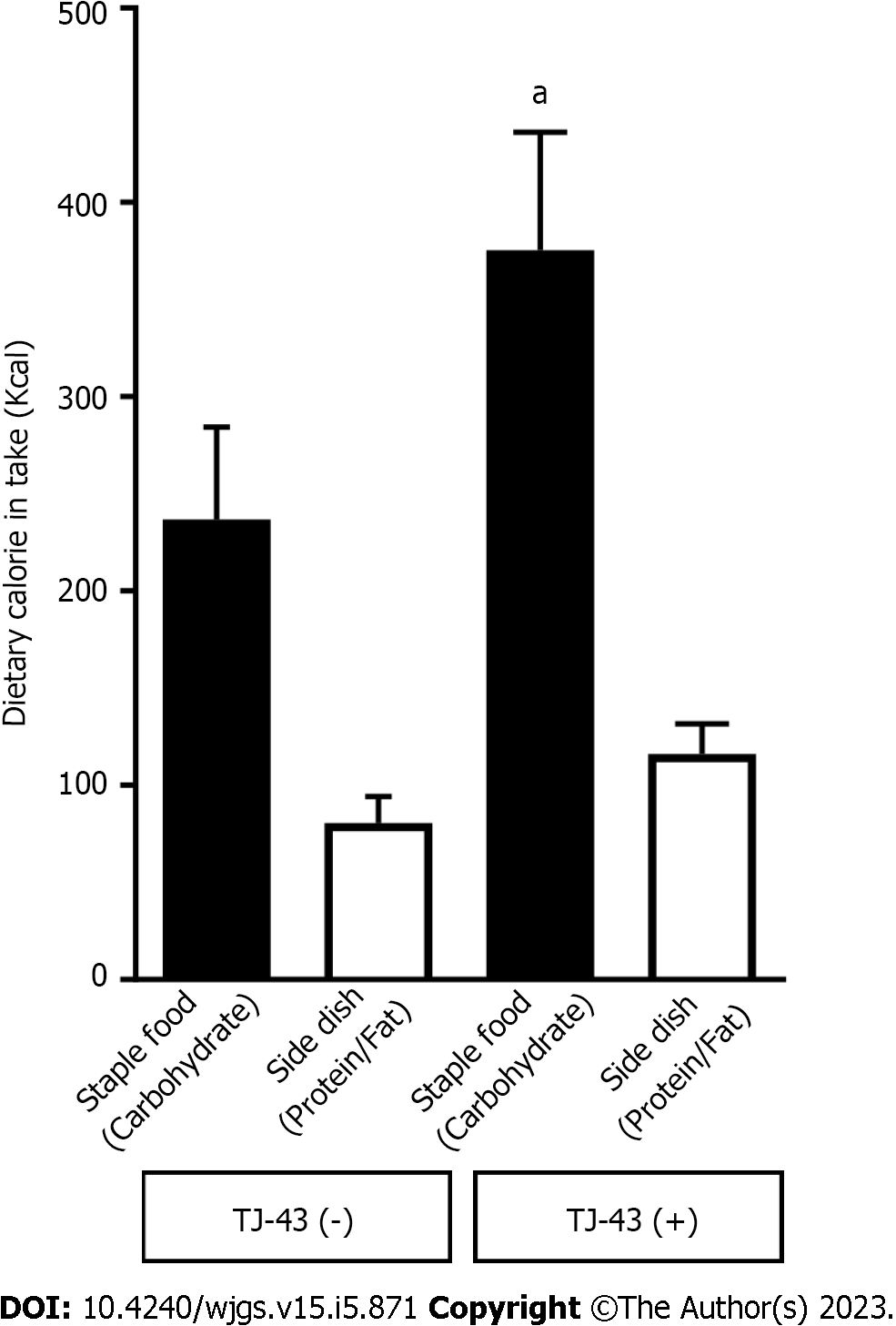
Consistent with the analysis of acylated ghrelin, total food consumption was more significant in patients treated with TJ-43 compared with patients without TJ-43 administration (patients treated with TJ-43, 491.5 ± 59.2 Kcal; and patients without TJ-43 treatment, 317.5 ± 52.3 Kcal, respectively). In addition, dietary intake from staple food was significantly greater in patients treated with TJ-43 compared with patients without TJ-43 treatment (patients treated with TJ-43, 375.3 ± 62.3 Kcal; and patients without TJ-43 treatment, 236.9 ± 49.7 Kcal, respectively) (P < 0.05) (Figure 3).
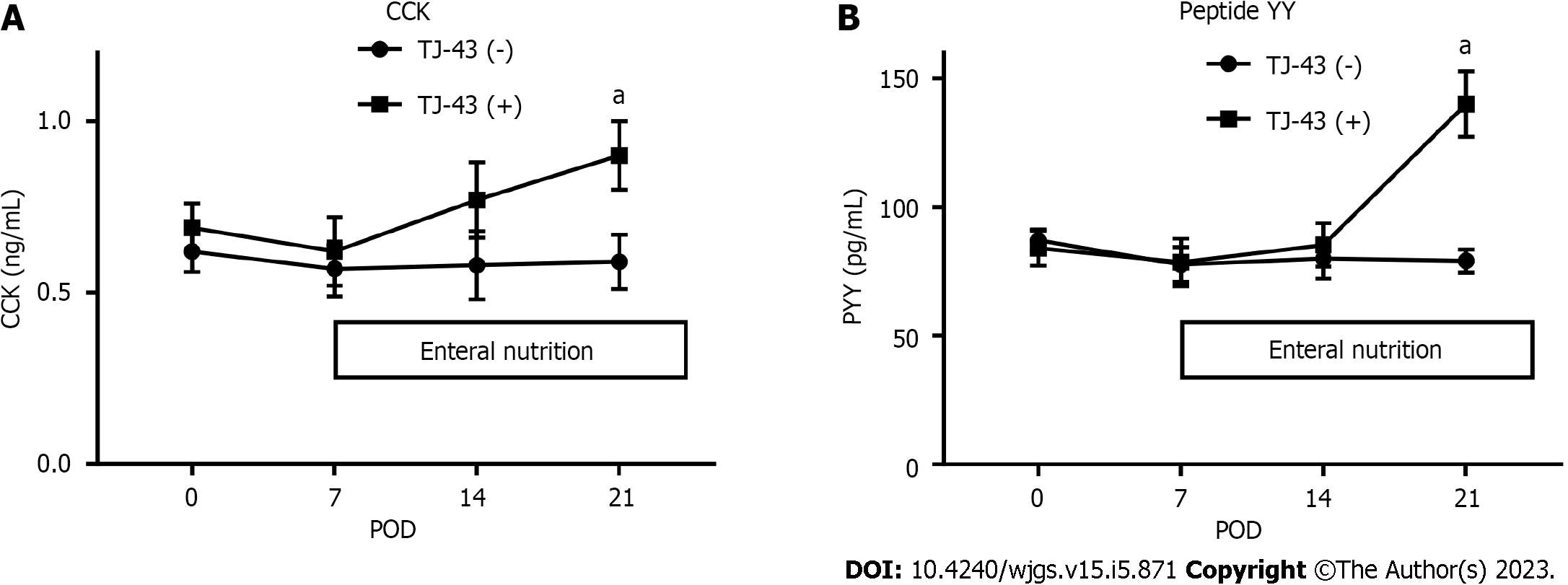
CCK and PYY were detected in plasma harvested before the operation (Figure 4). The levels of gastrointestinal hormones did not markedly change after the operation in patients without TJ-43 treatment; however, these levels were significantly increased at POD 21 in patients treated with TJ-43, compared with their levels before surgery (P < 0.05).
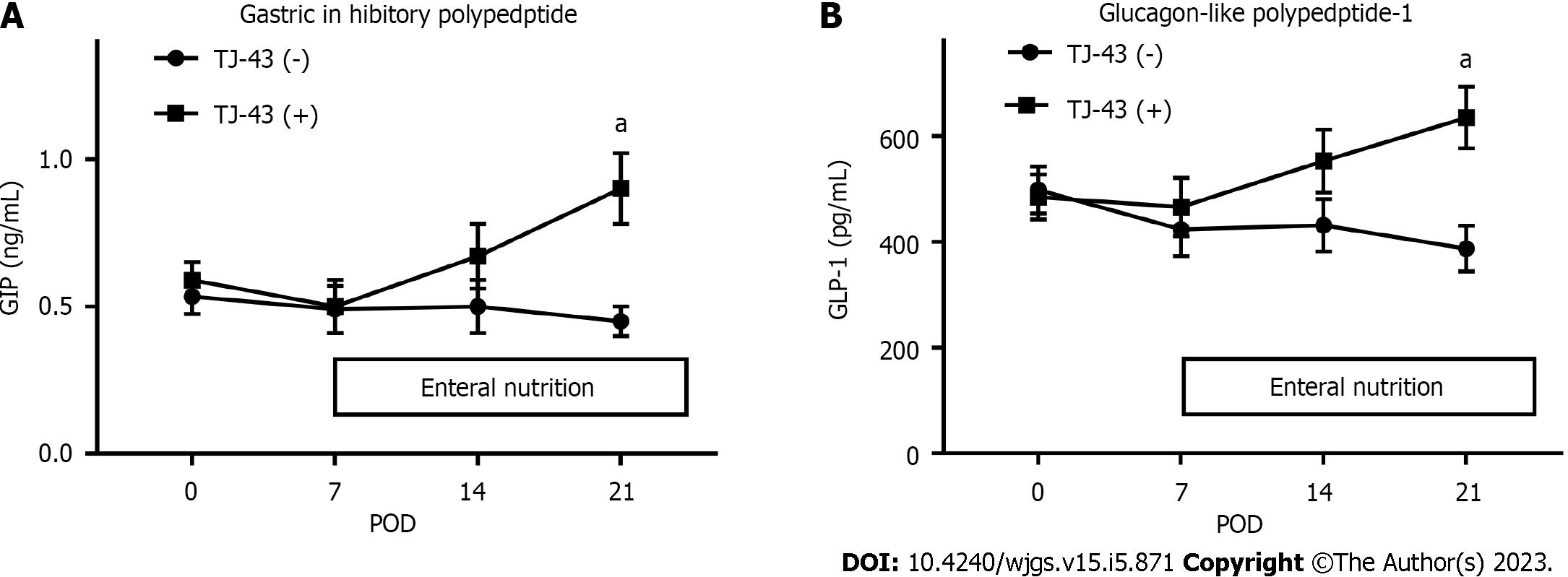
The plasma GIP and active GLP-1 Levels were not significantly changed after the operation in the TJ-43(-) group, compared with those before the operation. In contrast, these levels were significantly greater at POD 21 in the TJ-43(+) group, compared with those before the operation (P < 0.05) (Figure 5). These increases were significantly greater for the values of GIP than those of active GLP-1 (P < 0.05).
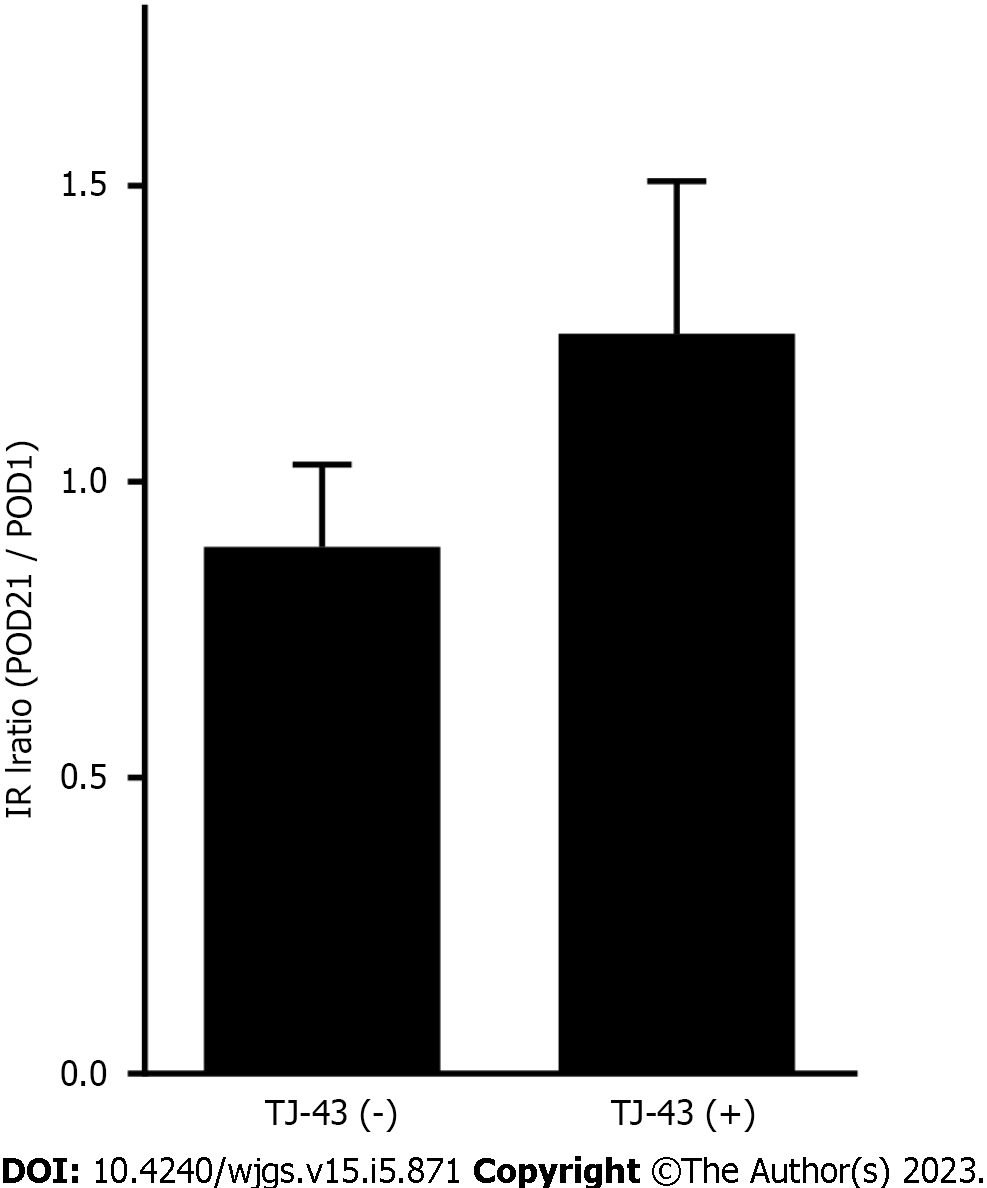
IRI levels were assessed at POD 21. Although no significant differences were observed in the IRI levels between the groups, levels in patients treated with TJ-43 were greater than those in patients without TJ-43 administration (Figure 6).
Blood glucose levels were 116.3 ± 18.5 in the TJ-43(-) group and 107.2 ± 15.3 in the TJ-43(+) group, 1 h after the meal. There were no significant differences between the two groups.
TJ-43 improves oral food intake by increasing the peripheral level of acylated ghrelin after PpPD. This study found that oral food intake in the TJ-43(+) group was significantly higher than that in the TJ-43(–) group after PpPD. Furthermore, the acyl ghrelin level in the TJ-43(+) group was significantly higher than that in the TJ-43(–) group at the same time points.
Patients undergoing PpPD with the reconstruction of the gastrointestinal tract have lower food intake in the early postoperative period. Reasons for this include vagal denervation and shrinkage of the residual stomach. Regarding the relationship between postoperative complications and oral food intake, DGE, POPF, and postoperative pneumonia are critical factors[24]. These complications may lead to loss of appetite. In this study, these complications were observed in both groups, without significant differences (Table 1).
Furthermore, levels of acylated ghrelin increased in patients treated with TJ-43 group compared with patients without TJ-43 administration after the operation (Figure 3). Since the only difference between the two groups was the start time of TJ-43 treatment, the reason for the increased acylated-ghrelin levels was most likely the early treatment with TJ-43, as reported previously[25]. Notably, oral meal consumption significantly increased in patients treated with TJ-43 than those in patients without TJ-43 treatment after the operation (Figure 2). Hence, TJ-43 increases acylated-ghrelin levels and improves food intake, mainly by promoting motility and mobility of the remnant stomach[7,18,26,27].
In addition to the enhancement of acylated-ghrelin levels by TJ-43[25], several other mechanisms contribute to the effects of TJ-43 on gastrointestinal function such as enhancement of gastric emptying, motility, and adaptive relaxation[28,29] The improvement in food consumption that was observed in this study may be mediated by these pathways, as well as ghrelin signaling, and thus, multiple actions of TJ-43 may improve oral food intake after PpPD. Moreover, in addition to acylated-ghrelin, TJ-43 increases plasma CCK, PYY, GIP, and GLP-1 after PpPD (Figures 2, 4 and 5). It has been reported that PYY and CCK cause DGE[30], which is opposite to the effects of acylated ghrelin. These results suggest that the body's response involves constant feedback mechanisms. Regarding appetite, acylated ghrelin contradicts CCK, PYY, and GLP-1. Furthermore, regarding gastric emptying, acylated ghrelin contradicts CCK and GLP-1. In this study, the ratio (POD 21/POD 0) for the change in the levels of acylated ghrelin, CCK, PYY, GIP, and GLP-1 was 2.14, 1.45, 1.61, and 1.37, respectively. Thus, the increase in acylated ghrelin was greater than that of CCK, PYY, GIP, and GLP-1. This result may support the effect of TJ-43 on an increase in food intake. Additionally, the effects of TJ-43 on acylated ghrelin may be more significant in the early period of TJ-43 treatment.
Moreover, gastrointestinal polypeptide hormone incretins secreted after meal intake[31] induce insulin secretion from the pancreas in a blood sugar-dependent manner, suggesting that hypoglycemia is unlikely to induce in the absence of food intake. Furthermore, it inhibits gastric acid secretion but does not affect gastric emptying[31]. The GIP is secreted from the K cells in the upper intestine and activates the islet β-cells. Furthermore, GLP-1 is secreted from the L cells in the lower intestine. Thus, incretin hormones are attracting interest for their application in clinical diabetes treatments. One new drug that has become recently available is a DPP-4 inhibitor. Collectively, these new drugs are called incretin-related drugs. By suppressing the action of this enzyme, incretin is less likely to be destroyed and blood glucose levels after eating are reduced. It was reported that herbal medicines can stimulate incretin secretion and regulate blood glucose in mice[32]. Furthermore, TJ-43 enhances insulin secretion after meals in humans[33]. In the present study, TJ-43 increased GIP and active GLP-1 Levels in patients who underwent PpPD. GIP and GLP-1 hormones can improve glucose tolerance and their effects are additive. GIP appears to quantitatively be the most important, particularly regarding insulin secretion, whereas the action of GLP-1 is mainly related to the inhibition of glucagon secretion[34]. The ratio of the change in GIP was greater than that of active GLP-1. These results may indicate that the effect of TJ-43 on the increase in GIP is more predominant than that in GLP-1.
Plasma levels of incretin hormones were increased by TJ-43 administration (Figure 4). In this study, insulin secretion tended to increase by TJ-43 administration (Figure 2), however, there were also no significant differences in blood glucose levels after a meal between the two groups (data not shown). Since undergoing PpPD involves reconstruction of the gastrointestinal tract, the effects also need to be investigated under normal physiological conditions. Considering that the increases in incretin hormone and IRI levels are most likely due to increases in oral food intake in the TJ-43(+) group, an animal study is necessary to resolve the clinical questions. Therefore, studies using animal models are currently underway. In one pilot study, the plasma and gastrointestinal expressions of incretin hormones significantly increased, and the expression of insulin in the pancreas was significantly enhanced by intragastric administration of TJ-43 for 28 d (unpublished data). Furthermore, activation of the islet cells increased with TJ-43 treatment. Thus, TJ-43 increases the incretin hormone levels in the blood after continuous intragastric administration and increases the expression of insulin in the pancreatic islet cells. Moreover, the administration of TJ-43 inhibits increased blood glucose levels during oral glucose tolerance tests in rats. These pilot studies in the animal model indicate that TJ-43 may increase insulin secretion after pancreatic surgery. Therefore, TJ-43 may benefit patients who undergo pancreatic resection.
In this study, TJ-43 increased the peripheral levels of acylated ghrelin and postoperative oral food intake. The findings indicate that TJ-43 may improve oral food intake by increasing the plasma acylated-ghrelin levels after PpPD. However, this study was small and non-randomized. A multicenter, randomized, placebo-controlled study is required to validate these findings. Furthermore, the effects of TJ-43 on incretin hormones need to be clarified in future studies.
Rikkunshito (TJ-43) improves gastrointestinal disturbances.
The effects of TJ-43 in patients undergoing pancreatic surgery have not been elucidated.
This study investigated the effects of TJ-43 in patients undergoing pylorus-preserving pancreatico-duodenectomy (PpPD).
Forty-one patients who underwent PpPD were divided into two groups; patients treated with daily doses of TJ-43 after surgery [TJ-43(+) group] or just on postoperative day (POD) 21 [TJ-43(-) group]. Plasma levels of acylated and desacylated ghrelin, cholecystokinin (CCK), peptide YY (PYY), gastric inhibitory peptide (GIP), and active glucagon-like peptide (GLP)-1 were evaluated. Oral calorie intake was assessed at POD 21 in both groups. The primary endpoint of this study was the total food intake after PpPD.
The acylated-ghrelin levels were significantly greater in patients treated with TJ-43 than those in patients without TJ-43 treatment at POD 21. Similarly, oral intake significantly increased in the TJ-43(+) group. The levels of CCK and PYY were significantly greater in patients treated with TJ-43 than those in patients without TJ-43 administration. Furthermore, the GIP and active GLP-1 levels increased and the values at POD 21 were significantly greater in patients treated with TJ-43 than those in patients without TJ-43 treatment. Insulin secretion tended to increase in patients treated with TJ-43.
TJ-43 may improve oral food intake in patients in the early phase after pancreatic surgery.
Further investigation is needed to clarify the effects of TJ-43 on incretin hormones.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lomperta K, Poland S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Murray CD, Kamm MA, Bloom SR, Emmanuel AV. Ghrelin for the gastroenterologist: history and potential. Gastroenterology. 2003;125:1492-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2466] [Cited by in RCA: 2457] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 3. | Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 5. | Ibrahim Abdalla MM. Ghrelin - Physiological Functions and Regulation. Eur Endocrinol. 2015;11:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D'Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Diéguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrère B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschöp MH. Ghrelin. Mol Metab. 2015;4:437-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 775] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 7. | Hoshino N, Nishizaki D, Hida K, Obama K, Sakai Y. Rikkunshito for upper gastrointestinal symptoms: A systematic review and meta-analysis. Complement Ther Med. 2019;42:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Esposito A, Criscitiello C, Gelao L, Pravettoni G, Locatelli M, Minchella I, Di Leo M, Liuzzi R, Milani A, Massaro M, Curigliano G. Mechanisms of anorexia-cachexia syndrome and rational for treatment with selective ghrelin receptor agonist. Cancer Treat Rev. 2015;41:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Yamada C, Hattori T, Ohnishi S, Takeda H. Ghrelin Enhancer, the Latest Evidence of Rikkunshito. Front Nutr. 2021;8:761631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Oka T, Okumi H, Nishida S, Ito T, Morikiyo S, Kimura Y, Murakami M; JOPM-EBM Working Team. Effects of Kampo on functional gastrointestinal disorders. Biopsychosoc Med. 2014;8:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Fujitsuka N, Uezono Y. Rikkunshito, a ghrelin potentiator, ameliorates anorexia-cachexia syndrome. Front Pharmacol. 2014;5:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Kawahara H, Kubota A, Hasegawa T, Okuyama H, Ueno T, Ida S, Fukuzawa M. Effects of rikkunshito on the clinical symptoms and esophageal acid exposure in children with symptomatic gastroesophageal reflux. Pediatr Surg Int. 2007;23:1001-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Kido T, Nakai Y, Kase Y, Sakakibara I, Nomura M, Takeda S, Aburada M. Effects of rikkunshi-to, a traditional Japanese medicine, on the delay of gastric emptying induced by N(G)-nitro-L-arginine. J Pharmacol Sci. 2005;98:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Tomono H, Ito Y, Watanabe T. [Successful antiemetic treatment of TSUMURA Rikkunshi-to Extract Granules for ethical use in addition to other antiemetic agents in neoadjuvant chemotherapy for an advanced breast cancer patient]. Gan To Kagaku Ryoho. 2006;33:1129-1131. [PubMed] |
| 15. | Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, Asaka M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Hattori T, Yakabi K, Takeda H. Cisplatin-induced anorexia and ghrelin. Vitam Horm. 2013;92:301-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Lüttges J. What's new? The 2010 WHO classification for tumours of the pancreas. Pathologe. 2011;32 Suppl 2:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Takiguchi S, Hiura Y, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Miyata H, Mori M, Hosoda H, Kangawa K, Doki Y. Effect of rikkunshito, a Japanese herbal medicine, on gastrointestinal symptoms and ghrelin levels in gastric cancer patients after gastrectomy. Gastric Cancer. 2013;16:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2328] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 20. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3512] [Article Influence: 175.6] [Reference Citation Analysis (34)] |
| 21. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2959] [Article Influence: 369.9] [Reference Citation Analysis (35)] |
| 22. | Matsumura T, Arai M, Yonemitsu Y, Maruoka D, Tanaka T, Suzuki T, Yoshikawa M, Imazeki F, Yokosuka O. The traditional Japanese medicine Rikkunshito increases the plasma level of ghrelin in humans and mice. J Gastroenterol. 2010;45:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13304] [Article Influence: 1108.7] [Reference Citation Analysis (0)] |
| 24. | Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast-track recovery programme after pancreatico- duodenectomy reduces delayed gastric emptying. Br J Surg. 2008;95:1387-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Takeda H, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Saegusa Y, Nahata M, Hattori T, Asaka M. Rikkunshito as a ghrelin enhancer. Methods Enzymol. 2012;514:333-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Yada T, Kohno D, Maejima Y, Sedbazar U, Arai T, Toriya M, Maekawa F, Kurita H, Niijima A, Yakabi K. Neurohormones, rikkunshito and hypothalamic neurons interactively control appetite and anorexia. Curr Pharm Des. 2012;18:4854-4864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Takahashi T, Endo S, Nakajima K, Souma Y, Nishida T. Effect of rikkunshito, a chinese herbal medicine, on stasis in patients after pylorus-preserving gastrectomy. World J Surg. 2009;33:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Fujitsuka N, Asakawa A, Amitani H, Fujimiya M, Inui A. Ghrelin and gastrointestinal movement. Methods Enzymol. 2012;514:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Yanai M, Mochiki E, Ogawa A, Morita H, Toyomasu Y, Ogata K, Tabe Y, Ando H, Ohno T, Asao T, Aomori T, Fujita Y, Kuwano H. Intragastric administration of rikkunshito stimulates upper gastrointestinal motility and gastric emptying in conscious dogs. J Gastroenterol. 2013;48:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Barreto SG, Windsor JA. Does the Ileal Brake Contribute to Delayed Gastric Emptying After Pancreatoduodenectomy? Dig Dis Sci. 2017;62:319-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 31. | Nauck MA, Meier JJ. Incretin hormones: Their role in health and disease. Diabetes Obes Metab. 2018;20 Suppl 1:5-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 530] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 32. | Suh HW, Lee KB, Kim KS, Yang HJ, Choi EK, Shin MH, Park YS, Na YC, Ahn KS, Jang YP, Um JY, Jang HJ. A bitter herbal medicine Gentiana scabra root extract stimulates glucagon-like peptide-1 secretion and regulates blood glucose in db/db mouse. J Ethnopharmacol. 2015;172:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Tanaka K, Urita Y, Nara K, Miura O, Sugimoto M. Effects of the traditional Japanese medicine Rikkunshito on postprandial glucose and lipid metabolism. Hepatogastroenterology. 2011;58:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Holst JJ. The incretin system in healthy humans: The role of GIP and GLP-1. Metabolism. 2019;96:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |