Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.812
Peer-review started: December 28, 2022
First decision: February 4, 2023
Revised: February 18, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 27, 2023
Processing time: 149 Days and 2.8 Hours
Total gastrectomy with splenectomy is the standard treatment for advanced proximal gastric cancer with greater-curvature invasion. As an alternative to splenectomy, laparoscopic spleen-preserving splenic hilar lymph node (LN) dissection (SPSHLD) has been developed. With SPSHLD, the posterior splenic hilar LNs are left behind.
To clarify the distribution of splenic hilar (No. 10) and splenic artery (No. 11p and 11d) LNs and to verify the possibility of omitting posterior LN dissection in laparoscopic SPSHLD from an anatomical standpoint.
Hematoxylin & eosin-stained specimens were prepared from six cadavers, and the distribution of LN No. 10, 11p, and 11d was evaluated. In addition, heatmaps were constructed and three-dimensional reconstructions were created to visualize the LN distribution for qualitative evaluation.
There was little difference in the number of No. 10 LNs between the anterior and posterior sides. For LN No. 11p and 11d, the anterior LNs were more numerous than the posterior LNs in all cases. The number of posterior LNs increased toward the hilar side. Heatmaps and three-dimensional reconstructions showed that LN No. 11p was more abundant in the superficial area, while LN No. 11d and 10 were more abundant in the deep intervascular area.
The number of posterior LNs increased toward the hilum and was not neglectable. Thus, surgeons should consider that some posterior No. 10 and No. 11d LNs may remain after SPSHLD.
Core Tip: Recently, laparoscopic spleen-preserving splenic hilar lymph node (LN) dissection (SPSHLD) has emerged as a viable alternative to splenectomy for advanced proximal gastric cancer with greater curvature invasion. However, laparoscopic SPSHLD has been observed to leave behind the posterior splenic hilar LNs. In this study, we aimed to clarify the distribution of splenic hilar and splenic artery LNs by examining cadavers, and to evaluate the possibility of omitting posterior LN dissection in laparoscopic SPSHLD from an anatomical perspective. Our findings revealed that the number of posterior LNs increased towards the hilum and was not negligible. Therefore, it is crucial for surgeons to consider that some posterior LNs may remain after SPSHLD.
- Citation: Umebayashi Y, Muro S, Tokunaga M, Saito T, Sato Y, Tanioka T, Kinugasa Y, Akita K. Distribution of splenic artery lymph nodes and splenic hilar lymph nodes. World J Gastrointest Surg 2023; 15(5): 812-824
- URL: https://www.wjgnet.com/1948-9366/full/v15/i5/812.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i5.812
Total gastrectomy with splenectomy is a standard treatment for locally advanced proximal gastric cancer with greater-curvature infiltration in East Asian countries[1,2]. Splenectomy is performed with the aim of achieving complete retrieval of splenic hilar lymph nodes (LNs), as well as LNs along the splenic artery, although the procedure increases the incidence of postoperative pancreas-related complications[1,3-5]. To avoid these potentially fatal complications, a new surgical technique named laparoscopic spleen-preserving splenic hilar LN dissection (SPSHLD) has been developed, and its use is widespread in some countries[3,4,6-8].
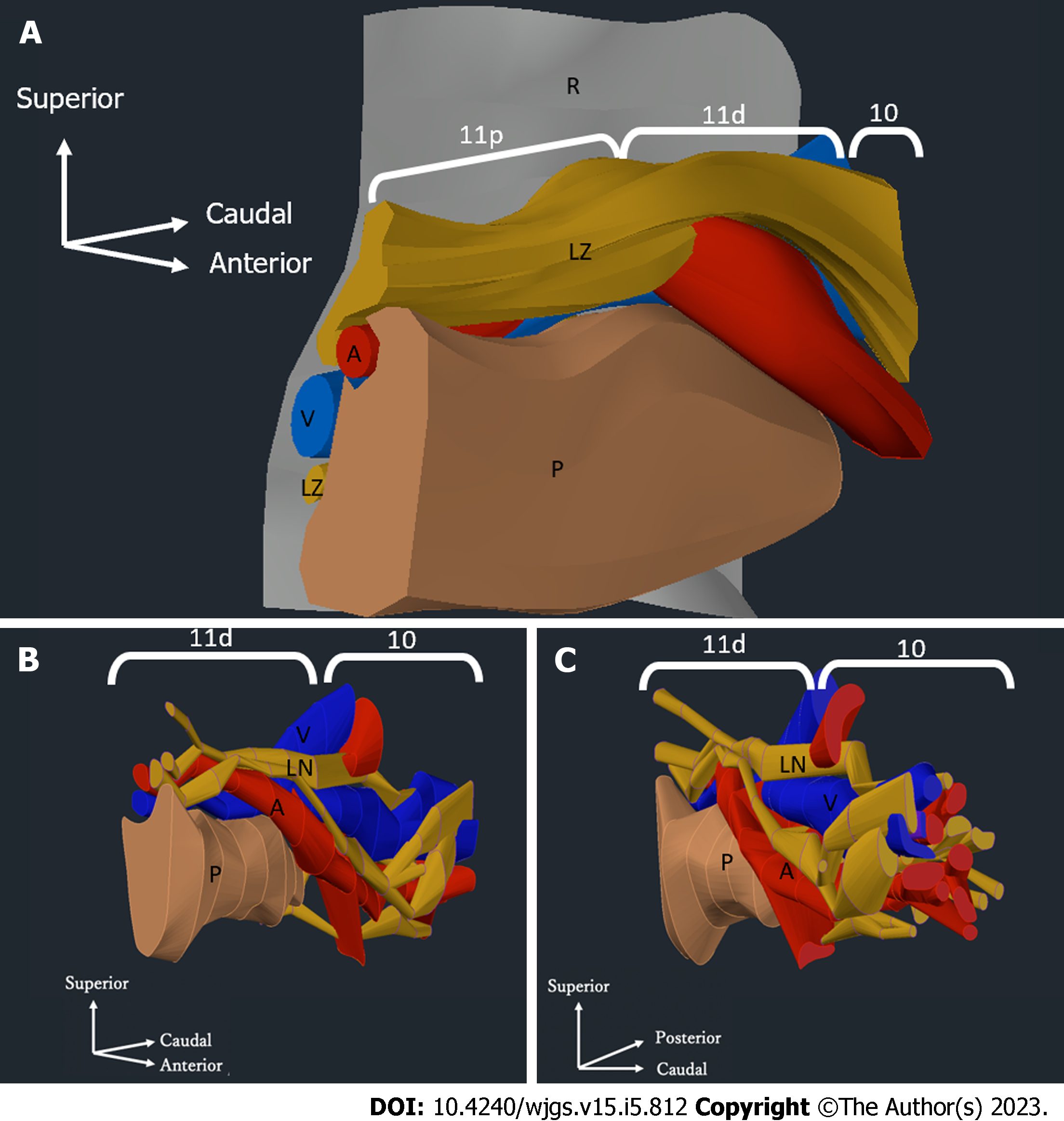
According to the Japanese Classification of Gastric Carcinoma[9], No. 10 LNs are defined as splenic hilar LNs adjacent to the splenic artery and distal to the pancreatic tail, as well as those on the roots of the short gastric arteries and those along the left gastroepiploic artery proximal to its gastric branch. The LNs along the splenic artery are referred to as No. 11 LNs, which are subdivided into No. 11p (proximal splenic artery LNs from the origin of the splenic artery to halfway between its origin and the pancreatic tail end) and No. 11d (distal splenic artery LNs from halfway between the origin of the splenic artery and the pancreatic tail end to the end of the pancreatic tail) LNs.
Both anterior and posterior splenic hilar LNs are completely retrieved with splenectomy; however, it is challenging to dissect the posterior LNs of the splenic hilum and the LNs along the splenic artery with laparoscopic SPSHLD in which the spleen is not mobilized[7,8]. For SPSHLD to be accepted as a standard procedure for locally advanced proximal gastric cancer with greater-curvature infiltration, omission of posterior splenic hilar LNs must be justified from both the clinical and anatomical standpoints.
Several reports have demonstrated the feasibility of laparoscopic SPSHLD from the clinical standpoint[8,10,11], but no studies have examined the distribution of splenic hilar LNs from the anatomical standpoint. Demonstrating the detailed distribution pattern of the anterior and posterior LNs would allow the feasibility of laparoscopic SPSHLD to be evaluated from an anatomical standpoint. Therefore, this study aimed to clarify the anatomical distribution of the splenic hilar (No. 10) and splenic artery (No. 11p and 11d) LNs. In addition, the number of anterior and posterior LNs was counted to clarify whether LN dissection without spleen mobilization can be justified from the anatomical standpoint. We also created three-dimensional (3D) images to clarify the anatomy around the splenic hilar and splenic artery LNs.
In this study, we examined six Japanese cadavers (four male and two female) aged between 74 and 99 years (Table 1). The cadavers were donated to the Department of Clinical Anatomy of Tokyo Medical and Dental University. All donors had previously signed documents agreeing to donate their bodies and had provided written consent for use in anatomical studies. The format of the document concurred with the Act on Body Donation for Medical and Dental Education under Japanese law. The study protocol was approved by our institutional review board (M2018-210). This study excluded the cadavers of individuals with esophageal, gastric, splenic, or pancreatic cancer. The cadavers were fixed by arterial perfusion with 8% formalin and preserved in 30% alcohol to prevent fungal growth and maintain the softness of the tissues.
| Case No. | 1 | 2 | 3 | 4 | 5 | 6 |
| Age (yr) | 75 | 81 | 79 | 96 | 99 | 85 |
| Sex | Male | Male | Male | Female | Female | Female |
| Weight (kg) | 38 | 59 | 40 | 41 | 30 | 42 |
| Cause of death | Bronchial pneumonia | DIC | Pneumonia | Senility | Senility | Liver failure |
| Patient history | Lung cancer | Miliary tuberculosis,post-CABG surgery | NA | Breast cancer, Melanoma | NA | Liver cancer, hepatic cirrhosis |
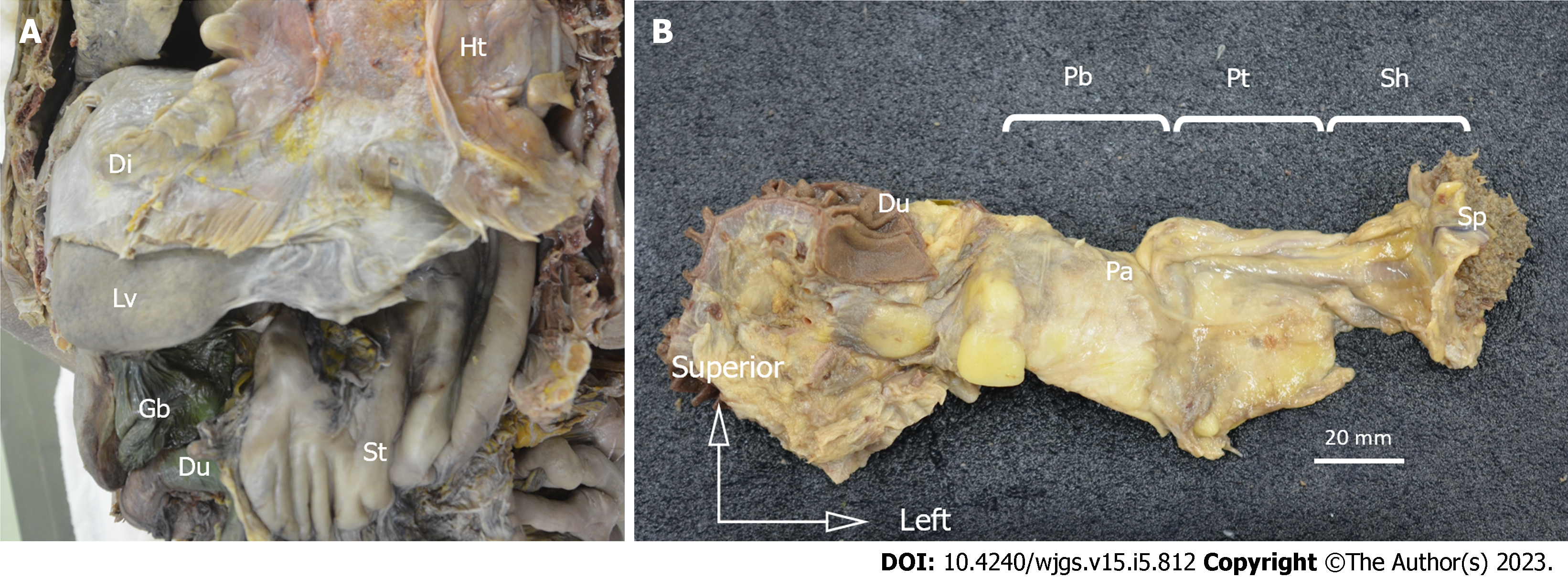
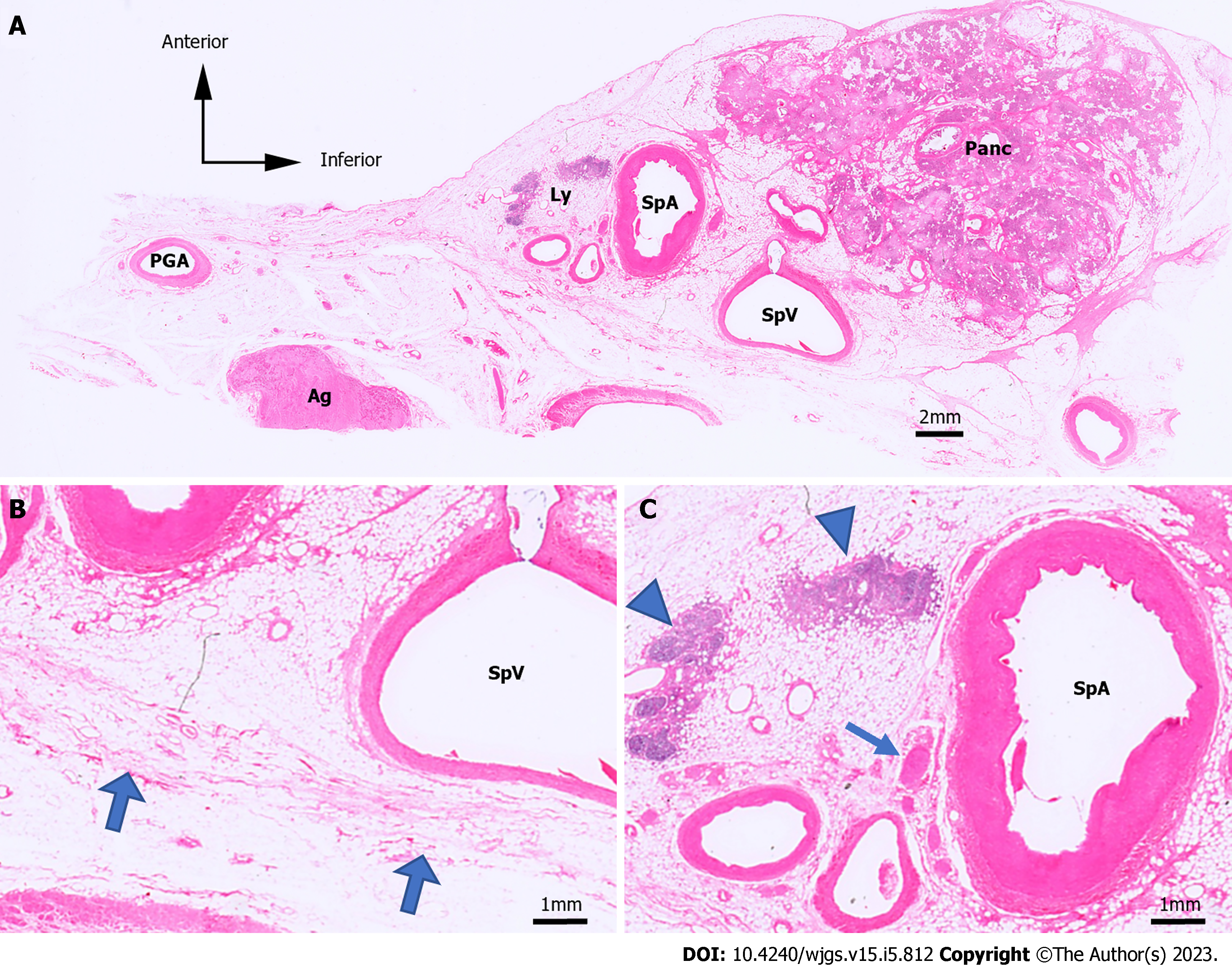
The esophagus, stomach, duodenum, spleen, pancreas, and vessels were resected from the cadavers in an en-bloc fashion (Figure 1A). Tissue blocks from the body of the pancreas to the splenic hilum were made from the specimens (Figure 1B). The short gastric arteries were dissected at the root, and the specimens consisted of the splenic hilum along with the pancreatic parenchyma. The tissue blocks were post-fixed, followed by defatting, demineralization, and paraffin embedding. Next, we made histological sections by cutting them into 5-µm-thick specimens at 1.0-mm intervals of width in the sagittal plane. Hematoxylin & eosin (H&E) staining was conducted to evaluate the structure of the organs and vasculature (Figure 2A and B). In H&E staining, LNs had a characteristic appearance with a dense core and were blue-purple because of hematoxylin staining, while the surrounding tissue was pink-red because of eosin staining. We also marked the splenic artery LNs and the splenic hilar LNs on H&E-stained sections (Figure 2C). We then evaluated the distribution of the splenic hilar LNs and the splenic artery LNs on H&E-stained specimens. We counted the number of anterior and posterior splenic artery LNs in each of the six cadavers, followed by the number of anterior and posterior splenic hilar LNs in five of these cadavers.
To assess whether the number of LNs that could not be dissected by laparoscopic SPSHLD was acceptable, we determined the number of anterior and posterior LNs.
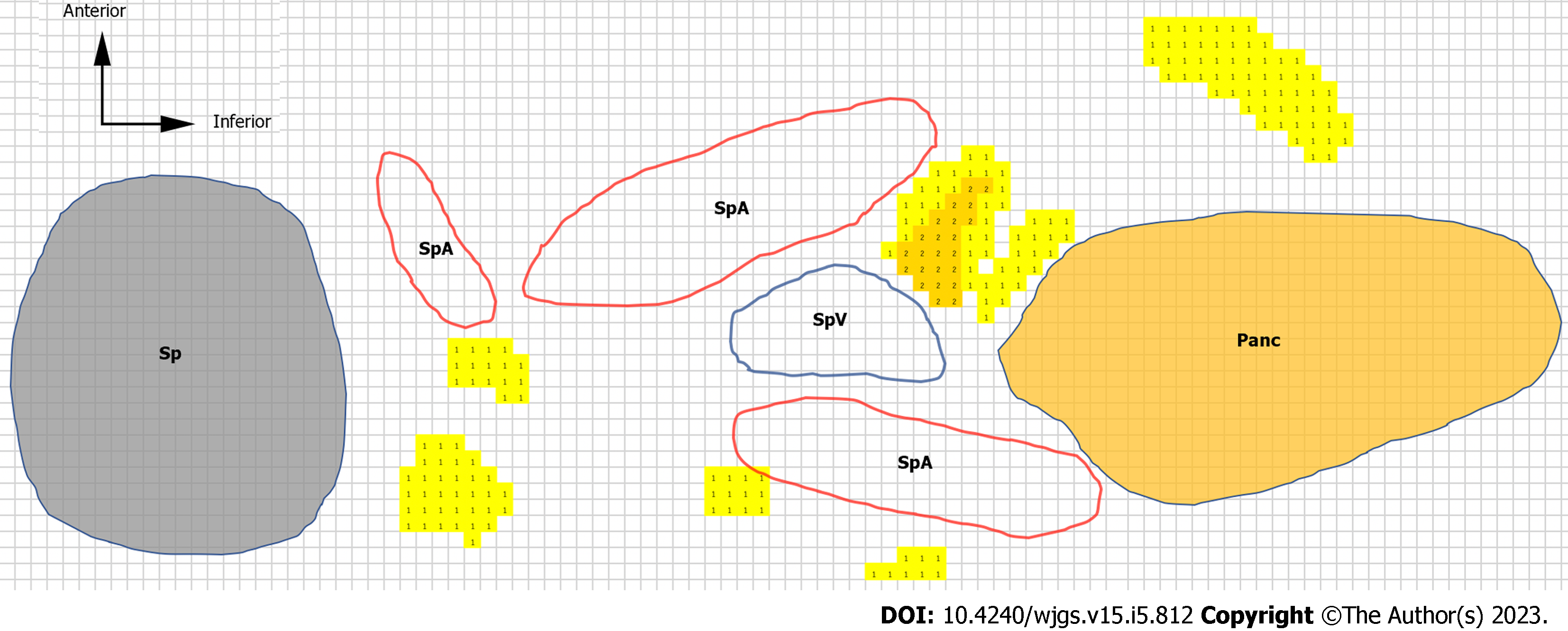
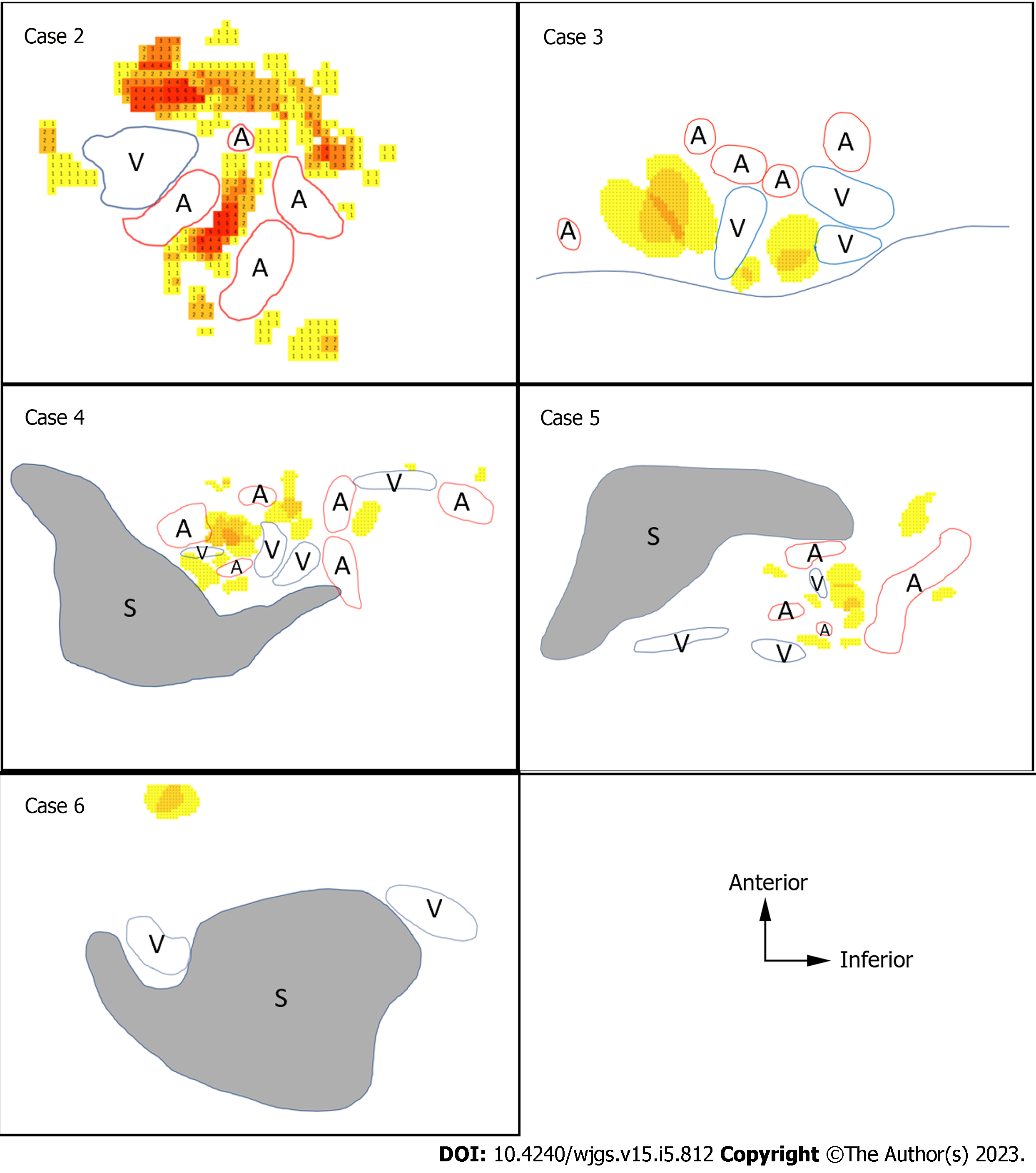
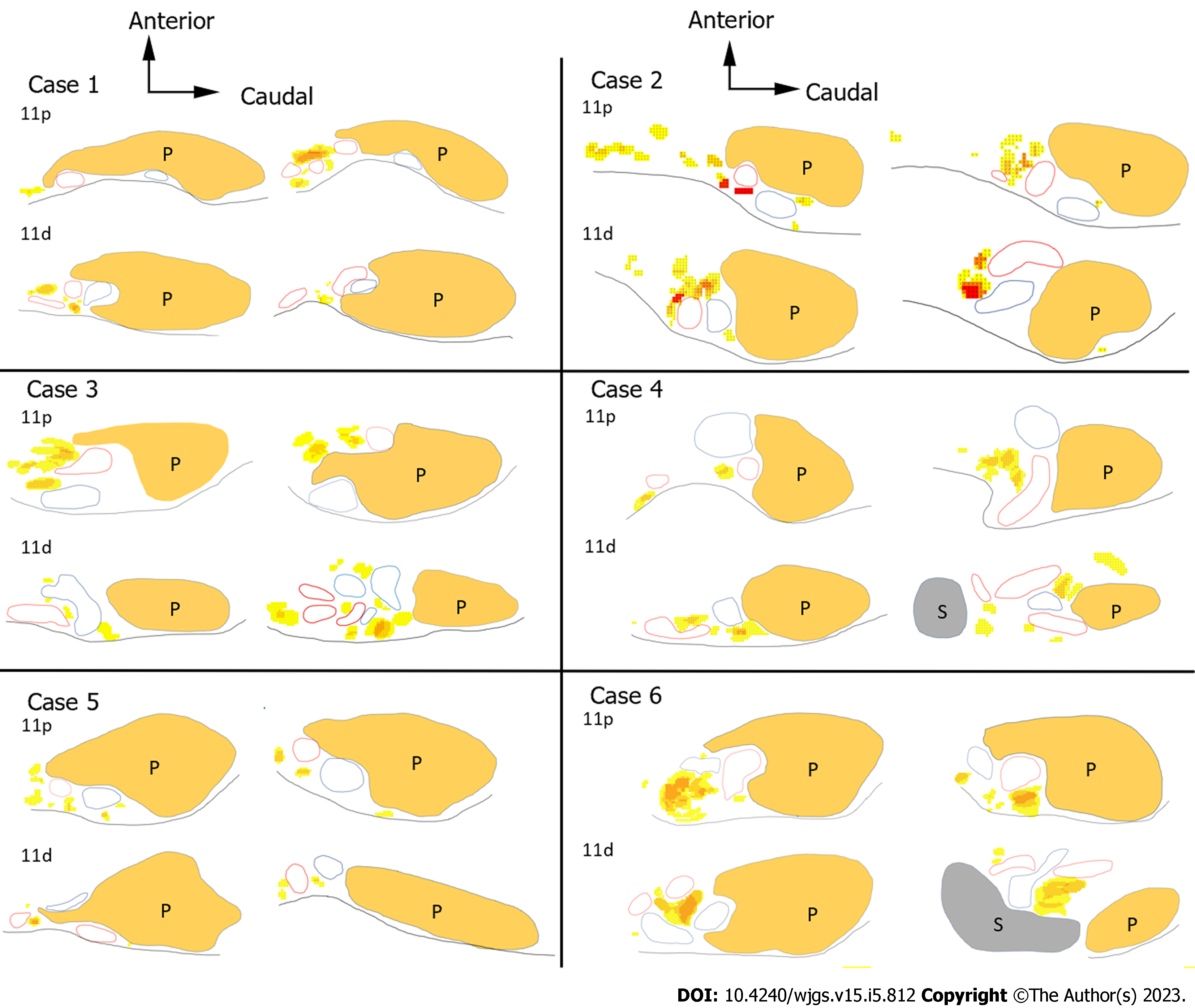
To clarify the difficulty in dissecting each LN, we created heatmaps and 3D images to objectively show the locations of the LNs and surrounding organs. The contours of the pancreas, spleen, blood vessels, and LNs were traced on H&E-stained sections. Anatomically similar sections from the same cadaver were overlaid to create schemas, and the frequency of the LNs was represented as heatmaps (Figure 3). We made the heatmaps using the color scale function of Microsoft Excel for Mac (version 16.16.27[202012]; Microsoft Corp., Redmond). The scale was set to show that the number of LNs increased as the color changed from yellow to red.
We used serial tissue sections from one of the six cadavers to perform 3D reconstruction. First, the traced images from a typical case were superimposed to create 3D images of the blood vessels and LNs. In addition, we generated 3D images by extracting only the regions of the distal splenic artery LNs and the splenic hilar LNs using Autodesk AutoCAD for Mac (product version R.47.M193).
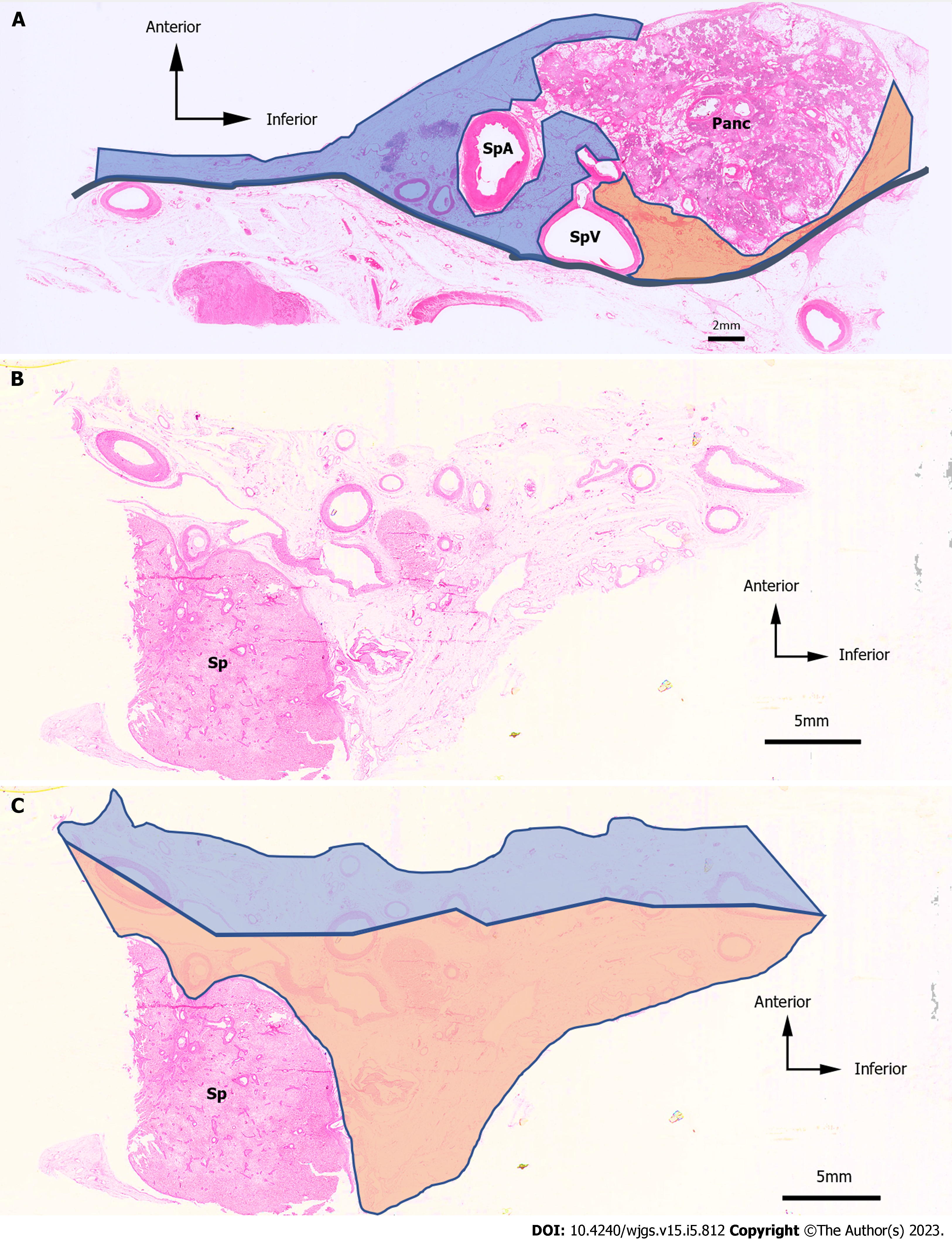
We defined the LNs within the range of laparoscopic LN dissection as anterior and posterior LNs. In the region of the No. 11 LNs, the splenic artery was divided into two patterns: one in which the splenic artery was located anterior to the splenic vein, and the other in which the splenic artery was located posterior to the splenic vein. When the splenic artery was shallower than the splenic vein, the posterior side was defined as the area bounded by the pancreatic parenchyma, splenic vein, and retroperitoneum (Figure 4A). In the region of the No. 10 LNs (Figure 4B), the anterior side was defined as the area ventral to the line connecting the midpoint of vessels with a long diameter of ≥ 1.5 mm, while the dorsal region was defined as the posterior side (Figure 4C).
The number of LNs on the anterior and posterior sides of the No. 10 and No. 11 LNs in each case are shown in Table 1. The No. 11 LNs of all of the cadavers were more frequently anterior than posterior. The number of No. 10 LNs varied from case to case. In three cadavers (cases 2, 4, and 6), there were more No. 10 LNs on the anterior side than on the posterior side. In two cadavers (cases 3 and 5), there were more No. 10 LNs on the posterior side than on the anterior side.
The mean number of No. 10 LNs per case was 11.5 on the anterior side and 10.7 on the posterior side. The mean number of No. 10 LNs per section was 1.08 on the anterior side and 1.00 on the posterior side. There was little difference in the number of No. 10 LNs between the anterior and posterior sides.
Next, we examined the No. 11p and No. 11d LNs separately. The number of anterior and posterior No. 11p and No. 11d LNs is shown in Table 2. The mean number of No. 11p LNs per case was 27.8 on the anterior side and 3.7 on the posterior side, and the mean number of No. 11d LNs per case was 25.3 on the anterior side and 7.7 on the posterior side. The mean number of No. 11p LNs per section was 1.46 on the anterior side and 0.19 on the posterior side, and the mean number of No. 11d LNs per section was 0.89 on the anterior side and 0.27 on the posterior side. For No. 11p and No. 11d LNs, all cases had more LNs on the anterior side than on the posterior side.
| Case No. | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Avg. (LN/case) | Avg. (LN/slice) | |
| No. 10 | ||||||||
| No. of slices | 0 | 18 | 4 | 18 | 19 | 5 | ||
| Anterior nodes | NA | 40 | 3 | 12 | 12 | 2 | 11.5 | 1.08 |
| Posterior nodes | NA | 25 | 4 | 6 | 29 | 0 | 10.7 | 1.00 |
| No. 11 | ||||||||
| No. of slices | 52 | 51 | 44 | 31 | 43 | 66 | ||
| Anterior nodes | 31 | 94 | 61 | 20 | 36 | 77 | 53.2 | 1.23 |
| Posterior nodes | 8 | 10 | 19 | 7 | 10 | 14 | 11.3 | 0.26 |
The number of No. 11p, No. 11d, and No. 10 LNs per section, as shown in Table 2 and Table 3, are summarized in Table 4. The anterior side had a larger number of No. 11p, No. 11d, and No. 10 LNs than the posterior side. In addition, the number of posterior LNs increased as they moved to the hilar (distal) side. Therefore, we calculated the ratio of posterior LNs to anterior LNs for LN No. 11p, 11d, and 10 for all cases. We also calculated the ratio of the total number of posterior LNs to the total number of anterior LNs for LN Nos. 11p, 11d, and 10 for all cases. These ratios are summarized in Table 5. The number of posterior LNs increased toward the splenic hilum (distal side).
| Case No. | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Avg. (LN/case) | Avg. (LN/slice) | |
| No. 11p | ||||||||
| No. of slices | 14 | 27 | 15 | 9 | 16 | 33 | ||
| Anterior nodes | 14 | 52 | 20 | 8 | 21 | 52 | 27.8 | 1.46 |
| Posterior nodes | 2 | 6 | 2 | 1 | 5 | 6 | 3.7 | 0.19 |
| No. 11d | ||||||||
| No. of slices | 38 | 24 | 29 | 22 | 27 | 33 | ||
| Anterior nodes | 17 | 42 | 41 | 12 | 15 | 25 | 25.3 | 0.88 |
| Posterior nodes | 6 | 4 | 17 | 6 | 5 | 8 | 7.7 | 0.27 |
| No. 11p | No. 11d | No. 10 | |
| Anterior node | 1.46 | 0.88 | 1.08 |
| Posterior node | 0.19 | 0.27 | 1.00 |
| → | Hilar side | ||
| Lymph node | Case No. | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Total | ||
| ↓ | No. 11p | 0.14 | 0.12 | 0.10 | 0.13 | 0.24 | 0.12 | 0.13 |
| No. 11d | 0.35 | 0.10 | 0.41 | 0.50 | 0.33 | 0.32 | 0.30 | |
| Hilar side | No. 10 | NA | 0.63 | 1.33 | 0.50 | 2.42 | 0.00 | 0.93 |
The heatmaps for the No. 10 LNs of the five cases are shown in Figure 5. The arrangement of the hilar vessels varied widely, and some No. 10 LNs existed intravascularly. In terms of the mean number of LNs, there were more anterior LNs than posterior LNs. However, some LNs existed deeply between the splenic hilar vessels.
The heatmaps for the No. 11 LNs are shown in Figure 6. We distinguished the No. 11 LNs into No. 11p and No. 11d LNs and evaluated them separately because the localization of these LNs differed between the proximal and distal sides. The number of posterior LNs tended to increase toward the spleen. As shown in the heatmap of the No. 11p LNs in Figure 6, in case 6, the No. 11p LNs tended to be more abundant in the superficial location, while the No. 11d LNs tended to be more common in more deeply intervascular locations. Some No. 11d LNs were located on the posterior side or were surrounded by the hilar vessels.
We generated 3D images of the No. 11 and No. 10 LNs (Figure 7A). The splenic hilum side had an intricate structure of vessels and LNs. Figure 7B shows that the No. 11p LNs existed on the surface of a single splenic artery. For the No. 11d and No. 10 LNs, the vascular branch was complicated, and the splenic hilar vessels covered the posterior LNs.
In this study, we report the results of an anatomical study on six cadavers in which we examined the distribution of No. 10 and No. 11 LNs. Importantly, we observed that the number of posterior LNs increased toward the hilum.
Lymphatic flow of proximal gastric cancers, especially those located along the greater curvature, drains to splenic hilar LNs via the left gastroepiploic artery, short gastric artery, celiac artery, and posterior gastric artery[12]. Therefore, the incidence of metastasis in No. 10 and splenic hilar LNs in advanced proximal gastric cancer were 9.8%-20.9% and 8.1%-27.9%, respectively[12,13]. Total gastrectomy with splenectomy is the standard treatment for locally advanced proximal gastric cancer with greater-curvature invasion[1,2]. However, this procedure has rarely been performed with the laparoscopic approach. Recently, large randomized controlled trials from Asia have demonstrated the non-inferiority of laparoscopic surgery for locally advanced gastric cancer[14-16], and laparoscopic surgery has thus become more popular recently. With the development of laparoscopic instruments and techniques, laparoscopic total gastrectomy became possible. Since then, outstanding surgeons in East Asia have started to perform laparoscopic SPSHLD[6,7]. Previous reports have insisted that the majority of No. 10 LNs are located on the anterior side, while posterior No. 10 LNs are rarely found[8,17]. However, the present study found that the number of posterior No. 10 LNs was not neglectable and was almost equal to, or even outnumbered, the number of anterior No. 10 LNs in some cases.
This discrepancy is probably because small LNs, which were counted as LNs in this study, might be overlooked in clinical practice. In clinical practice, surgeons visually remove the LNs from surgically resected specimens. Therefore, small LNs, which can be recognized under the microscope, but that are difficult to identify with the naked eye, are rarely counted. Indeed, small LNs of < 1 mm in size, which are usually ignored in clinical practice, were counted as LNs in the present study. Another probable reason is that in previous reports, some LNs, which were assumed to be on the posterior side in this study, might have been dissected as anterior LNs due to certain manipulations, such as elevation of the stomach, leading to displacement of the LNs from the posterior to the anterior side. As a result, it became difficult to completely distinguish the anterior LNs from the posterior LNs intraoperatively. Therefore, the distribution of the anterior and posterior LNs in this study (in which anatomical findings were considered) was different from that of previous studies (in which surgical findings were considered).
The novelty of this study lies in the use of autopsy to define the posterior and anterior LNs in detail and to determine the distribution and number of LNs based on serial sections. Furthermore, in this study, the distribution and number of LNs were visualized using a new analysis method of heatmapping and 3D reconstruction of serial sections, and the variability and complexity of the LN distribution were qualitatively verified.
The No. 10 and No. 11d LNs varied in their distribution, as shown in Figures 5 and 6. This variation could be attributed to the variety of vascular running and branching patterns in this region.
Studies have reported that the running patterns of the distal splenic artery and the splenic hilar vessels vary greatly[18,19]. Indeed, the branching patterns were not identical among the cadavers in the present study, as shown in Figures 5 and 6. This tendency became more obvious toward the distal end of the splenic artery. The traditional running pattern of the splenic artery is that it divides from a single splenic artery trunk into two or three branching vessels, which further branch into smaller vessels at the hilum[18]. These small vessels also have multiple branching patterns and can be divided into eight major categories. Although rare, the splenic artery may also branch near the celiac trunk[19]. Compared with the number of reports on the branching patterns of blood vessels, the branching patterns of lymphatic vessels have seldom been reported. However, as the lymphatic vessels usually run along the blood vessels, they are thought to have equally as numerous running patterns and LN distribution patterns in the distal splenic artery and splenic hilar regions as the splenic vessels.
The No. 10 and No. 11d LNs can be dissected as en-bloc specimens with splenectomy, regardless of their distribution. However, the posterior LNs may be left behind in vascular-sparing SPSHLD.
Our study showed that the number of posterior LNs increased toward the splenic hilum. This means that the number of LNs that may be left behind without splenectomy was high. Although a magnified view of laparoscopic surgery could enable surgeons to perform meticulous LN dissection by skeletonizing the blood vessels, retrieval of posterior LNs surrounded by multiple blood vessels or intertwined with each other is still technically demanding and difficult to perform, even with laparoscopic SPSHLD. Thus, surgeons should be cautious about omitting posterior LN dissection from the anatomical viewpoint.
Although the technical safety of SPSHLD has been demonstrated previously[8,20], the oncological safety of SPSHLD remains unclear. The efficacy of LN dissection should be comprehensively examined, and a variety of factors, such as the rate of metastasis, procedure-related complications, the local control rate, and survival outcomes, should be considered. Nevertheless, a survival analysis needs to be conducted to clarify these points.
The anterior/posterior ratio of No. 10 LNs, as well as the number of No. 10 LNs, differed widely among the cadavers examined in this study. The number of No. 10 LNs was small in cases 3 and 6, which was probably due to the short distance between the pancreatic tail and the spleen in these cases. Moreover, the number of sections in these cases was smaller than in the other cases.
Different from the No. 10 LNs, most of the No. 11p LNs were located in the anterior region. In addition, the splenic artery running pattern was relatively simple and did not branch at this level in most cases. Different from the No. 10 and No. 11d LNs, which were located deep between the vessels, the No. 11p LNs were identified at a resectable depth using the typical laparoscopic procedure without pancreatic mobilization.
To separate No. 10 LNs into those that were difficult to dissect by laparoscopic SPSHLD without pancreatic mobilization and those that were not, LNs behind vessels greater than 1.5 mm in diameter were defined as posterior in this study. However, anterior and posterior as defined in this study do not necessarily correspond to clinical anterior and posterior. The number of LNs in each may vary depending on how the definitions are determined. However, even qualitative assessment methods such as heat maps showed more LNs deep in the posterior compared with previous studies. Therefore, the conclusion that there were more posterior splenic hilar LNs compared with previous studies remains unchanged.
Another limitation is that this study did not investigate lymphatic flow from the gastric side to posterior splenic hilar LNs in proximal advanced gastric cancer. Therefore, the results do not directly relate the dissection of posterior LNs to recurrence. Cadavers with gastric cancer were excluded from assessing the neutral distribution of hilar LNs. If posterior No. 10 LNs receive little lymphatic flow from the stomach, the anterior LNs may be more often positive for metastases in gastric cancer. Further studies focusing only on proximal advanced gastric cancer are expected.
In this anatomical study of six cadavers, we found that several splenic hilar and distal splenic artery LNs might be left behind following anterior LN dissection by SPSHLD, as the anterior/posterior LN ratio of these areas was lower than expected. Most of the No. 11p LNs were located at the anterior side, and conventional laparoscopy would be sufficient for No. 11p LN dissection. Our results suggest that surgeons should consider that some posterior No. 10 and No. 11d LNs may be left behind after SPSHLD when applying this procedure in clinical practice. The feasibility of the procedure should be reviewed if future clinical trials show an increase in hilar LN recurrence in laparoscopic SPSHLD cases.
In East Asian countries, the standard treatment for locally advanced proximal gastric cancer with invasion of the greater-curvature is total gastrectomy with splenectomy. The splenic hilar and splenic artery lymph nodes (LNs) are usually dissected in this procedure. However, this procedure increases the risk of postoperative pancreatic complications. To avoid these complications, laparoscopic spleen-preserving splenic hilar LN dissection (SPSHLD) has been developed and is widely used in some countries.
Performing laparoscopic SPSHLD without spleen mobilization makes it challenging to dissect posterior splenic hilar LNs and LNs along the splenic artery. While previous studies have demonstrated the clinical feasibility of laparoscopic SPSHLD, anatomical studies have not been performed. Therefore, we sought to justify the omission of the posterior splenic portal LN from an anatomical perspective.
To evaluate the feasibility of laparoscopic SPSHLD from an anatomical standpoint, this study aimed to demonstrate the detailed distribution pattern of the anterior and posterior LNs, clarify the anatomical distribution of the splenic hilar (No. 10) and splenic artery (No. 11p and 11d) LNs, and count the number of anterior and posterior LNs.
This study examined six Japanese cadavers fixed by arterial perfusion with 8% formalin and preserved in 30% alcohol. The distribution of the splenic hilar LNs and splenic artery LNs was evaluated by creating histological sections, followed by hematoxylin & eosin staining to assess the structure of the organs and vasculature. In addition, the number of anterior and posterior LNs was counted, and three-dimensional reconstructions of their distributions were created.
This research uncovered a pattern where No. 11 LNs exhibited a greater frequency on the anterior side than on the posterior side, whereas No. 10 LNs showed minimal variability in number. The mean LN count was observed to be higher on the anterior side for No. 11p, No. 11d, and No. 10 LNs. Additionally, the number of LNs on the posterior side tended to increase toward the splenic hilum. Heat maps and three-dimensional images were generated to illustrate the spatial distribution and location of the LNs, showing that some LNs were intravascular or surrounded by the hilar vessels.
The ratio of anterior to posterior splenic hilar and splenic artery LNs may be lower than expected, and the number of posterior LNs increased toward the hilum. Our study suggests that surgeons should be aware that some posterior No. 10 and 11d LNs may be left behind after SPSHLD when using this procedure in clinical practice.
In laparoscopic SPSHLD, some LNs may not be retrieved, which should be considered by surgeons.
The authors thank all of the donors who donated their bodies for use in this anatomical study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li DH, China; Luo W, China S-Editor: Zhang H L-Editor: A P-Editor: Cai YX
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1329] [Article Influence: 332.3] [Reference Citation Analysis (2)] |
| 2. | Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg. 2017;265:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 3. | Kinoshita T, Okayama T. Is splenic hilar lymph node dissection necessary for proximal gastric cancer surgery? Ann Gastroenterol Surg. 2021;5:173-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Usui S, Tashiro M, Haruki S, Arita K, Ito K, Matsumoto A, Takiguchi N. Spleen preservation versus splenectomy in laparoscopic total gastrectomy with D2 lymphadenectomy for gastric cancer: A comparison of short-term outcomes. Asian J Endosc Surg. 2016;9:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Wanebo HJ, Kennedy BJ, Winchester DP, Stewart AK, Fremgen AM. Role of splenectomy in gastric cancer surgery: adverse effect of elective splenectomy on longterm survival. J Am Coll Surg. 1997;185:177-184. [PubMed] |
| 6. | Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008;207:e6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Kinoshita T, Shibasaki H, Enomoto N, Sahara Y, Sunagawa H, Nishida T. Laparoscopic splenic hilar lymph node dissection for proximal gastric cancer using integrated three-dimensional anatomic simulation software. Surg Endosc. 2016;30:2613-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Kinoshita T, Sato R, Akimoto E, Yoshida M, Harada J, Nishiguchi Y. Can laparoscopic spleen-preserving splenic hilar lymph node dissection replace prophylactic splenectomy for proximal advanced gastric cancers that invade the greater curvature? Eur J Surg Oncol. 2021;47:1466-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2869] [Article Influence: 204.9] [Reference Citation Analysis (0)] |
| 10. | Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M. Is it necessary to dissect the posterior lymph nodes along the splenic vessels during total gastrectomy with D2 lymphadenectomy for advanced gastric cancer? Eur J Surg Oncol. 2017;43:2357-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ma Z, Shi G, Chen X, Zhao S, Yang L, Ding W, Wang X. Laparoscopic splenic hilar lymph node dissection for advanced gastric cancer: to be or not to be. Ann Transl Med. 2019;7:343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Guner A, Hyung WJ. Advantages of Splenic Hilar Lymph Node Dissection in Proximal Gastric Cancer Surgery. J Gastric Cancer. 2020;20:19-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Zuo CH, Xie H, Liu J, Qiu XX, Lin JG, Hua X, Qin A. Characterization of lymph node metastasis and its clinical significance in the surgical treatment of gastric cancer. Mol Clin Oncol. 2014;2:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 529] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 15. | Kinoshita T, Uyama I, Terashima M, Noshiro H, Nagai E, Obama K, Tamamori Y, Nabae T, Honda M, Abe T; LOC-A Study Group. Long-term Outcomes of Laparoscopic Versus Open Surgery for Clinical Stage II/III Gastric Cancer: A Multicenter Cohort Study in Japan (LOC-A Study). Ann Surg. 2019;269:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 16. | Shimada M, Amaya S, Munemoto Y, Mitsui T. Laparoscopic lymph nodes dissection for advanced gastric cancer: The current status and the perspective. Mini-invasive Surg. 2019;3:7. [DOI] [Full Text] |
| 17. | Zhong Q, Chen QY, Xu YC, Zhao G, Cai LS, Li GX, Xu ZK, Yan S, Wu ZG, Xue FQ, Sun YH, Xu DP, Zhang WB, Wan J, Yu PW, Hu JK, Su XQ, Ji JF, Li ZY, You J, Li Y, Fan L, Zheng CH, Xie JW, Li P, Huang CM. Reappraise role of No. 10 lymphadenectomy for proximal gastric cancer in the era of minimal invasive surgery during total gastrectomy: a pooled analysis of 4 prospective trial. Gastric Cancer. 2021;24:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Liu DL, Xia S, Xu W, Ye Q, Gao Y, Qian J. Anatomy of vasculature of 850 spleen specimens and its application in partial splenectomy. Surgery. 1996;119:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Pandey SK, Bhattacharya S, Mishra RN, Shukla VK. Anatomical variations of the splenic artery and its clinical implications. Clin Anat. 2004;17:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Zheng C, Xu Y, Zhao G, Cai L, Li G, Xu Z, Yan S, Wu Z, Xue F, Sun Y, Xu D, Zhang W, Wan J, Yu P, Hu J, Su X, Ji J, Li Z, You J, Li Y, Fan L, Lin J, Li P, Huang C. Outcomes of Laparoscopic Total Gastrectomy Combined With Spleen-Preserving Hilar Lymphadenectomy for Locally Advanced Proximal Gastric Cancer: A Nonrandomized Clinical Trial. JAMA Netw Open. 2021;4:e2139992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |