Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.799
Peer-review started: January 26, 2023
First decision: February 20, 2023
Revised: March 6, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 27, 2023
Processing time: 120 Days and 2.4 Hours
Esophagogastric stricture is the troublesome long-term complication of corrosive ingestion with a significant adverse impact on the quality of life. Surgery remains the mainstay of therapy in patients where endoscopic treatment is not feasible or fails to dilate the stricture. Conventional surgical management of esophageal stricture is open esophageal bypass using gastric or colon conduit. Colon is the commonly used esophageal substitute, particularly in those with high pharyngoesophageal strictures and in patients with accompanying gastric strictures. Traditionally colon bypass is performed using an open technique that requires a long midline incision from the xiphisternum to the suprapubic area, with adverse cosmetic outcomes and long-term complications like an incisional hernia. As most of the affected patients are in the second or third decade of life minimally invasive approach is an attractive proposition. However, minimally invasive surgery for corrosive esophagogastric stricture is slow to evolve due to the complex nature of the surgical procedure. With advancements in laparoscopic skills and instrumentation, the feasibility and safety of minimally invasive surgery in corrosive esophagogastric stricture have been documented. Initial series have mainly used a laparoscopic-assisted approach, whereas more recent studies have shown the safety of a total laparoscopic approach. The changing trend from laparoscopic assisted procedure to a totally minimally invasive technique for corrosive esopha
Core Tip: Most patients with corrosive esophagogastric stricture are young adults in the most productive period of their lives. Hence, minimally invasive surgery for corrosive stricture is an attractive proposition. However, minimally invasive surgery for corrosive esophagogastric stricture is slow to evolve due to complex operative steps. With advances in laparoscopic technology, there is a changing trend from laparoscopic-assisted approaches to totally minimally invasive techniques. However, assessing the patency of the vascular arcade remains a challenge during the laparoscopic approach. The challenges and limitations highlighted in the present review could guide future research on minimally invasive surgery in corrosive esophagogastric stricture.
- Citation: Kalayarasan R, Durgesh S. Changing trends in the minimally invasive surgery for corrosive esophagogastric stricture. World J Gastrointest Surg 2023; 15(5): 799-811
- URL: https://www.wjgnet.com/1948-9366/full/v15/i5/799.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i5.799
Consumption of corrosive substances remains a significant public health concern, especially in developing countries. Stricture of the upper aerodigestive tract is one of the most feared long-term complications of corrosive ingestion. Children and young adults in the most productive period of their lives make up about 80% of the population affected by corrosive injuries globally[1]. While less common in western countries, corrosive esophageal injuries are the most frequent cause of benign esophageal obstruction in developing countries[2]. The social and economic repercussions of severe corrosive injuries are substantial. Consequences include a significant negative social impact due to the stress of prolonged treatment, delayed recovery, and negative body image secondary to unpleasant laparotomy scars, which cause families to dissolve and increase the chances of repeat suicidal ideation in patients.
Innovations and advancements in minimally invasive surgery allowed its application in various gastrointestinal surgical disorders. The short-term benefits of minimally invasive surgery are well known and include early ambulation and postoperative recovery, decreased postoperative pain, and analgesic requirement[3-5]. Also, the excellent cosmetic outcome is an added advantage considering the age group of the affected population. The long-term benefits of minimally invasive surgery are reduced incision-related complications such as incisional hernia. However, minimally invasive surgery for corrosive esophageal injury is slow to evolve due to the complexity of the esophageal bypass procedure[5,6]. Also, surgeons primarily focused on restoring oro-enteral continuity and reestablishing a euphagic state rather than the minimally invasive approach. However, growing experience in various minimally invasive gastrointestinal surgical procedures allowed its application in corrosive esophagogastric strictures.
Initial minimally invasive series for corrosive esophagogastric strictures reported hybrid approaches (hand-assisted or mini-laparotomy) because of limited instrumentation and experience[7-9]. However, with the advancement of laparoscopic technology, energy sources, and better instrumentation, a few series reported the safety and feasibility of the total minimally invasive procedure[10,11]. As the number of patients reported in each series is limited, the present review aims to provide an overview of the changing trends in minimally invasive surgery for corrosive esophagogastric strictures. Also, the current challenges and future perspectives in the minimally invasive management of corrosive esophagogastric strictures will be highlighted in this review.
All the authors did a PubMed search of relevant articles. Further, the articles’ reference lists were also searched for additional appropriate studies. The keywords and combinations included in the search were: “Caustics”; ”stricture”; “bypass”; “corrosive stricture” and “laparoscopic”; “colon conduit” and “Laparoscopic”; “colon bypass” and “robotic”; “gastric stricture” and “Laparoscopic”; “esophageal stricture” and “ laparoscopic”; “gastric conduit” and “Laparoscopic”; “gastric stricture” and “robotic”; “corrosive stricture” and “esophagectomy” and “laparoscopic ”. The search was limited to publications in English literature. All the authors agreed that the articles selected for review were relevant.
The two most frequently utilized conduits have been the stomach and the colon. While the colon has traditionally been the favored option, comparable results have been reported with gastric conduits in selected patients[3,7-9]. The use of a gastric conduit for the esophageal bypass procedure would be contingent upon the presence of a normal well-vascularized stomach, with no evidence of any stricture in the body or antrum and the absence of a high esophageal or hypopharyngeal stricture. The retrosternal gastric pull-up is an acceptable alternative to colon bypass in these patients. Traditionally, gastric transposition in corrosive stricture has been carried out using an open surgical method.
Javed et al[10] reported the technical feasibility and safety of the laparoscopic approach in four patients with corrosive stricture of the esophagus who had failed endoscopic therapy. The suitability of the stomach as a prospective conduit was evaluated based on the barium swallow. A computed tomography (CT) scan of the abdomen was used in patients for whom a complete barium examination was not possible because of a tight esophageal stricture. Findings from previous surgical records at the time of the feeding jejunostomy also guided decision-making. However, the final decision regarding the appropriateness of the stomach as a conduit was decided at the time of surgery after direct evaluation with a laparoscope.
The procedure was performed using four laparoscopic ports, and the conduit was based on right gastroepiploic vessels. An adequate retrosternal tunnel is created under vision till the level of the thoracic inlet. The stomach is delivered into the neck with the help of Ryle’s tube placed through the neck incision and advanced into the abdomen via the retrosternal tunnel. Gentle handling of the conduit and preserving vascular supply are vital principles to ensure an excellent postoperative outcome.
The mean duration of the surgery was 260 min, and none of the patients required blood transfusions or postoperative ventilation. All patients were ambulated on postoperative day one. Oral liquids were started between the fifth and seventh postoperative days after a gastrograffin study. One patient had transient hoarseness and another a mild chest infection, but none had an anastomotic leak. The mean hospital stay was 7.75 d. Long-term follow-up results are not available. However, all patients were euphagic to a solid diet at a mean follow-up of 6.5 mo[10].
In most patients with corrosive injury, using gastric conduit is not a viable option due to the frequent involvement of the stomach in the scarring process, particularly after acid ingestion. As a reliable evaluation of the stomach is not feasible in patients with high-grade esophageal stricture, gastric stricture manifesting in the postoperative period is not uncommon. In patients with high pharyngeal strictures, the length of the stomach conduit may be a concern. Also, functional results of gastric conduit tend to deteriorate with time, with symptomatic reflux, stricture, and columnar metaplasia of the residual squamous epithelium above the anastomosis. Additionally, when a gastric conduit is utilized, the function of the gastric reservoir is lost.
The colon is the most frequently used esophageal substitute in corrosive injury patients, particularly those with high pharyngoesophageal strictures and accompanying gastric strictures[11-14]. Such substantial lesions of the upper aerodigestive tract necessitate a longer and more versatile conduit, which the colon can offer[15,16]. Relative vascular impairment at the tip of the gastric conduit predisposes to anastomotic complications. At the same time, a robust blood supply of the colon conduit minimizes the risk of anastomotic leaks and strictures[15-18]. However, using the colon requires a minimum of three anastomoses instead of one needed with gastric conduit[17,18].
Various centers have used different parts of the colon as a conduit which may be the right, left, or mid-colon. Ananthakrishnan et al[14] have previously documented good long-term outcomes with open mid-colon esophageal bypass, a variation of the traditional left colon esophageal bypass. The mid-colon bypass offers a longer colon segment than other standard colon bypasses, thereby enabling tension-free cervical anastomoses. Additionally, using the terminal ileum to deliver the colon through the retrosternal tunnel to the neck protects the vascular pedicle and arcade from trauma. Also, the initial passage of the widest portion of the colon (cecum), through the retrosternal region minimizes the trauma on the subsequent colon segments. Resection of the terminal ileum and cecum in the neck permits using a colon segment that is comparatively unaffected by the trauma caused by conduit transfer[19].
An esophageal bypass without esophagectomy provides numerous advantages over esophageal resection. Corrosive injuries, especially those caused by acid, result in extensive periesophageal fibrosis, making esophageal resection hazardous with complications including bleeding, laryngeal nerve injury, thoracic duct damage, and tracheal lacerations[20-23]. Esophageal resection disproportionately increases the complexity and morbidity of the surgery than its claimed benefit of preventing cancer, whose prevalence is exaggerated[16,17,22]. Additional benefits include a reduction in operation duration and the avoidance of thoracotomy or blind hiatal dissection[17,18,21].
Traditionally colon bypass is performed using an open technique that requires a long laparotomy incision, frequently linked to suboptimal cosmetic results and long-term complications like incisional hernia. These consequences can be mitigated by adopting a minimally invasive approach, which has been recently demonstrated to be both safe and feasible[15,19-21]. While the initial series used hand-assisted and laparoscopic-assisted techniques, the feasibility of total laparoscopic colon bypass has been recently documented.
The hand-assisted laparoscopy combines traditional laparoscopy with the capability of putting a hand intraperitoneally. The hand-assisted technique improved exposure and allowed manual exploration, blunt dissection, and immediate control of hemostasis[20]. The hand port offers abdominal access for the surgeon’s hand while maintaining the pneumoperitoneum. After colon mobilization, gastrointestinal continuity and extracorporeal anastomosis (coloenterostomy and colocolostomy) were carried out through the incision to implant the hand port device[21].
Lin et al[21] demonstrated the safety and feasibility of Hand-assisted laparoscopic colonic mobilization for esophageal reconstruction in seven patients with esophageal stricture secondary to caustic ingestion. The procedure was performed with the patient in the lithotomy posture, and the surgeon stood between the patient’s legs (Table 1). The pneumo sleeve device was attached to the 7-cm supraumbilical midline incision. The camera port was positioned around 5 cm below the umbilicus, with two additional 10-mm ports placed into the bilateral flanks. The surgeon’s right hand, wrapped with a conventional glove, was inserted into a plastic sleeve with perforated fingers (Pneumo Sleeve), and then covered with a second traditional glove. The colon was mobilized using the ultrasonic shears, the pneumo device was subsequently removed, and the colon was exteriorized via the wound protector. The conventional left colon esophagocoloplasty extending from the hepatic flexure to the splenic flexure based on the ascending branch of the left colic artery was performed.
| Ref. | Total No. patients | Surgery | Colon used | Arterial pedicle | Route | Blood loss (mL) | Operative duration |
| Lin et al[21], 2002 | 7 | Hand-assisted laparoscopic colon mobilization | Transverse colon | Ascending branch of left colic artery | Retrosternal | 100 (50-350) | 3.9 h (3.2-5.0) |
| Esteves et al[4], 2010 | 3/5 corrosive | Laparoscopic-assisted esophagectomy and colonic interposition | Transverse colon | Double vascular pedicle (left colic artery and marginal paracolic arcade) | Posterior mediastinal | - | 3.6 h (3.0-4.0) |
| Maurer et al[5], 2013 | 20 | Laparoscopic transhiatal esophagectomy + laparoscopic assisted colonic transposition | Transverse colon | - | Posterior mediastinal | - | 8.3 h |
| Javed et al[18], 2013 | 4 | Total laparoscopic esophageal bypass | Ascending, transverse colon | Left colic artery | Retrosternal | 100 (80-120) | 6.1 h (5.3-7.0) |
| Banerjee et al[24], 2017 | 10 | Laparoscopic-assisted colonic transposition | Ascending, transverse colon | Left colic artery | Retrosternal | 150 (100-200) | 240 m (210-300) |
| Gurram et al[19], 2020 | 7 | Laparoscopic midcolon esophageal bypass | Mid colon | Left colic artery | Retrosternal | 200 (100-400) | 440 m (390-600) |
In the study by Lin et al[21], the mean (range) operative time was 3.9 (3.2-5.0) h, with the mean (range) operative time of 62 (45-75) min for hand-assisted laparoscopic colonic mobilization. Two of the seven patients had cologastric anastomosis combined with gastrojejunostomy due to the associated gastric antropyloric stricture (Table 2). Postoperatively, no patients experienced any anastomotic complications, and all are euphagic at 18 mo follow-up. One patient had a mild abdominal wound infection. The mean (range) hospital stay was 9.1 (8.0-13.0) d[21].
| Ref. | Anastomotic leak | Anastomotic stricture | Wound infection | Other complications | Hospital stay (d) | Follow up (mo) |
| Lin et al[21], 2002 | Absent | Absent | 1 | None | 9.1 (8.0-13.0) | 18.2 |
| Esteves et al[4], 2010 | Absent | 1 (cervical dilatation) | Absent | 1-atelectasis; 1-pneumonia; 2-pneumothorax; 1-cervical stenosis (persistent fibrotic esophagus) | 6 (9-18) | 20.4 (10.0-29.0) |
| Maurer et al[5], 2013 | 6-cervical anastomotic leak (resolved spontaneously) | 11-anastomotic stricture | - | 6-pneumothorax; 5-pleural effusion; 3-atelectasis; 6-vagal nerve injury | 26.8 | - |
| Javed et al[18], 2013 | 1 minor leak (cervical) | Absent | Absent | None | - | 5 (3-6) |
| Banerjee et al[24], 2017 | 1 (resolved spontaneously) | 1 (dilatation thrice) | Absent | 1-transient left recurrent laryngeal nerve paresis | - | 11 (5-24) |
| Gurram et al[19], 2020 | 1 (segmental conduit ischemia) | Absent | Absent | 2- postoperative pneumonia | 9 (8-42) | 14 (7-42) |
Proponents of laparoscopic-assisted techniques believe that intracorporeal suturing, a rate-limiting step in laparoscopy, can be eliminated. Also, adding a minilaparotomy does not contribute to the disfigurement because most patients have incision scars due to the earlier feeding procedure while offering comfort to the surgeon[24].
Banerjee et al[24] utilized laparoscopy for left colic artery-based colonic mobilization and retrosternal tunneling in ten patients with acid-induced esophageal strictures. The assessment of the adequacy of conduit perfusion and subsequent colonic transection was performed through a mini laparotomy (Table 1). The laparoscopic procedure was performed with the patient in the supine posture with legs split and the neck extended to the right. Four laparoscopic trocars were used. During descending and ascending colon mobilization, the surgeon stood to the patient’s right and left, respectively.
Lateral to medial mobilization of the descending, ascending, and transverse colon was performed sequentially. After releasing the falciform ligament from the anterior abdominal wall, the Xiphisternum was palpated with the non-dominant hand and pressed down to visualize its location on the monitor. At the junction of xiphisternum and sternum, the diaphragm was incised using ultrasonic shears to create a retrosternal tunnel. A U-shaped collar incision was used if the patient were to undergo a pharyngolaryngectomy. Otherwise, a left pre-sternomastoid incision was made for exposure of the esophagus alone. In patients where the larynx was salvageable, anastomosis to the pyriform fossa helped in preventing laryngectomy.
A mini-laparotomy was performed through the previous upper abdominal incision for feeding jejunostomy. The right colic, middle colic, and ileocolic vessels were clamped with bulldog clamps to confirm adequate perfusion by the left colic vessels. As per the required length of the colon for transposition transection was done at the transverse/ascending/ileo-ascending colon to create an isoperistaltic conduit based on the left colic artery. Using a camera sleeve as a retrosternal sheath and a Foley catheter attached to the cranial tip of the conduit with a silk suture, the conduit was delivered atraumatically into the neck with gentle traction[25].
The cervical esophagus was longitudinally opened, and a side-to-side esophago-colic anastomosis was performed using an interrupted 4-0 polydioxanone suture to create a wide anastomosis. In patients with stricture immediately below the cricopharynx, the esophageal slit was widened by extending it superiorly into the hypopharynx. Those who underwent partial laryngectomy received a side-to-end pyriform fossa-colic anastomosis. On the other hand, those who underwent total pharyngolaryngectomy had end-to-end pharyngo-colic anastomosis constructed at the base of the tongue. The conduit’s slack was rectified and secured to the diaphragmatic hiatus. Beyond the DJ flexure, the conduit was anastomosed to the proximal jejunum using stapled/hand-sewn side-to-side anastomosis. Just distal to the anastomosis, the colon was divided while maintaining the integrity of the marginal arcade and the colonic continuity restored.
In the study by Banerjee et al[24], acid-induced strictures in ten adults (5 males and 5 females) were treated. Two individuals had duodenal involvement in addition to several esophageal and gastric strictures. Three patients had strictures of the pharynx and larynx, requiring suprahyoid pharyngolaryngectomy (n = 2) or partial laryngectomy (n = 1). The mean (range) operative time was 240 (210-300) min with a mean (range) blood loss of 150 (100-200) mL. Postoperatively one patient developed a cervical anastomotic leak on ninth postoperative day, which healed with conservative management (Table 2). One patient developed proximal anastomotic stricture, requiring dilatation. One patient had transient left recurrent laryngeal nerve paresis, which resolved spontaneously. All the patients were euphagic to solid oral diet[24].
Advancements in laparoscopic technology and instrumentation facilitated total laparoscopic colonic transposition, as documented in recent studies[18,19]. The critical steps of total laparoscopic colonic transposition are described here.
Patient and port position: After inducing general anesthesia, the patient was positioned in the supine position with a leg split. A soft wedge or sandbag is kept under the shoulders, the neck is extended to the right, and the arms are tucked to the side of the body. Pneumoperitoneum was established using a 12 mm infraumbilical trocar. Five more additional ports were installed. In addition to an epigastric port to create a retrosternal tunnel, two working and two assistant trocars were inserted in the pararectal area. The patient’s previous jejunostomy or gastrostomy, if present, would be removed if it interfered with the surgical procedure. Later in the surgery, the gastrostomy could be utilized to construct a side-to-side cologastric anastomosis.
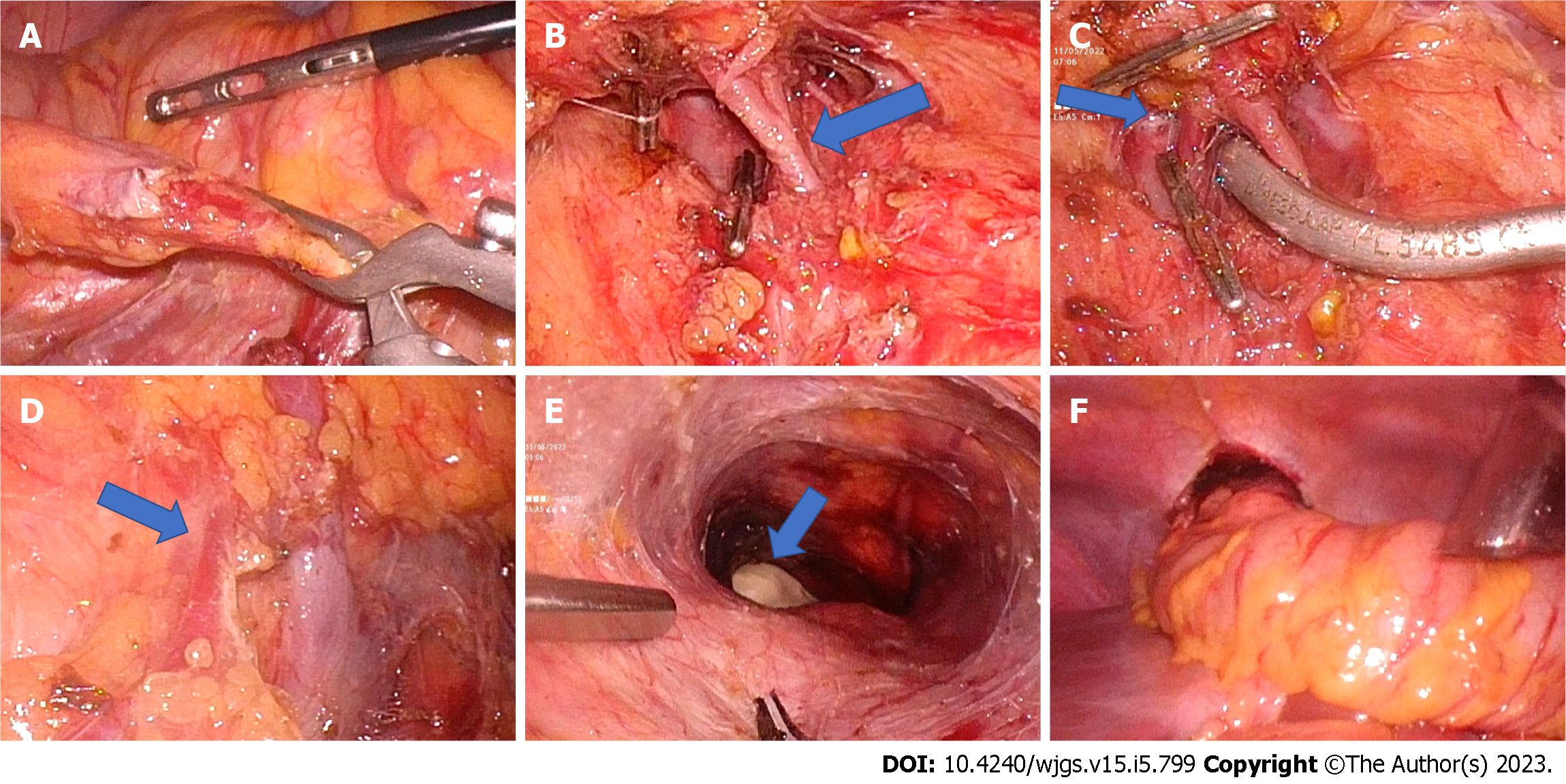
Trial clamping of vascular pedicles and assessment of the adequacy of perfusion: The operative surgeon stands on the patient’s left side and uses the two left pararectal ports as working trocars during the initial dissection. The ileocolic vessels are identified after providing traction to the terminal small bowel mesentery, looped, and secured with a laparoscopic vascular clamp (Figure 1). In patients with separate origin of the right colic artery; the vessel is dissected and clamped. After controlling the ileocolic pedicle and, if present, the right colic artery, the lesser sac is entered by dividing the gastrocolic omentum. Right colic veins draining to the gastrocolic trunk are divided. Middle colic vessels are dissected at their origin proximal to its bifurcation and clamped using a laparoscopic vascular clamp (Figure 1). To prevent retrograde perfusion of colonic conduit through the ileal arcade, a laparoscopic vascular clamp is applied to the mesentery of the terminal ileum. With the laparoscopic bulldog clamps in place, the sufficiency of the colonic vascular arcade is assessed. The surgeon briefly releases the pneumoperitoneum before proceeding to the neck to commence dissection.
Neck dissection: The neck is entered using a gently curved left-sided linear neck incision from the suprasternal notch to the upper part of the neck. Less frequently, a more cosmetic transverse skin crease neck incision may be used, although its application is restricted to lower esophageal strictures[18]. The incision is deepened further via the platysma while dividing the inferior belly of the omohyoid. The middle thyroid vein and occasionally the anterior jugular vein must be ligated because they impede the operative field. The thyroid gland is retracted medially away from the field with the use of a thyroid stitch. The cricoid cartilage is now palpated, the cervical esophagus is recognized, and its lumen is palpated either over a prepositioned Ryle’s tube or an esophageal bougie introduced by the anesthesiologist. Stay sutures are placed posteriorly above and below the targeted esophagotomy site to facilitate esophagotomy and subsequent anastomosis. Finger dissection is used to create a short retrosternal tunnel from the neck incision (Figure 1). The pneumoperitoneum is recreated after the incision has been covered with surgical gauze to prevent gas leakage.
Retrosternal tunnel creation: The surgeon shifts from the left side of the patient to stand between the patient’s legs and utilizes the upper two working trocars. The epigastric trocar will be used to finish the retrosternal tunnel creation and colonic transposition to the neck. In order to get sufficient exposure, the falciform ligament is split. Percutaneous insertion of a needle in the subxiphoid region facilitates identification of the most inferior point of the retrosternal region and prevent unintentional pleural or pericardial perforation. To enter the retrosternal space, the peritoneum is split at the needle’s entry point. The loose connective tissue of the retrosternal area opens easily with the blunt dissection aided by pneumoperitoneum. This region is bounded anteriorly by the posterior surface of the sternum, and posteriorly on both sides by the pericardium and bilateral pleurae (Figure 1). The camera light further transilluminates the sternum and guides the progression of retrosternal tunnel creation. Observing the assistant surgeon’s fingers placed into the neck incision verifies that the retrosternal tunnel has been completed (Figure 1).
Colonic transposition: With bulldog clamps in place, the conduit’s vascularity was examined by observing the cecum’s color and pulsations. An ideal colonic conduit should be pink, demonstrate peristalsis when stimulated, and have visible arterial pulsations to the terminal portion of the conduit with no obviously distended veins[19]. Once the surgeon was satisfied with the vascularity of the colon conduit, the previously clamped vessels were ligated at the origin and divided. The terminal ileum was divided near the ileocecal junction using a laparoscopic linear cutter. The umbilical tape placed through the retrosternal tunnel is tied to the ileum. The colonic conduit is transferred to the neck by gently pulling with umbilical tape and pushing it using endoscopic bowel graspers (Figure 1). The conduit should be handled carefully, and the mesocolon’s lie checked again. After delivering the conduit to the neck, the lower end of the colon was divided with a laparoscopic linear cutter where the colon meets the stomach or jejunum (in diffuse gastric stricture) without tension. The colonic conduit was checked for redundancy. During distal colonic transection, the mesocolon was opened close to the bowel wall to protect the marginal arcade.
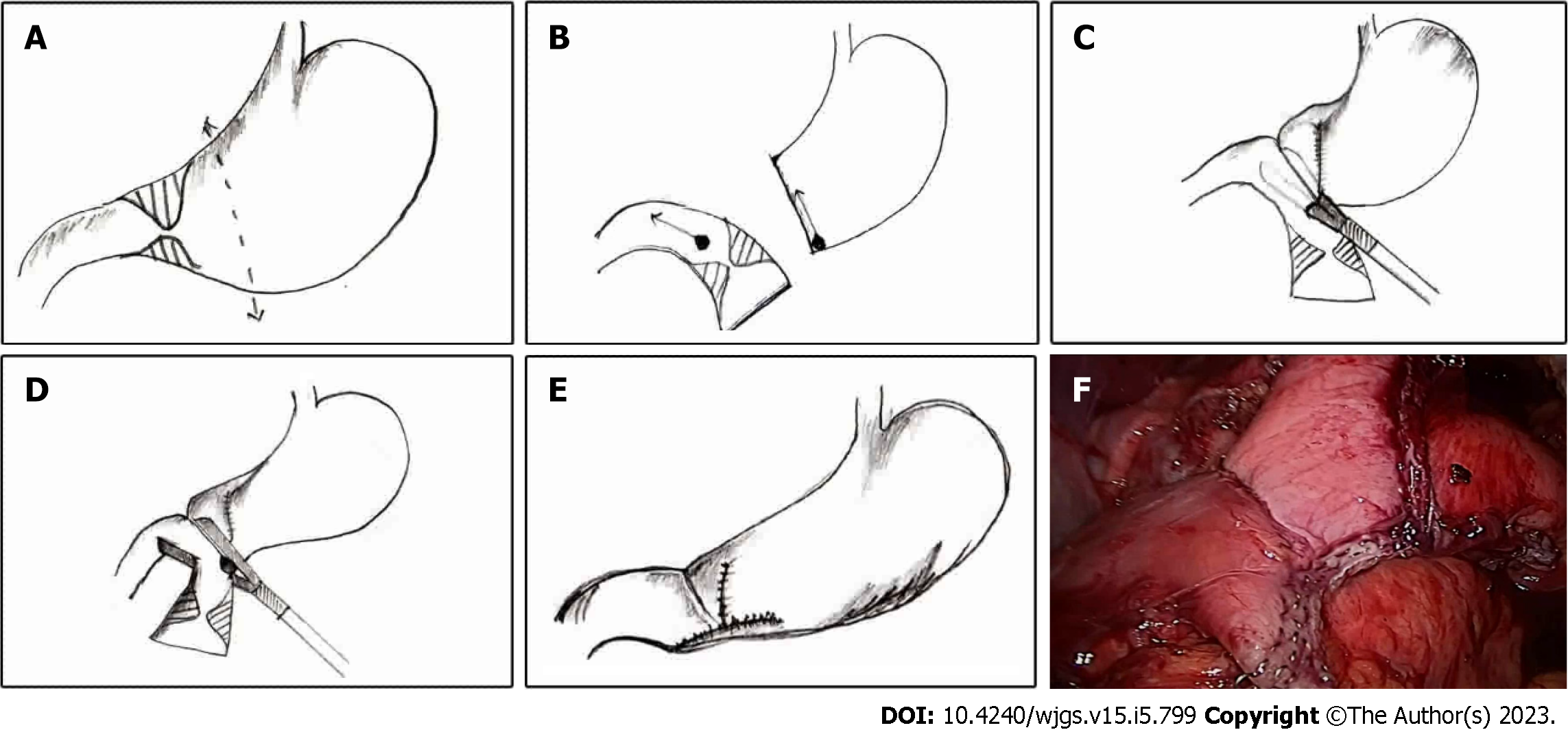
Reconstruction: A wide side-to-side, single-layer esophago/pharyngocolic anastomosis was created using interrupted polydioxanone 3-0 suture (Figure 2). The anastomosis should be as posterior and as wide as possible. The lower end of the colonic conduit is anastomosed to the anterior gastric wall in a side-to-side fashion using a laparoscopic linear cutter (Figure 2). The gastrostomy site may be used for cologastric anastomosis if the patient had a previous feeding gastrostomy. The stapler entry site is closed with a 3-0 polydioxanone suture. Laparoscopic Billroth-I gastrectomy or gastrojejunostomy is performed in patients with associated antropyloric stricture. The lower end of the colonic conduit is anastomosed to the jejunum in patients with a diffuse gastric stricture. The procedure concludes with the restoration of the colonic continuity with the aid of a stapled side-to-side ileocolic anastomosis (Figure 2).
In the series published by Javed et al[18] four patients with suicidal acid ingestion underwent total laparoscopic colon transposition. The average duration of surgery was 370 min, with an average blood loss of 100 mL (Table 2). None of the patients required any blood transfusions or ventilator support after surgery. In the laparoscopic group, all patients were ambulated earlier, and considerably fewer analgesics were required (P = 0.01)[18]. In a retrospective study, Gurram et al[19] analyzed 15 patients who underwent esophagacoloplasty for caustic stricture. Seven patients who underwent the laparoscopic procedure were compared with the eight patients who had an open mid-colon esophageal bypass. The laparoscopic group required considerably less postoperative analgesia (3 d vs 5 d, P = 0.02) and lesser intraoperative blood loss (200 mL vs 350 mL, P = 0.03). Operative time was shorter in the laparoscopic group (440 min vs 510 min), but the difference was not statistically significant (P = 0.93). Surgical complications, intensive care unit length of stay, total postoperative hospital stay, and medium-term swallowing outcomes were comparable between the two groups[19].
Esophagocoloplasty is a technically challenging surgery that necessitates advanced laparoscopic skills. A colonic bypass requires a minimum of three anastomoses, whereas a laparoscopic gastric bypass requires only one. In addition, the median operation time is more for laparoscopic colonic pull-up compared to laparoscopic gastric pullup (370 min vs 180 min)[18]. Transillumination of the mesocolon is used to evaluate the vascular arcade during an open mid-colon bypass, which is not possible during laparoscopy and necessitates a preoperative cross-sectional imaging to rule out substantial vascular anomalies. Also, unlike open approach adequacy of perfusion can be evaluated only by visual assessment of arterial pulsations in the marginal arcade and appendices epiploicae. Hence, it is essential to understand the limitations of the laparoscopic colonic transposition and to have a low threshold for conversion if the vascular anatomy cannot be precisely identified. The dangers of misjudgment and poor evaluation during a laparoscopic procedure greatly outweigh the benefits of a laparoscopic technique.
Acid ingestion is more common in developing countries compared to alkali ingestion. The corrosive gastric stricture is a late complication of corrosive-induced gastric injury and is usually linked to acid consumption. Acids cause coagulation necrosis, while alkalis cause liquefaction necrosis[26]. The adage, “acids lick the esophagus and bite the pyloric antrum”, highlights the risk of gastric stricture secondary to acid ingestion[27]. The antropyloric region of the stomach is most frequently affected because of reflux pylorospasm, which results in a prolonged contact time. Antropyloric stricture in the absence of high-grade esophageal stricture manifests as gastric outlet obstruction. Endoscopic assessment and barium studies can typically determine the extent of gastric involvement in patients with isolated gastric strictures[28]. However, neither of these procedures can be utilized to evaluate the type of gastric injury in individuals with absolute dysphagia. Often, a CT scan would be required in such a circumstance. A plain abdomen radiograph performed after an overnight fast is frequently a less expensive alternative if it reveals a gastric air-fluid level suggestive of gastric outlet obstruction[29]. As with esophageal strictures, the importance of gastrectomy to avoid malignant transformation has been exaggerated[2]. In more than 750 esophageal and 2000 gastric stricture patients, Hsu et al[30] observed no cases of cancer resulting from corrosive intake. Endoscopic therapy of gastric stricture is often associated with poor long-term outcomes, necessitating surgery in most patients.
For management purposes, gastric strictures are divided into five categories. Of this type I and type II represent localized short prepyloric strictures and strictures extending proximally up to the antrum, respectively[2]. The surgery of choice for patients with isolated type I and II gastric strictures is a limited resection with gastroduodenal reconstruction, maintaining physiological continuity while avoiding dumping syndrome and bile reflux. Loop gastrojejunostomy should be performed on patients in whom an esophageal stricture can be ruled out with certainty, as gastrojejunostomy precludes the use of the stomach as a conduit. However, if gastrojejunostomy is required retrocolic route should be avoided, since it increases the technical complexity of future colonic bypass by interfering with the vascular arcade[2].
Type III strictures are mid-gastric strictures involving the body and sparing the proximal and distal portion of the stomach, often requiring distal gastrectomy with Polya reconstruction. As the anastomosis is typically non-dependent in type III stricture, gastrojejunostomy should generally be avoided.
Type IV are diffuse gastric strictures producing a linitis plastica like appearance. A substantial amount of the corrosive swallowed will cause a widespread stomach contraction. In healthy, fit patients, total gastrectomy with esophagojejunostomy and feeding jejunostomy is the recommended surgical procedure.
Type V gastric strictures are associated with a stricture of the first part of the duodenum. The management of type V gastric stricture is a challenge. Since aggressive resection is associated with severe problems in these individuals, antecolic dependent gastrojejunostomy is considered a safer treatment option[2].
Compared to the esophageal stricture laparoscopic approach is feasible in a significant proportion of patients with isolated gastric stricture due to less complex surgical steps.
Shah et al evaluated the surgical outcomes of 30 patients with corrosive-induced gastric outlet obstruction who underwent laparoscopic-assisted (n = 15) versus open gastrojejunostomy (n = 15)[31]. The intraoperative findings and postoperative complications were recorded and compared between approaches. Shah et al[31] reported that the laparoscopic assisted gastrojejunostomy group required a smaller incision (4-5 cm vs 9-10 cm) and fewer intraoperative blood transfusions (2 patients vs 8 patients). Postoperatively, patients in the laparoscopic-assisted group experienced lesser post-operative pain with no incidence of wound infections (0 patient vs 4 patients). Early drain and suture removal result in quicker hospital discharge with minimal post-operative morbidity and no significant increase in the total duration or cost of the operation[31].
Raikwar et al[32] analyzed demographic characteristics, injury grade, location, mode of consumption, performed surgery, parameters before and after surgery, body weight, nutritional status, and mortality in a series of 35 patients. One patient underwent laparotomy, and Billroth II gastrectomy at the initial visit, while the rest underwent feeding jejunostomy for nutritional optimization. Thirteen patients subsequently underwent gastrojejunostomy, of which two underwent a laparoscopic procedure. Subasinghe et al[33] demonstrated the effectiveness of laparoscopic gastrojejunostomy in two patients with homicidal corrosive acid ingestion resulting in antral stricture. Both patients underwent laparoscopic stapled gastrojejunostomy with a mean operative time of 3 h. Oral intake was allowed by the fourth and sixth postoperative day, which they tolerated well, demonstrating the efficacy of laparoscopic gastrojejunostomy[33]. The safety and feasibility of laparoscopic gastrojejunostomy have also been documented in a few case reports[34,35].
Traditionally, corrosive-induced gastric outlet obstruction secondary to short pyloric stricture is managed with a Billroth I gastrectomy or bypass gastrojejunostomy. Heineke Mickulicz pyloroplasty is occasionally sufficient in individuals with partial gastric outlet obstruction due to moderate mucosal damage[36]. Following the footsteps of laparoscopic repair of neonatal duodenal atresia, some studies described and demonstrated the feasibility of laparoscopic diamond antroduodenostomy as a less invasive alternative for managing pyloric cicatricial obstruction in five pediatric patients[37,38]. For laparoscopic antroduodenostomy, monopolar cautery is used to make a transverse enterotomy in the healthy pyloric antrum, and a longitudinal one in the first portion of the duodenum. A diamond antroduodenostomy was created using single-layered interrupted intracorporeal sliding tumble-square knots. No abdominal drains were placed. A diluted contrast agent was administered through the nasogastric tube under the C-arm 24 h after surgery to evaluate anastomotic integrity and gastric motility. Patients were gradually allowed oral fluids when deemed appropriate and were discharged home the next day.
In the study by Seleim et al[38], the operation time ranged from 72 min to 89 min, with an average of 81 min. Contrast examinations on postoperative day 1, ruled out any radiological leaks, with delayed gastric emptying demonstrated in two cases. Seleim et al[38], demonstrated an average weight gain of 2.35 kg (almost 24% of preoperative weight)in the postoperative period. At a mean follow-up of 13.5 mo, no recurrence of obstructive symptoms nor dumping was observed, with excellent cosmetic outcomes[38].
In patients with isolated antropyloric stricture, Billroth I gastrectomy is preferred over gastrojejunostomy as it restores physiological alimentary continuity, thereby maintaining autocrine and paracrine signaling and feedback mechanisms. Also, in patients with a concurrent esophageal stricture Billroth I gastrectomy does not interfere with future colon conduit formation and esophageal bypass[39].
Nagaraj et al[40] reported two patients with corrosive acid ingestion managed with total laparoscopic double-stapled Billroth-I gastrectomy. One patient presented with progressive dysphagia and, on evaluation, was found to have antropyloric stricture with concomitant esophageal stricture amenable to endoscopic dilatation. The second patient had isolated antropyloric stricture with symptoms of gastric outlet obstruction[40].
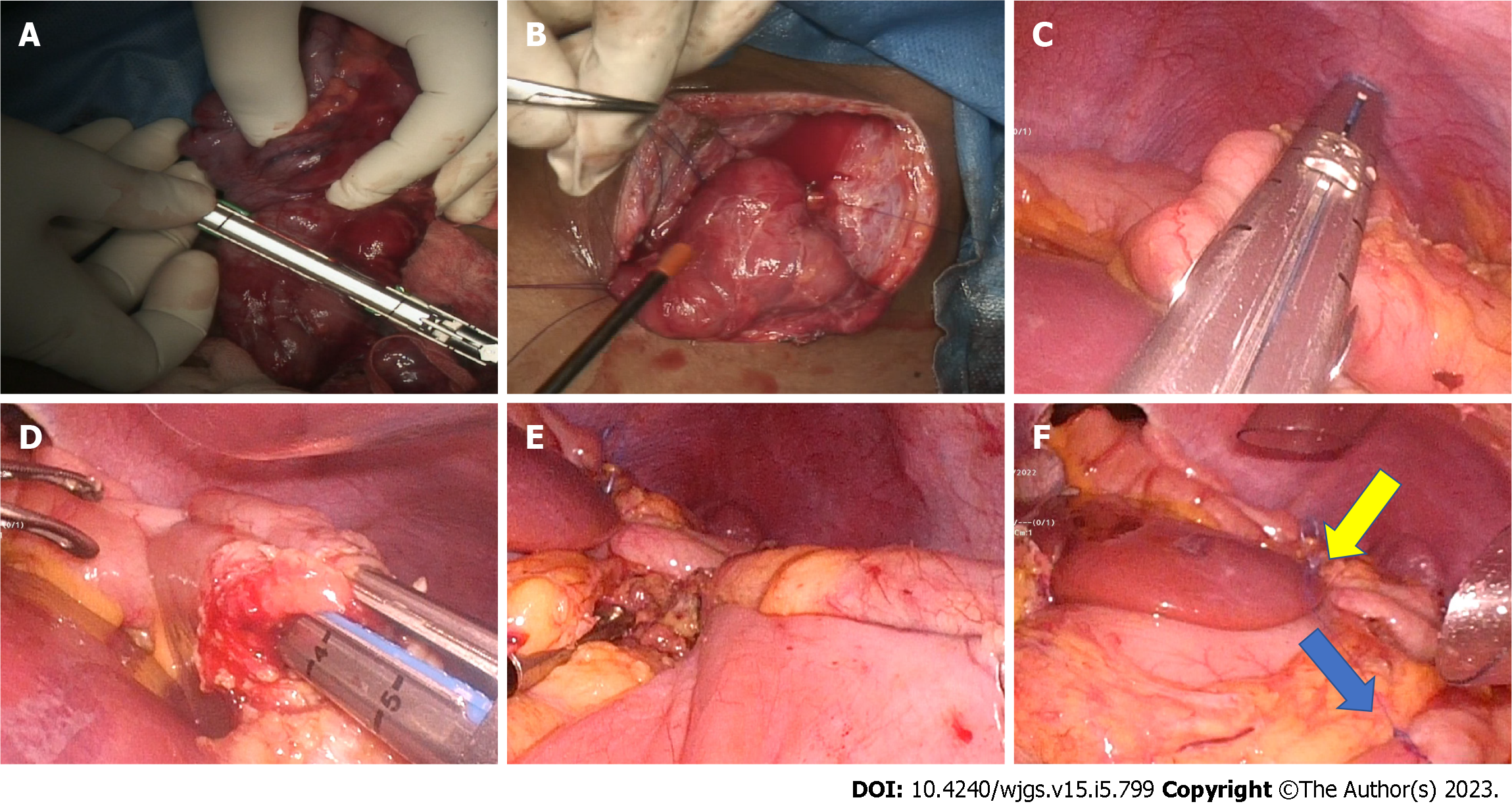
The patient was positioned supine with her legs split. The procedure was performed with five laparoscopic ports like any upper gastrointestinal laparoscopic surgery. The primary surgeon stands to the patient’s left, the assistant surgeon to the right, and the camera surgeon between the legs. The lesser sac is entered by dividing the gastrocolic omentum using ultrasonic shears. Subsequently right gastroepiploic vessels were clipped and divided. Creation of a retrogastric tunnel is completed by dividing the gastrohepatic ligament and right gastric artery branches using ultrasonic shears. An umbilical tape placed through the retro gastric tunnel is snugly tied to the antropyloric region to facilitate duodenal mobilization and provide traction during stapler application.
Stapled transection of the healthy portion of the stomach proximal to the stricture was performed with green or black reloads depending upon the stomach thickness (Figure 3). After gastric transection, one gastrotomy was made on the greater curvature proximal to the stapler line and a second one at the pyloric level. One jaw of a 60-mm blue laparoscopic linear cutter was positioned through the greater curve gastrotomy, and the stomach was brought anterior to the duodenum. The other stapler jaw was placed through the pyloric gastrotomy into the duodenum. The umbilical tape traction facilitates the stapling procedure. The firing of the stapler generates a V-shaped gastroduodenostomy between the stomach’s posterior wall and the duodenum’s anterior wall. The stapler entry site was closed with a laparoscopic linear cutter to complete the gastroduodenostomy using a double-stapled technique (Figure 3).
The modified linear stapling approach described by Nagaraj et al[40], as opposed to a circular stapler for gastroduodenostomy, does not require a mini-laparotomy. The most prevalent approach employing a linear stapler is a delta-shaped gastroduodenostomy, which is technically challenging and requires more staplers[41]. The technical difficulties of performing a completely laparoscopic gastroduodenal anastomosis are primarily due to the challenge in aligning gastric remnant and duodenal stump. In the technique described by Nagaraj et al[40], the duodenum is not cut until the very end, which allows for improved traction and orientation during the stapling procedure. The last stapler used to seal the gastroduodenostomy simultaneously transect the duodenum.
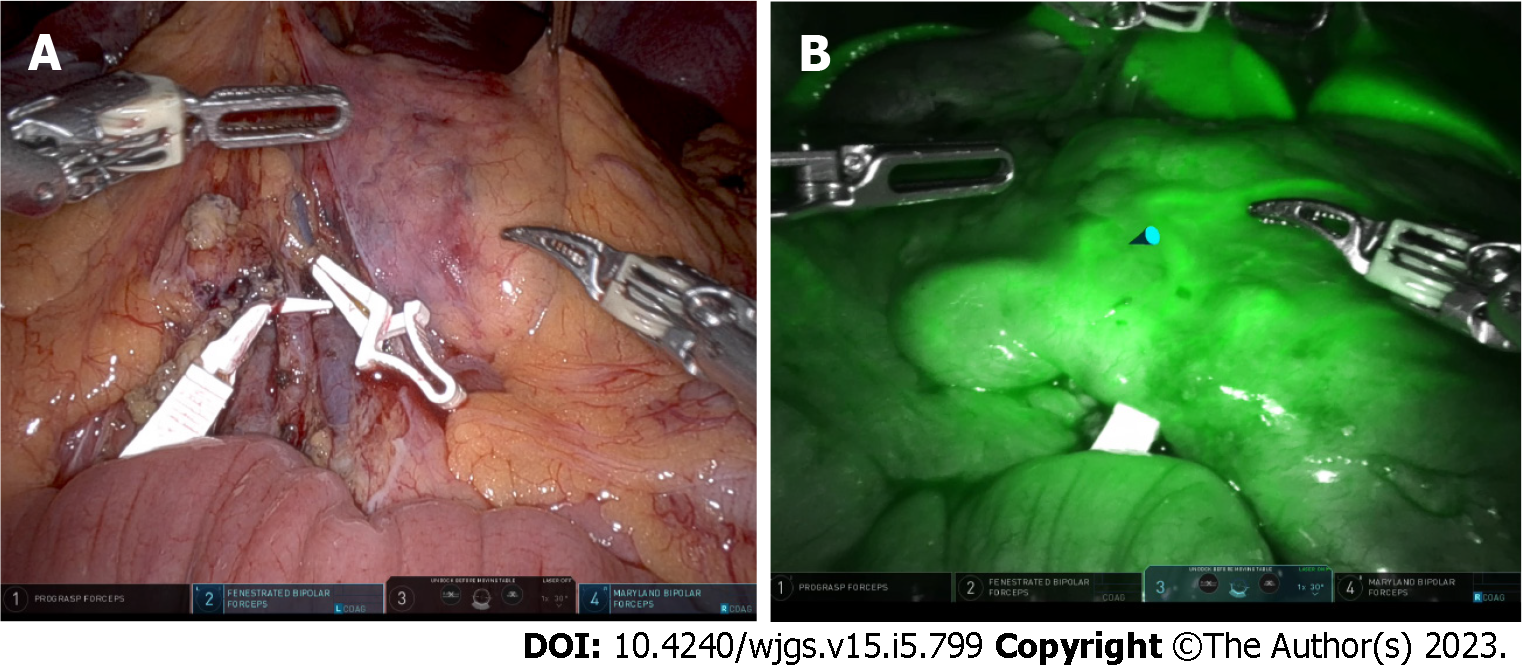
In a minimally invasive colon bypass, transillumination of the elevated colon to evaluate the vascular arcade is not possible. However, the limitation can be partially overcome by utilizing the near-infrared light for Indocyanine green fluorescence angiography, which is a real-time method to evaluate the organ perfusion. With the advent of a robotic platform and laparoscopic camera with inbuilt indocyanine green fluorescence technology, it should be feasible to objectively evaluate the vascular arcade (Figure 4). Also, minimally invasive approach can be potentially used for ischemic preconditioning. In this technique one of the major vessels, usually the ileocolic or middle colic pedicle, is ligated to promote vascular flow through marginal arcade.
The creation of a retrosternal tunnel in a laparoscopic approach is technically challenging because of the absence of any conventional landmarks. Insertion of a long needle percutaneously at the level of the xiphisternum facilitates precise identification and creation of a retrosternal tunnel without inadvertent damage to the pleura or pericardium.
Most patients with corrosive stricture will be referred for definitive management after a feeding procedure usually feeding jejunostomy. Hence, care should be taken to avoid iatrogenic bowel injury during initial trocar insertion and adhesiolysis. Performing feeding jejunostomy laparoscopically could minimize the adhesion-related challenges during definitive surgery.
As most patients with corrosive esophagogastric stricture are young adults minimally invasive approach is an attractive proposition. However, due to complexity of the surgery and technical challenges, minimally invasive surgery for corrosive esophagogastric stricture has lagged compared to other benign gastrointestinal orders. With improvements in laparoscopic instrumentation and technological advances, minimally invasive surgery for corrosive stricture is gaining momentum. There is a changing trend from laparoscopic assisted procedure to totally minimally invasive approach. However, carefully disseminating a minimally invasive approach for corrosive esophagogastric stricture is imperative to preclude adverse long-term outcomes. Technological advances like indocyanine green fluorescence help to improve the safety of the surgical procedure using a minimally invasive approach. However, well-designed trials with long-term follow-ups are required to document the superiority of minimally invasive surgery for corrosive esophagogastric stricture.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: IHPBA, No. M02056; APHPBA, No. M02056; SSAT, No. 227815.
Specialty type: Surgery
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shalli K, United Kingdom; Xiao YH, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Contini S, Scarpignato C. Caustic injury of the upper gastrointestinal tract: a comprehensive review. World J Gastroenterol. 2013;19:3918-3930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 273] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (7)] |
| 2. | Ananthakrishnan N, Kalayarasan R, Kate V. Corrosive injury of esophagus and stomach. In: Mishra PK, editor. Textbook of surgical gastroenterology. 1st ed. Jaypee: New Delhi, 2016: 194-206. [DOI] [Full Text] |
| 3. | Marujo WC, Tannuri U, Maksoud JG. Total gastric transposition: an alternative to esophageal replacement in children. J Pediatr Surg. 1991;26:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Esteves E, Sousa-Filho HB, Watanabe S, Silva JF, Neto EC, da Costa AL. Laparoscopically assisted esophagectomy and colon interposition for esophageal replacement in children: preliminary results of a novel technique. J Pediatr Surg. 2010;45:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Vasseur Maurer S, de Buys Roessingh A, Reinberg O. Comparison of transhiatal laparoscopy versus blind closed-chest cervicotomy and laparotomy for esophagectomy in children. J Pediatr Surg. 2013;48:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Hugh TB, Kelly MD. Corrosive ingestion and the surgeon. J Am Coll Surg. 1999;189:508-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Javed A, Pal S, Dash NR, Sahni P, Chattopadhyay TK. Outcome following surgical management of corrosive strictures of the esophagus. Ann Surg. 2011;254:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Spitz L, Kiely E, Pierro A. Gastric transposition in children--a 21-year experience. J Pediatr Surg. 2004;39:276-81; discussion 276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Gupta NM, Gupta R. Transhiatal esophageal resection for corrosive injury. Ann Surg. 2004;239:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 10. | Javed A, Agarwal AK. Laparoscopic retrosternal bypass for corrosive stricture of the esophagus. Surg Endosc. 2012;26:3344-3349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 11. | DeMeester SR. Colon interposition following esophagectomy. Dis Esophagus. 2001;14:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Kochhar R, Sethy PK, Kochhar S, Nagi B, Gupta NM. Corrosive induced carcinoma of esophagus: report of three patients and review of literature. J Gastroenterol Hepatol. 2006;21:777-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Okonta KE, Tettey M, Abubakar U. In patients with corrosive oesophageal stricture for surgery, is oesophagectomy rather than bypass necessary to reduce the risk of oesophageal malignancy? Interact Cardiovasc Thorac Surg. 2012;15:713-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Ananthakrishnan N, Subbarao KS, Parthasarathy G, Kate V, Kalayarasan R. Long Term Results of Esophageal Bypass for Corrosive Strictures without Esophageal Resection Using a Modified Left Colon Esophagocoloplasty--A Report of 105 Consecutive Patients from a Single Unit Over 30 Years. Hepatogastroenterology. 2014;61:1033-1041. [PubMed] |
| 15. | Mathiesen D, Morse CR. Techniques of esophageal reconstruction. In: Yeo CJ, Matthews JB, McFadden DW, editors. Shakleford’s Surgery of the Alimentary Tract. 7th ed. Philadelphia: Saunders, 2012; 518-536. [DOI] [Full Text] |
| 16. | Han Y, Cheng QS, Li XF, Wang XP. Surgical management of esophageal strictures after caustic burns: a 30 years of experience. World J Gastroenterol. 2004;10:2846-2849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Gvalani AK, Deolekar S, Gandhi J, Dalvi A. Antesternal colonic interposition for corrosive esophageal stricture. Indian J Surg. 2014;76:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Javed A, Agarwal AK. Total laparoscopic esophageal bypass using a colonic conduit for corrosive-induced esophageal stricture. Surg Endosc. 2013;27:3726-3732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Gurram RP, Kalayarasan R, Gnanasekaran S, Pottakkat B. Minimally Invasive Retrosternal Esophageal Bypass Using a Mid-Colon Esophagocoloplasty for Corrosive-Induced Esophageal Stricture. World J Surg. 2020;44:4153-4160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 20. | Kim CN. What is the role of hand-assisted laparoscopic surgery in the single-port surgery era? Ann Coloproctol. 2013;29:217-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Lin TS, Kuo SJ, Chou MC. Hand-assisted laparoscopic colon mobilization for esophageal reconstruction. Surg Endosc. 2003;17:115-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Bonavina L, Chirica M, Skrobic O, Kluger Y, Andreollo NA, Contini S, Simic A, Ansaloni L, Catena F, Fraga GP, Locatelli C, Chiara O, Kashuk J, Coccolini F, Macchitella Y, Mutignani M, Cutrone C, Poli MD, Valetti T, Asti E, Kelly M, Pesko P. Foregut caustic injuries: results of the world society of emergency surgery consensus conference. World J Emerg Surg. 2015;10:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Boukerrouche A. Colonic esophageal reconstruction by substernal approach for caustic stricture: what is the impact of the enlargement of the thoracic inlet on cervical anastomotic complications? J Surg. 2014;10:10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 24. | Banerjee JK, Saranga Bharathi R. Minimally invasive substernal colonic transposition for corrosive strictures of the upper aerodigestive tract. Dis Esophagus. 2017;30:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Bharathi RS. Efficacy of camera sleeve in conveyance of conduits. Pol Przegl Chir. 2017;89:76-83. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Lahoti D, Broor SL. Corrosive injury to the upper gastrointestinal tract. Indian J Gastroenterol. 1993;12:135-141. [PubMed] |
| 27. | Gumaste VV, Dave PB. Ingestion of corrosive substances by adults. Am J Gastroenterol. 1992;87:1-5. [PubMed] |
| 28. | Ananthakrishnan N, Subba Rao KSVK, Rajendran P. Delayed gastric outlet obstruction after esophagocoloplasty: clinical presentation with massive megacolon. Indian J Thorac Cardiovasc Surg. 1991;7:99-100. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Ananthakrishnan N, Parthasarathy G, Kate V. Gastric fluid level after overnight fast: Test to diagnose gastric outlet obstruction in corrosive esophageal stricture. Indian J Gastroenterol. 2006;25:269-270. [PubMed] |
| 30. | Hsu CP, Chen CY, Hsu NY, Hsia JY. Surgical treatment and its long-term result for caustic-induced prepyloric obstruction. Eur J Surg. 1997;163:275-279. [PubMed] |
| 31. | Shah S, Patel C, Mehta S, Gohil V. A prospective study of comparison between open gastrojejunostomy and laparoscopic assisted gastrojejunostomy in patients of post corrosive ingestion pyloric stenosis. Nat J Med Res. 2016;6:48-50. |
| 32. | Raikwar RS, Mathur RK, Ahirwar R. Retrospective and prospective study of corrosive poisoning and its effects on gastro intestinal tract and surgical management in tertiary care centre. Int J Med Biomed Studies. 2019;3:211-215. [DOI] [Full Text] |
| 33. | Subasinghe D, Rathnasena BG. Early laparoscopic gastro jejunostomy for corrosive injury of upper gastrointestinal tract. Trop Gastroenterol. 2011;32:333-335. [PubMed] |
| 34. | Balaji P, Rathinasamy R, Selvakumar S. Liquid fire in the stomach a minimal invasive approach. Glob J Res Analysis. 2018;7:9-13. [DOI] [Full Text] |
| 35. | Tayyem R, Siddiqui T, Musbahi K, Ali A. Gastric stricture following zinc chloride ingestion. Clin Toxicol (Phila). 2009;47:689-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Tekant G, Eroğlu E, Erdoğan E, Yeşildağ E, Emir H, Büyükünal C, Yeker D. Corrosive injury-induced gastric outlet obstruction: a changing spectrum of agents and treatment. J Pediatr Surg. 2001;36:1004-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Bax NM, Ure BM, van der Zee DC, van Tuijl I. Laparoscopic duodenoduodenostomy for duodenal atresia. Surg Endosc. 2001;15:217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Seleim HM, Wishahy AMK, Abouelfadl MH, Farouk MM, Elshimy K, Fares AE, Kaddah SN, Eltagy G, Elbarbary MM. Laparoscopic Diamond Antroduodenostomy for Postcorrosive Pyloric Cicatrization: A Novel Approach. J Laparoendosc Adv Surg Tech A. 2019;29:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Ananthakrishnan N, Parthasarathy G, Kate V. Chronic corrosive injuries of the stomach-a single unit experience of 109 patients over thirty years. World J Surg. 2010;34:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Nagaraj K, Kalayarasan R, Gnanasekaran S, Pottakkat B. Total laparoscopic Billroth-I gastrectomy for corrosive-induced antropyloric stricture. J Minim Access Surg. 2018;15:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 10.3] [Reference Citation Analysis (0)] |