Published online Apr 27, 2023. doi: 10.4240/wjgs.v15.i4.712
Peer-review started: November 8, 2022
First decision: November 23, 2022
Revised: December 1, 2022
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 27, 2023
Processing time: 166 Days and 0.7 Hours
Acute pancreatitis is the most common complication of endoscopic retrograde cholangiopancreatography (ERCP). Currently, there is no suitable treatment for post-ERCP pancreatitis (PEP) prophylaxis. Few studies have prospectively evaluated interventions to prevent PEP in children.
To assess the efficacy and safety of the external use of mirabilite to prevent PEP in children.
This multicenter, randomized controlled clinical trial enrolled patients with chronic pancreatitis scheduled for ERCP according to eligibility criteria. Patients were randomly divided into the external use of mirabilite group (external use of mirabilite in a bag on the projected abdominal area within 30 min before ERCP) and blank group. The primary outcome was the incidence of PEP. The secondary outcomes included the severity of PEP, abdominal pain scores, levels of serum inflammatory markers [tumor necrosis factor-alpha (TNF-α) and serum interleukin-10 (IL-10)], and intestinal barrier function markers [diamine oxidase (DAO), D-lactic acid, and endotoxin]. Additionally, the side effects of topical mirabilite were investigated.
A total of 234 patients were enrolled, including 117 in the external use of mirabilite group and the other 117 in the blank group. The pre-procedure and procedure-related factors were not significantly different between the two groups. The incidence of PEP in the external use of mirabilite group was significantly lower than that in the blank group (7.7% vs 26.5%, P < 0.001). The severity of PEP decreased in the mirabilite group (P = 0.023). At 24 h after the procedure, the visual analog scale score in the external use of mirabilite group was lower than that in the blank group (P = 0.001). Compared with those in the blank group, the TNF-α expressions were significantly lower and the IL-10 expressions were significantly higher at 24 h after the procedure in the external use of mirabilite group (P = 0.032 and P = 0.011, respectively). There were no significant differences in serum DAO, D-lactic acid, and endotoxin levels before and after ERCP between the two groups. No adverse effects of mirabilite were observed.
External use of mirabilite reduced the PEP occurrence. It significantly alleviated post-procedural pain and reduced inflammatory response. Our results favor the external use of mirabilite to prevent PEP in children.
Core Tip: This was a multicenter, prospective, randomized controlled study, which aimed to assess the efficacy and safety of the external use of mirabilite to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP) in children. Our study showed that the external use of mirabilite can reduce the incidence of PEP, relieve post-procedural pain, and regulate inflammatory mediator expression to reduce the inflammatory response. This study suggests that the external use of mirabilite is a safe, effective, and more acceptable option for the prevention of PEP prophylaxis in pediatric patients.
- Citation: Zeng JQ, Zhang TA, Yang KH, Wang WY, Zhang JY, Hu YB, Xiao J, Gu ZJ, Gong B, Deng ZH. External use of mirabilite to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis in children: A multicenter randomized controlled trial. World J Gastrointest Surg 2023; 15(4): 712-722
- URL: https://www.wjgnet.com/1948-9366/full/v15/i4/712.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i4.712
Endoscopic retrograde cholangiopancreatography (ERCP) is one of the crucial procedures for the diagnosis and treatment of biliary and pancreatic diseases in children[1,2]. Post-ERCP pancreatitis (PEP) is the most common adverse event; it can be a serious complication following ERCP, occurring in approximately 6.0%-20.7% of children. The rate of PEP varies between case series as it depends on potential patient- and procedure-related risk factors, such as a history of PEP, visualization of the pancreatic duct, guide-wire insertion into the pancreatic duct, diagnostic ERCP, suspected sphincter of Oddi dysfunction, difficult cannulation, pancreatic sphincterotomy, and others[3-7]. Most episodes of PEP are mild and moderate; however, severe pancreatitis still occurs, accounting for a prolonged hospital stay and can be potentially fatal[6,8]. To a certain extent, the occurrence of PEP limits the application of ERCP in children.
To date, only non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to be effective in preventing PEP in adults[9-11]. The role of rectal indomethacin, which is widely used in adults and is often not utilized in children as the method of rectal administration is not acceptable for children, remains questionable. Meanwhile, few reports have investigated prophylactic medicine for PEP in children. Finding an ideal, effective, less invasive, and safe prevention strategy for children is desirable.
Mirabilite, a white granular mineral medicine, primarily composed of hydrous sodium sulfate (
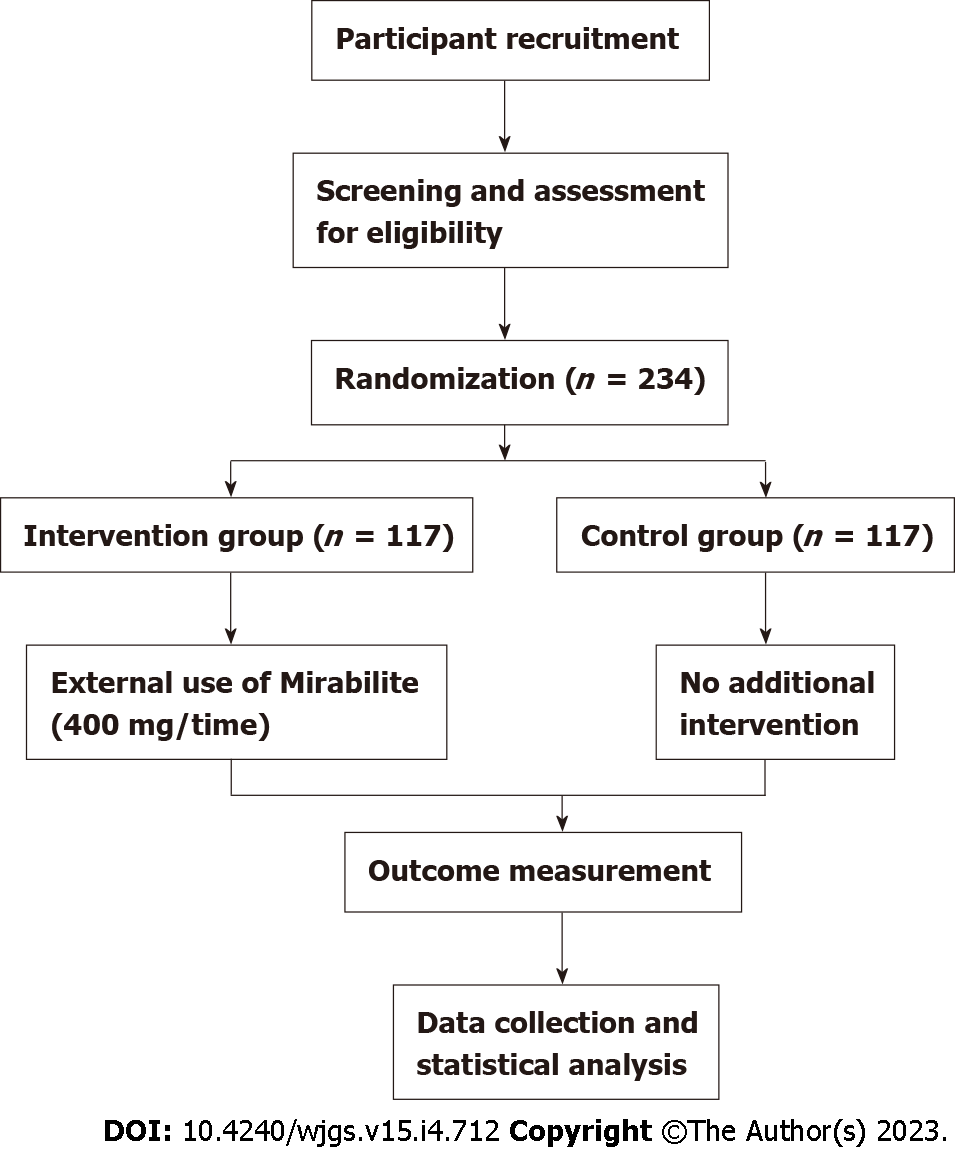
This was a multicenter, prospective, randomized controlled study. Patients were recruited from Shanghai Children’s Medical Center and Shanghai Shuguang Hospital in China between October 1, 2019 and December 30, 2021 after approval from the human studies review committee at each institution (see Figure 1 for flow diagram).
An investigator, who was blinded to the treatment allocation, recorded the patient demographics, post-ERCP adverse events, follow-up data, and procedure-related parameters, including sphincterotomy, stricture dilation, pancreatic stone extraction, number of cannulation attempts, and contrast agent dose. A screening session and physical examination prior to inclusion were conducted by a medical doctor according to the following inclusion criteria: (1) Age, 0-14 years; (2) Received therapeutic ERCP for chronic pancreatitis; (3) Blood amylase and lipase levels before ERCP were within the normal limits (amylase, 30-110 U/L; blood lipase, 23-300 U/L); and (4) Informed consent was obtained from the patient’s guardians, and assent was obtained from patients aged > 8 years. The exclusion criteria were as follows: (1) Organic gastrointestinal disease, such as upper digestive tract stenosis or obstruction; (2) Pancreatitis or use of pancreatic enzyme medication within 7 d; (3) Cardiovascular, hepatic, renal, cerebrovascular, or hematopoietic system disease; (4) Dermatological disorders, such as fresh abdominal wounds, skin lesions, or angioma; and (5) Allergy to contrast agents or mirabilite.
The diagnostic criteria for chronic pancreatitis were the same as those of the International Study Group of Pediatric Pancreatitis: In Search for a Cure[15], which included children with irreversible structural changes in the pancreas, with or without abdominal pain, exocrine pancreatic insufficiency, or diabetes.
We calculated the sample size according to our primary study. The PEP incidence rate in the control group was estimated to be 21% based on historical data from the study institution[6]. Assume that preoperative prevention can reduce the risk of PEP by 50%, the target incidence of PEP in mirabilite external application group was estimated to not exceed 7%. Set α = 0.05, two-sided test, β = 0.20. Calculated by PASS15.0 software, each group needed 99 participants, the estimated dropout rate was 15%, and 117 patients would be included in each group. Institutional review board approval was obtained (the Ethics Committee of Shanghai Children’s Medical Center). This study registered with Clinical Trials ChiCTR1900022642. Registered on April 19, 2019, http://www.chictr.org.cn.
Procedure details: All patients underwent a comprehensive review and specialist consultation before ERCP. This process aimed to ensure an objective and comprehensive analysis, to determine if ERCP was appropriate, and rule out contraindications to endoscopy. Patients were asked to undergo routine preoperative laboratory testing (complete blood count, coagulation, blood amylase and lipase concentrations, and hepatic function markers), upper abdominal ultrasonography, magnetic resonance cholangiopancreatography or computed tomography (CT), and iodine allergy testing.
Each patient was required to fast for 12 h before surgery. Duodenoscopy was performed using a JF240V device (Olympus Corp., Tokyo, Japan). ERCP was conducted by an experienced digestive endoscopy specialist who performed > 30000 ERCPs. The following procedures were performed under radiographic guidance.
Standard post-ERCP treatment: Standard treatment was administered for PEP in both groups, including fasting, pancreatic enzyme control, and maintenance of fluid and electrolyte balance. Complications, such as infection, bleeding, or perforation within 1 mo of discharge were treated accordingly.
Patients in the external use of mirabilite group were administered a topical application of mirabilite (Chinese Medicine Institute, Shanghai, China), in a bag, on the middle and upper abdomen within 30 min before ERCP and until 24 h after ERCP, during which time the mirabilite was replaced every 4 h, whereas those in the blank group did not receive any additional intervention.
The external application of mirabilite (400 g each time) was packaged in custom-made topical bags. Mirabilite bags were designed with a rectangular shape and in two different sizes based on the projected area of the pancreas in pediatric patients. Children aged ≤ 6 years received bags with dimensions of 17 mm × 14 mm, whereas those aged > 6 years received bags with dimensions of 24 mm × 14 mm. Two layers of medical gauze were sewn into rectangular bags, and four 8-cm attachment bands were sewn to the two longer sides. The bags were used for topical application of mirabilite to the abdomen and were attached to the patient’s backs using attachment bands.
In this study, patients were randomly assigned to two groups in a 1:1 ratio using block randomization stratified by centers. Randomization was performed before ERCP (about 7 h before ERCP). Mirabilite was administered in the procedure room before or after ERCP by one investigator at each site who did not participate in data collection and analysis. The mirabilite external application package was removed before entering the operation room, the operator and assistant who participated in the ERCP procedures were blinded to the group allocation. Furthermore, the investigator who collected demographic or procedure-related data or who participated in the assessment of post-ERCP complications was blinded to the group allocation.
At 24 h after the procedure, abdominal pain scores [visual analog scale (VAS) scores][16] were recorded. Communicative patients were asked to indicate their level of pain unaided. Pain assessment was completed by legal guardians in non-communicative patients. Serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-10 (IL-10), diamine oxidase (DAO), blood D-lac, and endotoxin were measured in all patients 3 h before ERCP and 24 h after ERCP. Serum TNF-α and IL-10 levels were determined using enzyme linked immune assay (ELISA; Shanghai Hengyuan Bioengineering Institute, Shanghai, China). Moreover, serum DAO, D-lactic, and endotoxins were determined using ELISA (Shanghai Hengyuan Bioengineering Institute, Shanghai, China). According to the criteria[17] for the diagnosis of PEP, abdominal pain, serum amylase levels, and upper abdominal ultrasonography were measured 24 h after ERCP.
According to Cotton’s criteria[17], PEP is classified into mild, moderate, and severe pancreatitis (mild, additional hospitalization for 1-3 d; moderate, additional hospitalization for 4-10 d; and severe, hospitalization for > 10 d and in cases of hemorrhagic pancreatitis, phlegmon, or pseudocysts).
Primary outcome: The primary outcome was the incidence of PEP after the procedure. According to consensus criteria[17], PEP was diagnosed if a child met two of the three following criteria after ERCP: A new onset of classical abdominal pain, a plasma amylase or lipase concentration exceeding three times the normal upper limit at 24 h postoperatively, and radiographic (B-type ultrasonography or CT) findings suggestive of pancreatitis.
Secondary outcomes: Additionally, several secondary outcome measures were recorded, including the severity of PEP. Abdominal pain was measured 24 h after ERCP using a VAS as follows: 0 points, no tenderness, no pain; 1-3 points, mild but tolerable discomfort and pain; 4-6 points, sleep quality affected by tolerable discomfort and pain; and 7-10 points, severe discomfort and intolerable pain that severely affect sleep quality. Inflammatory cytokines were assessed by serum TNF-α and IL-10; and intestinal barrier function was recorded by DAO, D-lactic, and endotoxin levels.
Safety endpoints: The patients were monitored for adverse reactions to mirabilite, including skin damage and diarrhea. The following adverse reactions to ERCP were further monitored: Intestinal perforation, bleeding, bile duct infection, and other procedure-related complications requiring extended hospital stay. Patients were followed up for 1 mo postoperatively.
The data of all patients who underwent randomization were analyzed. Counting data were presented as absolute numbers and proportions. Corresponding analysis was performed using the chi-square test or Fisher’s exact test when the actual frequency was < 5. Continuous variables without normal distribution in this study were presented with median and interquartile range, and comparisons were made using the Mann-Whitney U test between two groups. A two-sided P < 0.05 indicated statistical significance. All statistical analyses were performed using R version 3.5.1 (The R Foundation, Vienna, Austria).
A total of 234 patients were included in this study, with 117 in the external use of mirabilite group and 117 in the blank group. The baseline characteristics and ERCP procedure-related parameters were similar between the two groups (Table 1). Cannulation was successful in 234 patients (100%). The overall incidence of PEP in 234 patients was 17.1%; the incidence of PEP in children aged < 6 years was 15.1%, and in those aged ≥ 6 years was 18.8%. No statistical difference was found between the two age groups (P = 0.460).
| External use of mirabilite group | Blank group (n = 117) | P value | |
| Female, n (%) | 71 (60.7) | 60 (51.3) | 0.147 |
| BMI, kg/m2 (IQR) | 15.59 (14.12, 17.27) | 15.55 (14.37, 17.64) | 0.359 |
| Age, yr (IQR) | 6.8 (4.1, 11.8) | 7 (4.0, 7.0) | 0.971 |
| Medical history, n (%) | 0.609 | ||
| Idiopathic pancreatitis | 58 (49.6) | 62 (53.0) | |
| Pancreas divisum | 20 (17.1) | 24 (20.5) | |
| Pancreaticobiliary maljunction | 36 (30.8) | 27 (23.1) | |
| Pancreas divisum accompanied annular pancreas | 3 (2.6) | 4 (3.4) | |
| Procedure, n (%) | |||
| EPS (major papilla) | 25 (21.4) | 20 (17.1) | 0.407 |
| EPS (minor papilla) | 6 (5.1) | 5 (4.3) | 0.757 |
| EST | 9 (7.7) | 16 (13.7) | 0.139 |
| ERPD | 57 (48.7) | 57 (48.7) | 1.000 |
| ERBD | 30 (25.6) | 34 (29.1) | 0.557 |
| ENPD | 15 (12.8) | 15 (12.8) | 1.000 |
| ENBD | 10 (8.5) | 8 (6.8) | 0.624 |
| Contrast medium | 0.866 | ||
| Contrast agent dose < 5 mL | 96 (82.1) | 95 (81.2) | |
| Contrast agent dose ≥ 5 mL | 21 (17.9) | 22 (18.8) | |
| Attempts for successful Cannulation (≥ 5 times) | 20 (17.1) | 20 (17.1) | 1.000 |
| Pancreatogram | 103 (88.0) | 104 (88.9) | 0.838 |
The incidence of PEP in the external use of mirabilite group was significantly lower than that in the blank group (7.7% vs 26.5%, P < 0.001); the incidence of PEP in the mirabilite and blank groups was statistically significant in different age groups (Table 2). There were nine patients with mild pancreatitis but no moderate and severe pancreatitis in the mirabilite group, whereas in the blank control group, there were 16 patients with mild pancreatitis, 13 with moderate pancreatitis, and 2 with severe pancreatitis. The difference was statistically significant (P = 0.023) (Table 3). The VAS scores were significantly lower in the external use of mirabilite group than those in the blank group at 24 h after ERCP (P = 0.001) (Table 4). No statistically significant difference was noted in the expression levels of serum TNF-α and IL-10 between the two groups 3 h before the procedure. Compared with the values measured 3 h before the procedure, the expression levels of serum TNF-α and the expression levels of serum IL-10 increased in both groups at 24 h after procedure (P = 0.016 and P < 0.001, respectively). Compared with those in the blank group, the expression levels of serum TNF-α in the external use of mirabilite group were significantly lower, and the expression levels of serum IL-10 in the external use of mirabilite group were significantly higher at 24 h after the procedure. The differences were statistically significant (P = 0.032 and P = 0.011, respectively) (Table 5). No significant differences were found in the levels of serum D-lactate, DAO, and endotoxins before and after the procedure between the two groups (Table 6). No side effects due to the external use of mirabilite, such as skin allergy or diarrhea, were observed. Intestinal perforation, bleeding, bile duct infection, and other procedure-related complications, except PEP, did not occur during the 1-mo follow-up.
| External use of mirabilite group (n = 9) | Blank group (n = 31) | P value | |
| Severity of PEP | 0.0231 | ||
| Mild | 9 (100.0%) | 16 (51.6%) | |
| Moderate | 0 (0.0%) | 13 (41.9%) | |
| Severe | 0 (0.0%) | 2 (6.5%) |
| Group | VAS score | Z value | P value |
| External use of mirabilite group | 0 (0, 2) | -3.27 | 0.0011 |
| Blank group | 2 (0, 5) |
| External use of mirabilite group (n = 117) | Blank group (n = 117) | P value | ||
| TNF-α | Pre-ERCP 3 h | 1.78 (0.00, 3.39) | 1.30 (0.00, 4.25) | 0.622 |
| Post-ERCP 24 h | 2.42 (1.14, 6.53) | 3.41 (1.63, 8.26) | 0.0321 | |
| P value | 0.0161 | < 0.0011 | ||
| IL-10 | Pre-ERCP 3 h | 3.56 (1.49, 6.01) | 3.49 (1.24, 6.62) | 0.992 |
| Post-ERCP 24 h | 4.60 (2.99, 9.40) | 3.76 (1.74, 7.86) | 0.0111 | |
| P value | < 0.0011 | 0.282 |
| External use of mirabilite group (n = 117) | Blank group (n = 117) | P value | ||
| D-lactate | Pre-ERCP 3 h | 190.65 (164.37, 273.58) | 224.38 (187.29, 313.58) | 0.120 |
| Post-ERCP 24 h | 199.73 (180.57, 233.96) | 213.88 (192.04, 314.86) | 0.078 | |
| P value | 0.849 | 0.914 | ||
| Endotoxin | Pre-ERCP 3 h | 5.95 (4.06, 7.83) | 5.21 (3.94, 6.91) | 0.375 |
| Post-ERCP 24 h | 5.36 (4.09, 8.01) | 5.35 (4.50, 7.89) | 0.874 | |
| P value | 0.762 | 0.559 | ||
| DAO | Pre-ERCP 3 h | 0.65 (0.44, 2.47) | 0.63 (0.51, 0.97) | 0.635 |
| Post-ERCP 24 h | 1.04 (0.52, 2.47) | 0.79 (0.585, 0.79) | 0.536 | |
| P value | 0.410 | 0.129 |
ERCP has been a primary treatment method for biliary and pancreatic diseases and has gradually replaced traditional surgery[18,19]. Although ERCP has many advantages, the high incidence of complications still restricts its widespread use to a certain extent. Among the complications of ERCP, PEP was the most common adverse event. Prophylactic drugs are necessary to reduce PEP, and a safe, effective, and convenient way to administer them is readily acceptable in children. Currently, there is no standard of care for the prevention of PEP in the pediatric population, and adopting adult-based standards is controversial. In our study, the external use of mirabilite significantly lowered the incidence of PEP and improved post-procedural pain, suggesting that external mirabilite use was helpful in preventing PEP in children and was highly acceptable. Our study is the first to evaluate prophylactic medications for pediatric PEP in a multi-center, randomized controlled trial in China.
At our center, the prevalence of PEP was 26.5% in the blank group. This was higher than the incidence of PEP in adults and other countries[1,4,20]. The main reason for the high incidence of PEP in our study population maybe because of patient and disease spectrum selection. The incidence of PEP is not low in Asia, about 10%-20%[6,21]; in a study of Chinese children, the incidence of PEP was 20.7%[6]. Some previous studies have reported a low PEP rate in adults; the spectrum of diseases were mainly biliary diseases, including choledocholithiasis, cholangitis, or biliary stricture[20,22]. In contrast, previous studies have confirmed that bile duct disease is not a high-risk factor for PEP, but young age, sphincterotomy are high-risk factors for PEP[6,21]. However, this study included children with chronic pancreatic diseases, and most surgeries conducted in the pancreatic duct may account for the high PEP occurrence.
Mirabilite is a well-known traditional Chinese medicine for the treatment of acute pancreatitis[12]. The medicine is applied externally, absorbed through the skin, and does not go through the digestive system; thus, the procedure is simple and safe to conduct, with no risk of an adverse reaction. Research indicates that mirabilite plays a role in moisture absorption, reduction of swelling, heat clearance, toxicity removal, and anti-inflammatory action. In addition, mirabilite has been shown to improve the levels of amylase in the blood, improve pancreatic blood circulation, promote the absorption of necrotic tissue, promote gastrointestinal peristalsis, and decrease a variety of complications, improving the overall prognosis. Wang et al[12] have applied mirabilite to reduce pancreatic leakage in severe acute pancreatitis, which lowered intra-abdominal pressure, reduced the secretion of pancreatic amylase, eliminated inflammatory edema, and reduced IL-6 levels in the blood. Animal experiments have shown that Dachengqi decoction, a famous formula in China that comprises mirabilite as the principal component, increased cell viability, reduced acinar necrosis, and provided protection from injury to the pancreas in vivo and in vitro[23]. However, few reports have researched the efficacy of mirabilite for preventing PEP.
This study found that the incidence and PEP severity in the externally applied mirabilite group was significantly lower than that in the control group. This illustrated that mirabilite can treat acute pancreatitis and may prevent PEP and reduce the severity of PEP. Currently, NSAIDs have been shown to be the only effective drug in preventing PEP in adults, and few reports have investigated prophylactic medicine for PEP in children. In a meta-analysis of randomized controlled trials, the overall incidence of PEP was 7.64% (47/615 patients) in the rectal indomethacin group and 15.15% (95/627 patients) in the placebo group[24]. Similar results have been obtained with external application of mirabilite, which is more acceptable than rectal or oral administration in children. Therefore, this study would be informative to expand the field of prospective research on the prevention of pediatric PEP. Cytokines play a major role in the pathogenesis of acute pancreatitis as part of the underlying systemic inflammatory response, tissue damage, and organ dysfunction. The severity of acute pancreatitis depends on an intricate balance between localized tissue damage with proinflammatory cytokine production and the systemic anti-inflammatory response[13]. TNF-α is a pro-inflammatory cytokine that promotes the inflammatory response, whereas IL-10 is an immunosuppressive cytokine that inhibits inflammation[25]. Anti-inflammatory cytokines and pro-inflammatory factors maintain a balance when no inflammatory responses are occurring. Disruption in this balance leads to an inflammatory response when the anti-inflammatory cytokines are insufficient against the pro-inflammatory factors. For restoration of immunological balance, a proinflammatory response is usually followed by secretion of anti-inflammatory mediators, such as IL-10, which suppress the synthesis and effects of proinflammatory cytokines. In this study, the expression levels of TNF-α and the expression levels of IL-10 increased after ERCP in both groups. However, the TNF-α expression in the external use of mirabilite group at 24 h after the ERCP procedure was significantly lower and the IL-10 expression was higher than that in the blank group, indicating that external use of mirabilite can improve anti-inflammatory function and reduce the inflammatory response by down-regulating the secretion of proinflammatory factors and up-regulating the anti-inflammatory factors to alleviate pancreatic injury.
Studies have shown that the release of inflammatory factors may lead to intestinal ischemic hypoxia, resulting in the destruction of the intestinal barrier function that shifts intestinal bacteria and toxins, further causing pancreatic damage[26]. When the intestinal barrier is damaged, DAO, D-lactate, and endotoxins are transferred into the blood circulation through the damaged mucosa in the early stage. In addition, the serum concentrations of D-lactate, DAO could reflect the intestinal permeability in patients with acute pancreatitis[27]. Thus, the detection of plasma levels of DAO and D-lactate can promptly reflect the extent of damage and permeability changes in the small intestine, which are the specificity and sensitivity indicators of intestinal barrier function to evaluate pancreatitis. The present study showed no statistically significant differences in the levels of D-lactate, DAO, and endotoxins in both groups before and after the procedure, suggesting that gastrointestinal mucosal barrier dysfunction was not obvious. This may be because the inflammatory response induced by the procedure is not strong enough to injure the gastrointestinal mucosal barrier function. In this study, no side effects of the external use of mirabilite, such as skin allergy and diarrhea, were observed.
The limitation of this study was that it was an unblinded study, with researcher subjective biases, and it was restricted to the Chinese population. In addition, a suitable control medicine should be used for further studies. Furthermore, the exact mechanisms of action remain relatively unknown and should be investigated further in future studies.
Our study showed that the external use of mirabilite can reduce the incidence of PEP, relieve post-procedural pain, and regulate inflammatory mediator expression to reduce the inflammatory response. Further, this study suggests that the external use of mirabilite is a safe, effective, and more acceptable option for the prevention of PEP prophylaxis in pediatric patients.
Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) is the most common adverse event following ERCP. Currently, only non-steroidal anti-inflammatory drugs have been shown to be effective in preventing PEP in adults. Few studies have prospectively evaluated interventions to prevent PEP in children.
The occurrence of PEP could limit the application of ERCP in children, for which finding an ideal, effective, less invasive, and safe prevention strategy is desirable.
The objective of this study was to assess the efficacy and safety of external use of mirabilite to prevent PEP in children.
We conducted a multicenter, randomized controlled clinical trial. Patients with chronic pancreatitis scheduled for ERCP were enrolled and randomly divided into the external use of mirabilite group and the blank group. The primary outcome was the incidence of PEP. The secondary outcomes included the severity of PEP, abdominal pain scores, levels of serum inflammatory markers, tumor necrosis factor (TNF)-α and interleukin (IL)-10, and intestinal barrier function markers, diamine oxidase (DAO), D-lactic acid, and endotoxin. Additionally, the side effects of topical mirabilite were investigated.
A total of 234 patients were enrolled, including 117 in the external use of mirabilite group and the other 117 in the blank group. The pre-procedure and procedure-related factors were not significantly different between the two groups. The incidence of PEP in the external use of mirabilite group was significantly lower than that in the blank group (7.7% vs 26.5%, P < 0.001). The severity of PEP decreased in mirabilite group (P = 0.023). At 24 h after the procedure, the visual analog score in the external use of mirabilite group was lower than that in the blank group (P = 0.001). Compared with those in the blank group, the TNF-α expressions were significantly lower and the IL-10 expressions were significantly higher at 24 h after the procedure in the external use of mirabilite group (P = 0.032 and P = 0.011, respectively). There were no significant differences in serum DAO, D-lactic acid, and endotoxin levels before and after ERCP between the two groups. No adverse effects of mirabilite were observed.
External use of mirabilite reduced the occurrence of PEP. Moreover, it significantly alleviated post-procedural pain and reduced inflammatory response. Our results favor the external use of mirabilite to prevent PEP in children.
This study illustrated that external use of mirabilite is a safe, effective, and more acceptable option for PEP prophylaxis in pediatric patients. Our findings would be informative to expand the field of prospective research on the prevention of pediatric PEP.
We thank Dr. Fu Li, the distinguished professor of Shanghai Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, for his review of the manuscript for optimal English language presentation. Moreover, we would like to thank all the staff for their valuable support in participant recruitment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Parvin S, Bangladesh; Saito H, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Giefer MJ, Kozarek RA. Technical outcomes and complications of pediatric ERCP. Surg Endosc. 2015;29:3543-3550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Felux J, Sturm E, Busch A, Zerabruck E, Graepler F, Stüker D, Manger A, Kirschner HJ, Blumenstock G, Malek NP, Goetz M. ERCP in infants, children and adolescents is feasible and safe: results from a tertiary care center. United European Gastroenterol J. 2017;5:1024-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Iorgulescu A, Sandu I, Turcu F, Iordache N. Post-ERCP acute pancreatitis and its risk factors. J Med Life. 2013;6:109-113. [PubMed] |
| 4. | Troendle DM, Abraham O, Huang R, Barth BA. Factors associated with post-ERCP pancreatitis and the effect of pancreatic duct stenting in a pediatric population. Gastrointest Endosc. 2015;81:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Usatin D, Fernandes M, Allen IE, Perito ER, Ostroff J, Heyman MB. Complications of Endoscopic Retrograde Cholangiopancreatography in Pediatric Patients; A Systematic Literature Review and Meta-Analysis. J Pediatr. 2016;179:160-165.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Deng Z, Zeng J, Lv C, Jiang L, Ji J, Li X, Hao L, Gong B. Prevalence and Factors Associated with Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis in Children. Dig Dis Sci. 2021;66:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Tryliskyy Y, Bryce GJ. Post-ERCP pancreatitis: Pathophysiology, early identification and risk stratification. Adv Clin Exp Med. 2018;27:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Åvitsland TL, Aabakken L. Endoscopic retrograde cholangiopancreatography in infants and children. Endosc Int Open. 2021;9:E292-E296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Fogel EL, Lehman GA, Tarnasky P, Cote GA, Schmidt SE, Waljee AK, Higgins PDR, Watkins JL, Sherman S, Kwon RSY, Elta GH, Easler JJ, Pleskow DK, Scheiman JM, El Hajj II, Guda NM, Gromski MA, McHenry L Jr, Arol S, Korsnes S, Suarez AL, Spitzer R, Miller M, Hofbauer M, Elmunzer BJ; US Cooperative for Outcomes Research in Endoscopy (USCORE). Rectal indometacin dose escalation for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography in high-risk patients: a double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Liu L, Li C, Huang Y, Jin H. Nonsteroidal Anti-inflammatory Drugs for Endoscopic Retrograde Cholangiopancreatography Postoperative Pancreatitis Prevention: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2019;23:1991-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA, Kapral C; European Society of Gastrointestinal Endoscopy. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Zhang X, Li C. Applying Hot Compresses with Rhubarb and Mirabilite to Reduce Pancreatic Leakage Occurrence in the Treatment of Severe Acute Pancreatitis. Iran J Public Health. 2017;46:136-138. [PubMed] |
| 13. | Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 14. | Wang Y, Zhang Y, Jiang R. Early traditional Chinese medicine bundle therapy for the prevention of sepsis acute gastrointestinal injury in elderly patients with severe sepsis. Sci Rep. 2017;7:46015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Gariepy CE, Heyman MB, Lowe ME, Pohl JF, Werlin SL, Wilschanski M, Barth B, Fishman DS, Freedman SD, Giefer MJ, Gonska T, Himes R, Husain SZ, Morinville VD, Ooi CY, Schwarzenberg SJ, Troendle DM, Yen E, Uc A. Causal Evaluation of Acute Recurrent and Chronic Pancreatitis in Children: Consensus From the INSPPIRE Group. J Pediatr Gastroenterol Nutr. 2017;64:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Petruzziello C, Marannino M, Migneco A, Brigida M, Saviano A, Piccioni A, Franceschi F, Ojetti V. The efficacy of a mix of three probiotic strains in reducing abdominal pain and inflammatory biomarkers in acute uncomplicated diverticulitis. Eur Rev Med Pharmacol Sci. 2019;23:9126-9133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2037] [Article Influence: 59.9] [Reference Citation Analysis (1)] |
| 18. | Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, Aithal GP, Anderloni A, Bruno M, Cantú P, Devière J, Domínguez-Muñoz JE, Lekkerkerker S, Poley JW, Ramchandani M, Reddy N, van Hooft JE. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy. 2019;51:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 19. | Baron TH. Endoscopic management of biliary disorders: diagnostic and therapeutic. Surg Clin North Am. 2014;94:395-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Fujita K, Yazumi S, Matsumoto H, Asada M, Nebiki H, Matsumoto K, Maruo T, Takenaka M, Tomoda T, Onoyama T, Kurita A, Ueki T, Katayama T, Kawamura T, Kawamoto H; Bilio-pancreatic Study Group of West Japan. Multicenter prospective cohort study of adverse events associated with biliary endoscopic retrograde cholangiopancreatography: Incidence of adverse events and preventive measures for post-endoscopic retrograde cholangiopancreatography pancreatitis. Dig Endosc. 2022;34:1198-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Parvin S, Islam MS, Majumdar TK, Azam MG, Begum MR, Hossain MA, Imam I, Ahmed F. Post-ERCP pancreatitis: Frequency and risk stratification from four tertiary care referral hospitals in South East Asia. Medicine (Baltimore). 2022;101:e30216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | ASGE Standards of Practice Committee; Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 536] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 23. | Zhao J, Tang W, Wang J, Xiang J, Gong H, Chen G. Pharmacokinetic and pharmacodynamic studies of four major phytochemical components of Da-Cheng-Qi decoction to treat acute pancreatitis. J Pharmacol Sci. 2013;122:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Shi N, Deng L, Altaf K, Huang W, Xue P, Xia Q. Rectal indomethacin for the prevention of post-ERCP pancreatitis: A meta-analysis of randomized controlled trials. Turk J Gastroenterol. 2015;26:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Singh P, Garg PK. Pathophysiological mechanisms in acute pancreatitis: Current understanding. Indian J Gastroenterol. 2016;35:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Luan ZG, Zhang H, Ma XC, Zhang C, Guo RX. Role of high-mobility group box 1 protein in the pathogenesis of intestinal barrier injury in rats with severe acute pancreatitis. Pancreas. 2010;39:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Huang Q, Wu Z, Chi C, Wu C, Su L, Zhang Y, Zhu J, Liu Y. Angiopoietin-2 Is an Early Predictor for Acute Gastrointestinal Injury and Intestinal Barrier Dysfunction in Patients with Acute Pancreatitis. Dig Dis Sci. 2021;66:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |