Published online Apr 27, 2023. doi: 10.4240/wjgs.v15.i4.674
Peer-review started: December 12, 2022
First decision: January 11, 2023
Revised: February 6, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: April 27, 2023
Processing time: 132 Days and 2.6 Hours
Pancreaticoduodenectomy combined with portal vein (PV) and/or superior mesenteric vein (SMV) resection in patients with pancreaticobiliary malignancy has become a common surgical procedure. There are various grafts currently used for PV and/or SMV reconstruction, but each of these grafts have certain limitations. Therefore, it is necessary to explore novel grafts that have an extensive resource pool, are low cost with good clinical application, and are without immune response rejection or additional damage to patients.
To observe the anatomical and histological characteristics of the ligamentum teres hepatis (LTH) and evaluate PV/SMV reconstruction using an autologous LTH graft in pancreaticobiliary malignancy patients.
In 107 patients, the post-dilated length and diameter in resected LTH specimens were measured. The general structure of the LTH specimens was observed by hematoxylin and eosin (HE) staining. Collagen fibers (CFs), elastic fibers (EFs), and smooth muscle (SM) were visualized by Verhoeff-Van Gieson staining, and the expression of CD34, factor VIII-related antigen (FVIIIAg), endothelial nitric oxide synthase (eNOS), and tissue type plasminogen activator (t-PA) were detected using immunohistochemistry in LTH and PV (control) endothelial cells. PV and/or SMV reconstruction using the autologous LTH was conducted in 26 patients with pancreaticobiliary malignancies, and the outcomes were retrospectively analyzed.
The post-dilated length of LTH was 9.67 ± 1.43 cm, and the diameter at a pressure of 30 cm H2O was 12.82 ± 1.32 mm at the cranial end and 7.06 ± 1.88 mm at the caudal end. Residual cavities with smooth tunica intima covered by endothelial cells were found in HE-stained LTH specimens. The relative amounts of EFs, CFs and SM in the LTH were similar to those in the PV [EF (%): 11.23 ± 3.40 vs 11.57 ± 2.80, P = 0.62; CF (%): 33.51 ± 7.71 vs 32.11 ± 4.82, P = 0.33; SM (%): 15.61 ± 5.26 vs 16.74 ± 4.83, P = 0.32]. CD34, FVIIIAg, eNOS, and t-PA were expressed in both LTH and PV endothelial cells. The PV and/or SMV reconstructions were successfully completed in all patients. The overall morbidity and mortality rates were 38.46% and 7.69%, respectively. There were no graft-related complications. The postoperative vein stenosis rates at 2 wk, 1 mo, 3 mo and 1 year were 7.69%, 11.54%, 15.38% and 19.23%, respectively. In all 5 patients affected, the degree of vascular stenosis was less than half of the reconstructed vein lumen diameter (mild stenosis), and the vessels remained patent.
The anatomical and histological characteristics of LTH were similar to the PV and SMV. As such, the LTH can be used as an autologous graft for PV and/or SMV reconstruction in pancreaticobiliary malignancy patients who require PV and/or SMV resection.
Core Tip: The anatomical, histological and clinical studies using the recanalized ligamentum teres hepatis (LTH) to reconstruct the portal vein (PV) and/or superior mesenteric vein (SMV) were studied. It was found that the post-dilated length and diameter of the LTH were suitable for PV and/or SMV reconstruction. The histological structure of the LTH wall was similar to the PV. High vascular patency rate and good clinical effects were acquired in clinical application. It was demonstrated that there is both basic and clinical rationale for the use of LTH in PV and/or SMV reconstruction since it does not cause additional injury or increase medical costs and has good clinical effects.
- Citation: Zhu WT, Wang HT, Guan QH, Zhang F, Zhang CX, Hu FA, Zhao BL, Zhou L, Wei Q, Ji HB, Fu TL, Zhang XY, Wang RT, Chen QP. Ligamentum teres hepatis as a graft for portal and/or superior mesenteric vein reconstruction: From bench to bedside. World J Gastrointest Surg 2023; 15(4): 674-686
- URL: https://www.wjgnet.com/1948-9366/full/v15/i4/674.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i4.674
Locally advanced carcinoma of the pancreatic head or ampulla of Vater often involves the portal vein (PV) and/or the superior mesenteric vein (SMV). Partial removal of the PV or SMV for complete surgical resection of the tumor is indicated[1-4]. A graft as a conduit or patch for PV and/or SMV reconstruction is needed. The most commonly used grafts include autogenous, homologous or artificial blood vessels[5-9]. However, these grafts have their own limitations. Therefore, it is necessary to explore more suitable grafts for PV and/or SMV reconstruction.
The ligamentum teres hepatis (LTH) is a fibrous remnant of the obliterated umbilical vein, which has the potential to be used as a graft[10,11]. Since the 1990s, the LTH has been described as a graft for reconstruction of the biliary tract, stomach or duodenum[12-14]. However, there are few studies on the use of LTH as a vascular graft during a pancreatoduodenectomy (PD) procedure, and systematic studies have not been performed. The aim of the present study was to understand the vascular characteristics of the LTH in the laboratory and assess clinical outcomes of the LTH as a vascular graft for PV and/or SMV reconstruction in patients with pancreaticobiliary malignancy.
Collection of LTH specimens: LTHs were obtained from 107 patients undergoing upper abdominal surgery in Binzhou Medical University Hospital. The surgical procedures included radical resections for gastric cancer (n = 54), hilar cholangiocarcinoma (n = 27) and pancreatic cancer (n = 26).
Specimen harvest and preparation: During the procedure, the entire LTH was excised. After removing the superficial fat layer, the LTH was placed in normal saline.
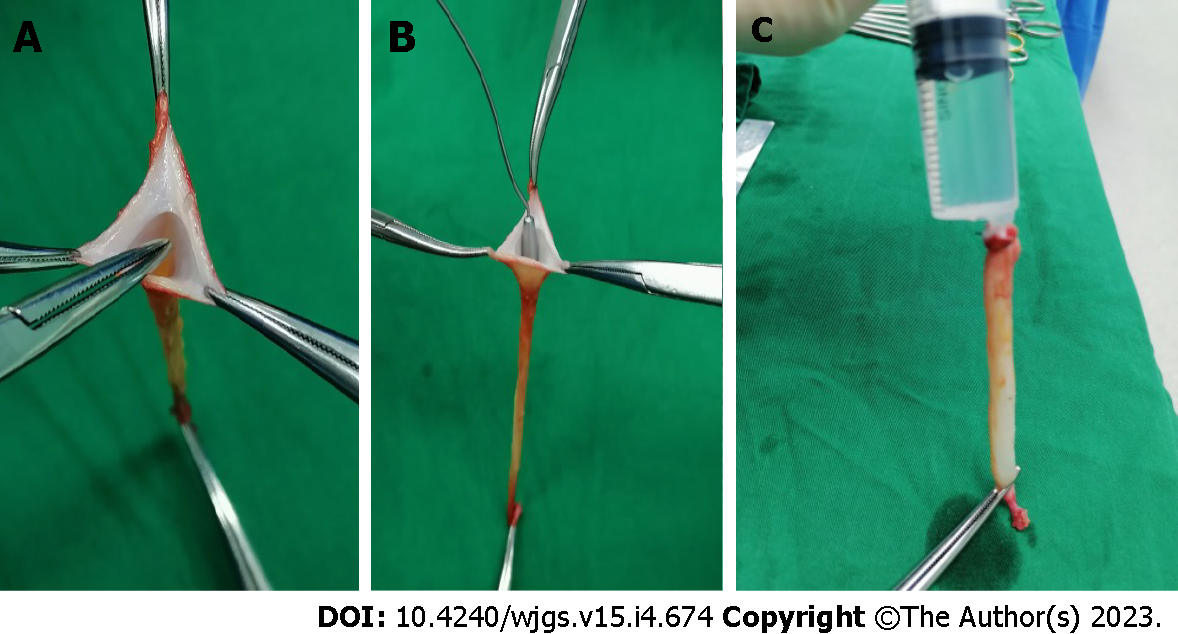
Recanalization of LTH: The remnant lumen of the LTH was identified and recanalized using a mosquito clamp (Figure 1A) and a 3 mm probe (Figure 1B), then gradually dilated using probes of 5-10 mm in diameter until the endothelial creases completely disappeared. The distal end of the LTH was tightly clamped, and a bolus of normal saline was injected into the lumen from the proximal end to further enlarge the lumen (Figure 1C).
Measurement of the recanalized LTH: The length and the outer diameter of the recanalized LTH were measured using a ruler and Vernier calipers, respectively, at a hydrostatic pressure of 30 cm H2O. The diameters of the LTH vessels were measured at 1 cm intervals over the entire length of the vessel.
Specimen harvest and preparation of LTH and PV: Forty LTH specimens were obtained from patients undergoing upper abdominal surgery at Binzhou Medical University Hospital, and PV specimens were obtained from 40 donor livers at The First Affiliated Hospital of Xi’an Jiaotong University. LTH and PV specimens were fixed with 10% neutral formaldehyde solution and embedded in paraffin. Cross sections (5 μm) were prepared for hematoxylin and eosin (HE), Verhoeff-Van Gieson (VVG), and immunohistochemistry staining.
The solutions purchased from Shanghai Sheng Gong Biological Engineering Technology Service (Shanghai, China) included hematoxylin, eosin, 10% and 2% FeCl3, Weigert’s iodine, 5% sodium thiosulfate, VVG stain and phosphate-buffered saline (PBS). Mouse monoclonal anti-human CD34, rabbit monoclonal anti-human factor VIII-related antigen (FVIIIAg), rabbit polyclonal anti-human endothelial nitric oxide synthase (eNOS), and rabbit polyclonal anti-human tissue type plasminogen activator (t-PA) antibodies were purchased from Santa Cruz Biotechnology (CA, United States). SP-9000 Histostain TM-plus kits and concentrated ZLI-9031 diaminobenzidine (DAB) kits were purchased from Beijing Zhongshan Jinqiao Biotechnology (Beijing, China).
Determination of the relative contents of collagen fibers (CFs), elastic fibers (EFs) and smooth muscle (SM) was performed in 40 sections of both LTHs and PVs. Sections were deparaffinized with xylene and immersed in VVG working solution to stain for 1 h. The sections were rinsed three times with distilled water and then immersed in 2% FeCl3 for 2 min for color separation. After immersion in 5% sodium thiosulfate solution for 1 min, the sections were washed with running water for 5 min, then restained with VVG staining solution for 5 min. The sections were rapidly dehydrated with a gradient alcohol series and cleared with xylene before sealing with Rhamsan gum. Under a light microscope, the left intersection point of the horizontal axis and LTH rings were used as the sampling window. Forty fields from 40 sections (one field/section) of both LTH and PV tissues were selected at a high magnification (× 400). Using the Motic medical image analysis system (MMD6.0 A), the relative content of EFs, CFs and SM in the wall of LTH and PV specimens were analyzed.
Detection of the distribution and function of LTH endothelial cells was also performed in 40 sections of both LTHs and PVs. Sections were deparaffinized with xylene and immersed in 3% H2O2 solution to inactivate endogenous enzymes. The sections were then washed with PBS prior to heat-induced antigen retrieval and incubated with normal goat serum at room temperature for 15 min. Then, the sections were incubated with primary antibodies, including anti-CD34 (1:100 dilution), anti-FVIIIAg (1:50 dilution), anti-eNOS (1:200 dilution) and anti-t-PA (1:200 dilution). After overnight incubation at 4 °C, sections were incubated with secondary antibodies (biotinylated universal secondary antibody, ready-to-use secondary antibody) for 15 min at 37 ºC. Next, sections were incubated with horseradish peroxidase streptavidin for 15 min at 37 ºC followed by the DAB reagent for 3-10 min to visualize color. The reaction time was controlled by observation under a light microscope. The sections were counterstained with hematoxylin for 5 min. After dehydration and clearing, the sections were sealed with Rhamsan gum and observed.
Subjects: Two hundred and sixty-four patients underwent a PD procedure at Binzhou Medical University Hospital from September 2003 to July 2019. Among the 264 patients, 39 patients underwent PD combined with PV and/or SMV resection. The vascular resection rate was 14.77%. Among these 39 patients, 26 patients underwent PD combined with PV and/or SMV resection and reconstruction using a recanalized LTH and were included in this study. Among the 26 patients, 25 patients underwent an open PD, and 1 patient underwent a laparoscopic PD. All 26 patients were evaluated preoperatively by physical examination and blood tests. Contrast-enhanced computed tomography (CT) was performed to assess the status of vascular infiltration.
Inclusion criteria included: (1) Patients who underwent PD combined with PV and/or SMV resection and reconstruction with LTH for malignant tumors of the bile duct, pancreas, Vater’s ampulla and duodenum; and (2) Clinical data were available.
Exclusion criteria included: (1) Patients who underwent PD combined with PV and/or SMV resection and reconstruction without grafts or using other grafts; and (2) Patients whose clinical data were not available.
Preparation of LTH grafts: Based on the intraoperative findings, if there was PV and/or SMV involvement of more than one-third of the circumference and 3 cm in length, then resection and reconstruction of the involved vein was performed. The LTH was then excised and recanalized as described above. After recanalization, LTH was trimmed into a tubular graft or patch according to the defect extent of the PV and/or SMV. The trimmed LTH was preserved in heparinized saline for grafting.
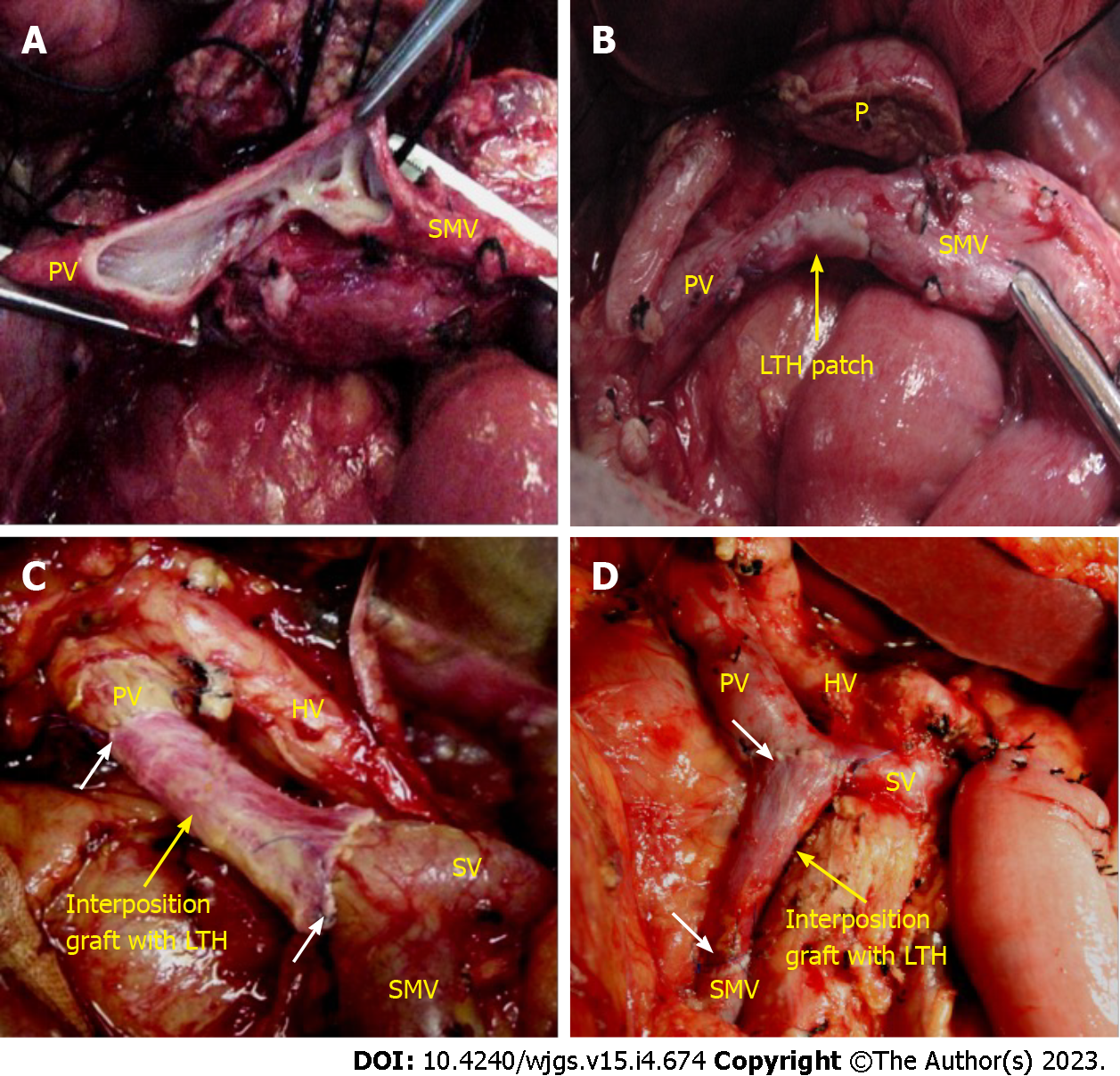
Vascular reconstruction: The proximal and distal portion of the involved vein was clamped with nontraumatic clips, and the venous occlusion time was documented. The splenic vein (SV) was also controlled if needed. The tumor along with the involved PV and/or SMV segment was resected en bloc. If the extent of the PV and/or SMV resection was more than one-third but less than one-half of the circumference of the vein, then a patch of LTH was used for reconstruction (Figure 2A and B, Video 1). If the resected segment of the involved PV and/or SMV was more than 3 cm in length, LTH was used as an interposition graft (Figure 2C and D, Video 2).
All anastomoses were performed using continuous 5-0 prolene sutures. Before completion of the anastomosis, both the stump of the recipient’s vein and the graft were rinsed with heparinized saline and flushed by release of the PV clamp to remove any clots. Upon completion, the anastomosis was checked for leaks and refilling. The types of surgical procedure, total operative time, estimated blood loss and vascular occlusion time were recorded. The operations were performed by the same surgeon with more than 15 years of experience in hepatobiliary surgery.
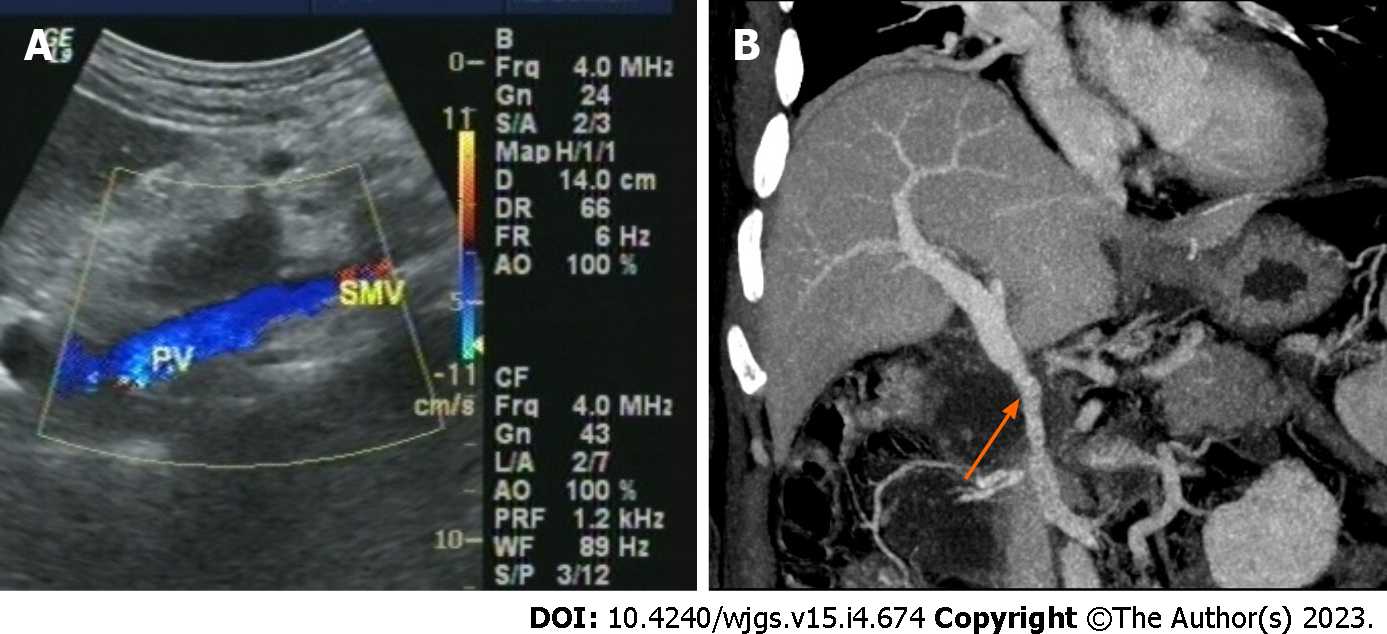
Postoperative management, complications and assessment of vascular patency: All patients received anticoagulant therapy with low molecular weight heparin (4100 IU; every 12 h) in the 1st postoperative week. Aspirin or low-dose warfarin was initiated from the 8th postoperative day in all patients and continued for 3 mo. The postoperative complications were recorded and classified using the International Study Group of Pancreatic Surgery and Clavien-Dindo classification[15,16]. PV/SMV blood flow was monitored using Doppler ultrasound. The patency of the reconstructed PV/SMV was evaluated by contrast-enhanced abdominal CT (Figure 3). The degree classification of reconstructed vein stenosis was based on the classification method of Kleive et al[17]. The date of last follow-up was June 2022.
This study was approved by the Ethics Committee of Binzhou Medical University Hospital (2019-LW-023). This study has been registered with the Chinese Clinical Trial Registry (Registration No. ChiCTR1900027098 https://www.chictr.org.cn). Written informed consent to participate was obtained from all patients.
The SPSS 18.0 software (SPSS, Chicago, IL, United States) was used for all statistical analyses. The variables were expressed as mean ± SD or medians with interquartile ranges. Intergroup comparisons of the data were made using independent sample t-tests. A P value of less than 0.05 was considered statistically significant. Reconstructed vascular lumen patency and overall survival were estimated using the Kaplan-Meier method.
The LTH was successfully dilated in 104 specimens yielding a success rate of 97.20%. The length of the dilated segments was 9.67 ± 1.43 cm (range: 6.9-13.0 cm). The diameters of the dilated LTH were 12.82 ± 1.32 mm (range: 10.9-17.2 mm) at the cranial end and 7.06 ± 1.88 mm (range: 3.0-12.1 mm) at the caudal end (Table 1).
Residual cavities with smooth tunica intima covered with endothelial cells were found in all 40 LTH specimens (Figure 4A and B).
VVG staining revealed that LTH specimens were composed of EFs, CFs and SM components similar to the PV specimens (Figure 4C and D). Analysis using the Motic medical image analysis system (MMD 6.0A) showed that there were no significant differences in the relative content of EFs, CFs and SM between LTHs and PVs, as shown in Table 2.
| EF (%) | CF (%) | SM (%) | C/E | |
| LTH | 11.23 ± 3.40 | 33.51 ± 7.71 | 15.61 ± 5.26 | 3.27 ± 1.22 |
| PV | 11.57 ± 2.80 | 32.11 ± 4.82 | 16.74 ± 4.83 | 3.94 ± 0.85 |
| P value | 0.62 | 0.33 | 0.32 | 0.16 |
Immunohistochemical staining revealed the expression of CD34, FVIIIAg, eNOS and t-PA in the cytoplasm of endothelial cells in both LTH and PV specimens (Figure 4E-L), suggestive of the synthesis of CD34, FVIIIAg, eNOS and t-PA in endothelial cells of the LTH.
Demographic characteristics: In a total of 26 cases, the proportion of males and females was equal. The median age was 62 years (interquartile range 53.25-69.25). This case series included patients with pancreatic cancer (n = 19), cholangiocarcinoma (n = 4) and ampullary carcinoma (n = 3).
Intraoperative parameters and complications: PV, SMV and PV plus SMV reconstructions were conducted in 16, 9 and 1 case(s), respectively. The intraoperative data are shown in Table 3.
| Variable | Value |
| Type of venous resection + reconstruction | |
| Tangential + patch, n (%) | 5 (19.23) |
| Segmental + interposition, n (%) | 21 (80.77) |
| Length of the segmental resected vein in mm | 40 (35-50) |
| Length of the interposition graft in mm | 40 (30-40) |
| Operative time in min | 485 (397.50-572.75) |
| Blood loss at surgery in mL | 200 (150-300) |
| Vein clamping time in min | 50 (40-60) |
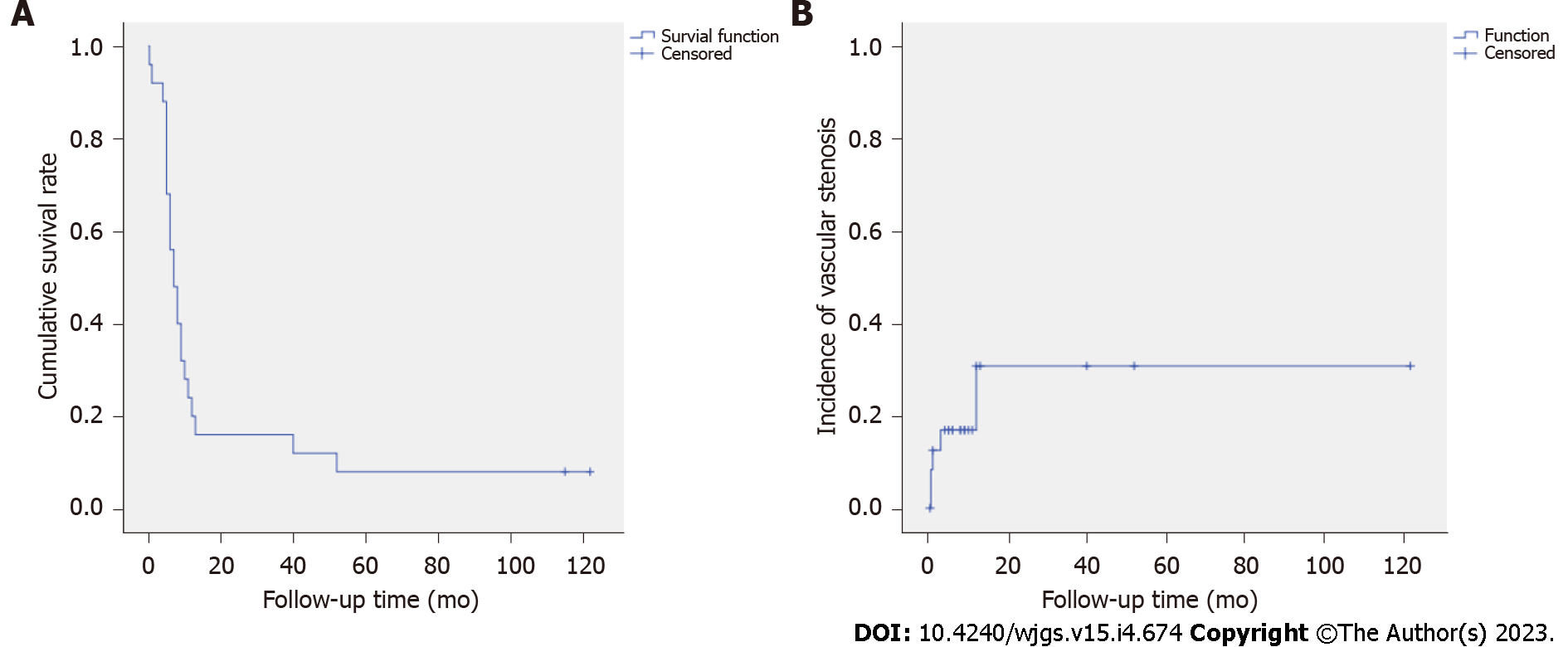
Postoperative outcomes: The overall morbidity rate was 38.46% (n = 10) (Table 4), and there were no graft-related complications. Two patients (7.69%) died within 30 d after surgery. One patient died of gastrointestinal hemorrhage caused by bleeding from the pancreatoenteric anastomosis, and the other died of pancreatic fistula-associated severe abdominal bleeding caused by gastroduodenal artery stump bleeding. The median postoperative hospital stay was 20 d (interquartile range: 16.75-25.00 d), and the median survival was 7 mo (interquartile range: 5.00-11.25 mo). No patients were lost to follow-up (Figure 5A). The vascular cumulative stenosis curve was shown in Figure 5B. Vascular stenosis was found within the 2nd postoperative week in 2 cases. One case of vascular stenosis was identified at 1 mo, one at 3 mo and one at 1 year. No vascular stenosis was identified later than 1 year postoperative, and the longest follow-up was 122 mo. The postoperative vein stenosis rates at 2 wk, 1 mo, 3 mo and 1 year were 7.69%, 11.54%, 15.38% and 19.23%, respectively. In all 5 patients, the degree of the vascular stenosis was less than half of the reconstructed vein lumen diameter (mild stenosis), and the vessels remained patent.
| Postoperative complications | Patients |
| Grade I | 3 |
| Bile leakage | 2 |
| Pulmonary infection | 1 |
| Grade II | 5 |
| Pancreatic leakage | 1 |
| Delayed gastric emptying, grade B | 1 |
| Pulmonary infection | 2 |
| Gastrointestinal hemorrhage | 1 |
| Grade III | 2 |
| Gastrointestinal bleeding | 1 |
| Lymphorrhea requiring abdominocentesis | 1 |
| Grade IV | 0 |
| Grade V, death | 2 |
| Overall morbidity, n/total (%) | 10/26 (38.46) |
| Overall mortality, n/total (%) | 2/26 (7.69) |
PD combined with PV and/or SMV resection and reconstruction may be required for locally advanced periampullary and pancreatic head carcinoma with PV and/or SMV involvement. This procedure has been confirmed to improve the R0 resection rate and patient survival[4,18,19]. Grafts used for vein reconstruction can be obtained from various veins, such as the internal jugular vein, femoral vein, external iliac vein, gonadal vein, great saphenous vein, splenic vein, left renal vein and the falciform ligament of the liver[20-28]. However, harvesting autologous grafts requires an additional surgery and increases risk of damage to the major vessels[22,27]. LTH, as a remnant derived from the obliterated umbilical vein, can be dilated to form a conduit with potential venous characteristics[13,14]. The LTH has been used as a vascular graft to reconstruct the PV and/or SMV since 2003 in our medical center and has achieved good clinical results[29]. Few successful cases have been subsequently reported[30,31], but no larger sample sizes are available.
In the present study, the morphometric findings of the LTH revealed that it is suitable for PV and/or SMV reconstruction in terms of its length and diameter, which were demonstrated in our previous study[32]. The LTH diameters were measured at a pressure of 30 cm H2O, which simulates the physiological status of the mean PV pressure of 18 cm H2O (13-24 cm H2O)[33].
Studying the structure of the LTH wall is essential to evaluate its potential as a graft for PV/SMV reconstruction. VVG staining revealed that EF, CF and SM content in the LTH wall were similar to PVs, which suggested that the LTH had characteristics similar to major abdominal vessels, such as vascular stiffness as well as contraction, and relaxation properties[34-38]. Therefore, the relative abundance of EFs, CFs and SM suggests that histologically LTH can be used as a graft to reconstruct the PV and/or SMV.
After PV and/or SMV reconstruction, vascular patency is key for a technically successful procedure[26,27]. Vascular endothelial cells act as a vascular barrier and mediate hemostatic and antithrombotic functions, which can affect the patency of blood vessels and reduce the risk of thrombosis[39,40]. HE staining showed that the inner surface of LTH was smooth and covered with endothelial cells. Immunohistochemical staining revealed the expression of FVIIIAg and CD34 at the inner surface of LTH, confirming the presence of vascular endothelial cells.
Nitric oxide and t-PA, which are synthesized by endothelial cells, play important roles in thrombosis prevention. Abnormal eNOS function is associated with an increased risk of endothelial dysfunction[40], which in turn can be mitigated by upregulating eNOS expression[41]. Previous studies demonstrated that increasing t-PA production reduced thrombus formation[42-44]. The current study found that eNOS and t-PA can be expressed in LTH endothelial cells, suggestive of its anti-thrombosis function.
The surgical procedure, operating time, blood loss, PV clamping time, overall perioperative morbidity rate and mortality rate in this case series was similar to previous studies[23,45,46]. Postoperative partial thrombosis led to vascular stenosis in 5/26 patients, which was less than that reported in previous studies[6,45]. No patients developed uncontrollable portal hypertension or liver dysfunction. It is suggested that LTH as a graft for PV and/or SMV reconstruction is safe and reliable.
We acknowledge that the clinical study is limited by its retrospective nature and the small number of patients. Larger prospective studies should be conducted in the future to validate the findings of this study. However, this study is one of the largest studies that exclusively focused on patients with venous reconstruction using a recanalized LTH graft during PD.
In conclusion, the recanalized length of the LTH was suitable for reconstruction of the PV and SMV, and the dilated diameter and histological characteristics of the LTH were similar to the PV and SMV. Using the LTH as an autologous graft to reconstruct these vessels has achieved good clinical results and fits ideal characteristics including a wide range of sources, low cost, good histocompatibility and does not cause additional damage to patients. Based on the present study, we recommend the LTH as an autologous graft for PV and or SMV reconstruction in patients suffering from pancreaticobiliary cancer with PV/SMV involvement.
Grafts may be required for portal vein (PV) and/or superior mesenteric vein (SMV) reconstruction during a pancreaticoduodenectomy (PD) procedure combined with PV and/or SMV resection. These grafts, including autogenous, homologous and artificial blood vessels, each have their own limitations. Therefore, it is necessary to explore more suitable grafts for PV and/or SMV reconstruction.
The ligamentum teres hepatis (LTH) is a fibrous remnant of the obliterated umbilical vein and can be recanalized. If the diameter and the histological characteristics of the dilated LTH tube wall are similar to the PV and SMV, and if the dilated LTH can be successfully used for PV and SMV reconstruction clinically, a novel PV and SMV graft will be acquired that has many sources, no additional medical costs and no immune rejection response.
To evaluate the feasibility of using the LTH as an autologous substitute for the reconstruction of the PV and/or SMV during PD and to provide basic and clinical evidence for using the LTH as an autologous graft for the PV and/or SMV reconstruction.
The dilated length, diameter, tube wall histological characteristics and endothelial cell function of the LTH were measured and observed, and the results were compared to the PV and SMV for the first time. The outcomes of 26 patients who underwent PD where the LTH was used for PV and/or SMV reconstruction were studied, which is the largest sample size to date that exclusively focused on patients with venous reconstruction using a recanalized LTH graft during PD. The patency of the reconstructed PV and/or SMV using LTH as the autologous graft was reported for the first time.
The length, diameter and histological characteristics of the LTH tube wall were similar to the PV and/or SMV. The tunica intima of the LTH was covered with endothelial cells, and these cells functioned normally. The LTH as an autologous graft for PV and/or SMV reconstruction was successfully used in the clinic. However, larger prospective studies should be conducted in the future to validate the findings of this study.
The LTH can be used as an autologous graft for PV and/or SMV reconstruction.
The establishment of a homologous blood vessel bank using the LTH as grafts is expected.
The funding bodies had no role in the design of the study, the collection, analysis, or interpretation of the data or writing the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fernández-Placencia RM, Peru; Shah OJ, India S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Riediger H, Makowiec F, Fischer E, Adam U, Hopt UT. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg. 2006;10:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Adham M, Mirza DF, Chapuis F, Mayer AD, Bramhall SR, Coldham C, Baulieux J, Buckels J. Results of vascular resections during pancreatectomy from two European centres: an analysis of survival and disease-free survival explicative factors. HPB (Oxford). 2006;8:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, Nakagawa N, Sueda T. Benefit of portal or superior mesenteric vein resection with adjuvant chemotherapy for patients with pancreatic head carcinoma. J Surg Oncol. 2013;107:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Ramacciato G, Mercantini P, Petrucciani N, Giaccaglia V, Nigri G, Ravaioli M, Cescon M, Cucchetti A, Del Gaudio M. Does portal-superior mesenteric vein invasion still indicate irresectability for pancreatic carcinoma? Ann Surg Oncol. 2009;16:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Wang X, Cai Y, Zhao W, Gao P, Li Y, Liu X, Peng B. Laparoscopic pancreatoduodenectomy combined with portal-superior mesenteric vein resection and reconstruction with interposition graft: Case series. Medicine (Baltimore). 2019;98:e14204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Kim PT, Wei AC, Atenafu EG, Cavallucci D, Cleary SP, Moulton CA, Greig PD, Gallinger S, Serra S, McGilvray ID. Planned vs unplanned portal vein resections during pancreaticoduodenectomy for adenocarcinoma. Br J Surg. 2013;100:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Evans DB, Farnell MB, Lillemoe KD, Vollmer C Jr, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1736-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Kim SM, Min SK, Park D, Min SI, Jang JY, Kim SW, Ha J, Kim SJ. Reconstruction of portal vein and superior mesenteric vein after extensive resection for pancreatic cancer. J Korean Surg Soc. 2013;84:346-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Yamamoto Y, Sakamoto Y, Nara S, Ban D, Esaki M, Shimada K, Kosuge T. Reconstruction of the portal and hepatic veins using venous grafts customized from the bilateral gonadal veins. Langenbecks Arch Surg. 2009;394:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Ikegami T, Wang H, Imai D, Bekki Y, Yoshizumi T, Yamashita Y, Toshima T, Soejima Y, Shirabe K, Maehara Y. Pathological analysis of opened round ligaments as venous patch grafts in living donor liver transplantation. Liver Transpl. 2013;19:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Stüben BO, Heumann A, Stürznickel J, Izbicki JR, Li J. Successful Use of the Recanalized Remnant Umbilical Vein as a Patch Graft for Venous Reconstruction in Abdominal Surgery. J Gastrointest Surg. 2019;23:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Costalat G, Alquier Y. Combined laparoscopic and endoscopic treatment of perforated gastroduodenal ulcer using the ligamentum teres hepatis (LTH). Surg Endosc. 1995;9:677-9; discussion 680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Ying DJ, Ho GT, Cai JX. Anatomic bases of the vascularized hepatic teres ligament flap. Surg Radiol Anat. 1997;19:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Hur MS, Kim HJ, Lee KS. Termination of the ligamentum venosum and the topographic relationship between the left portal vein, left hepatic artery, and ligamentum venosum in the fissures for the ligamentum teres and ligamentum venosum. Surg Radiol Anat. 2015;37:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Tan WJ, Kow AW, Liau KH. Moving towards the New International Study Group for Pancreatic Surgery (ISGPS) definitions in pancreaticoduodenectomy: a comparison between the old and new. HPB (Oxford). 2011;13:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24849] [Article Influence: 1183.3] [Reference Citation Analysis (0)] |
| 17. | Kleive D, Berstad AE, Sahakyan MA, Verbeke CS, Naper C, Haugvik SP, Gladhaug IP, Line PD, Labori KJ. Portal vein reconstruction using primary anastomosis or venous interposition allograft in pancreatic surgery. J Vasc Surg Venous Lymphat Disord. 2018;6:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Nakao A, Kanzaki A, Fujii T, Kodera Y, Yamada S, Sugimoto H, Nomoto S, Nakamura S, Morita S, Takeda S. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann Surg. 2012;255:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE, Pisters PW, Evans DB. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 446] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 20. | Kubota K, Makuuchi M, Sugawara Y, Midorikawa Y, Sakamoto Y, Takayama T, Harihara Y. Reconstruction of the hepatic and portal veins using a patch graft from the right ovarian vein. Am J Surg. 1998;176:295-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Fleming JB, Barnett CC, Clagett GP. Superficial femoral vein as a conduit for portal vein reconstruction during pancreaticoduodenectomy. Arch Surg. 2005;140:698-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Smoot RL, Christein JD, Farnell MB. An innovative option for venous reconstruction after pancreaticoduodenectomy: the left renal vein. J Gastrointest Surg. 2007;11:425-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Kaneoka Y, Yamaguchi A, Isogai M. Portal or superior mesenteric vein resection for pancreatic head adenocarcinoma: prognostic value of the length of venous resection. Surgery. 2009;145:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Zhiying Y, Haidong T, Xiaolei L, Yongliang S, Shuang S, Liguo L, Li X, Atyah M. The falciform ligament as a graft for portal-superior mesenteric vein reconstruction in pancreatectomy. J Surg Res. 2017;218:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Shao Y, Yan S, Zhang QY, Shen Y, Zhang M, Wang WL, Zheng SS. Autologous falciform ligament graft as A substitute for mesentericoportal vein reconstruction in pancreaticoduodenectomy. Int J Surg. 2018;53:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Yoshioka M, Uchinami H, Watanabe G, Iida M, Nakagawa Y, Miyazawa H, Yoshida M, Yamamoto Y. Domino Reconstruction of the Portal Vein Using the External Iliac Vein and an ePTFE Graft in Pancreatic Surgery. J Gastrointest Surg. 2017;21:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Kostov G, Dimov R. Portal vein reconstruction during pancreaticoduodenal resection using an internal jugular vein as a graft. Folia Med (Plovdiv). 2021;63:429-432. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Saglam K, Sahin TT, Usta S, Koc C, Otan E, Kayaalp C, Aydin C, Yilmaz S. Portal vein reconstruction with cryopreserved vascular grafts: A two-edged sword. Pediatr Transplant. 2022;26:e14206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Chen QP, Ou K, Guan QH, Zhang F, Lin XT. Feasibility study on reconstruction of portal vein/ superior mesenteric vein using ligmentum teres. Shandong Yi Yao. 2006;46:7-8. [DOI] [Full Text] |
| 30. | Gunasekaran G, Mosna LC, Savino JA. Portomesenteric reconstruction using an umbilical vein patch during pancreaticoduodenectomy (Whipple procedure). J Am Coll Surg. 2013;217:e9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Wei Q, Chen QP, Guan QH, Zhu WT. Repair of the portal vein using a hepatic ligamentum teres patch for laparoscopic pancreatoduodenectomy: A case report. World J Clin Cases. 2019;7:2879-2887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Zhu WT, Chen QP, Guan QH, Zhang F, Zhang CX, Huang Q, Wang HT. Comparative study of the length and diameter in recanalized adult ligamentum teres hepatic and their auto-portal vein and superior mesenteric vein. Zhonghua Putong Waike Xue Wenxian. 2012;6:386-393. [DOI] [Full Text] |
| 33. | Thompson SM, Fleming CJ, Yohanathan L, Truty MJ, Kendrick ML, Andrews JC. Portomesenteric Venous Complications after Pancreatic Surgery with Venous Reconstruction: Imaging and Intervention. Radiographics. 2020;40:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Fischer GM, Llaurado JG. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ Res. 1966;19:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 141] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Zhang J, Zhao X, Vatner DE, McNulty T, Bishop S, Sun Z, Shen YT, Chen L, Meininger GA, Vatner SF. Extracellular Matrix Disarray as a Mechanism for Greater Abdominal Versus Thoracic Aortic Stiffness With Aging in Primates. Arterioscler Thromb Vasc Biol. 2016;36:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Latimer CA, Nelson M, Moore CM, Martin KE. Effect of collagen and elastin content on the burst pressure of human blood vessel seals formed with a bipolar tissue sealing system. J Surg Res. 2014;186:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Steucke KE, Tracy PV, Hald ES, Hall JL, Alford PW. Vascular smooth muscle cell functional contractility depends on extracellular mechanical properties. J Biomech. 2015;48:3044-3051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Brozovich FV, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M, Morgan KG. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol Rev. 2016;68:476-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 351] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 39. | Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 476] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 40. | Luo Y, Wang Y, Luo W. C allele of -786 T>C polymorphism in the promoter region of endothelial nitric oxide synthase is responsible for endothelial dysfunction in the patients with rheumatoid arthritis. J Cell Biochem. 2020;121:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Chen J, Zhang J, Shaik NF, Yi B, Wei X, Yang XF, Naik UP, Summer R, Yan G, Xu X, Sun J. The histone deacetylase inhibitor tubacin mitigates endothelial dysfunction by up-regulating the expression of endothelial nitric oxide synthase. J Biol Chem. 2019;294:19565-19576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Larsson P, Alwis I, Niego B, Sashindranath M, Fogelstrand P, Wu MC, Glise L, Magnusson M, Daglas M, Bergh N, Jackson SP, Medcalf RL, Jern S. Valproic acid selectively increases vascular endothelial tissue-type plasminogen activator production and reduces thrombus formation in the mouse. J Thromb Haemost. 2016;14:2496-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Montt-Guevara MM, Palla G, Spina S, Bernacchi G, Cecchi E, Campelo AE, Shortrede JE, Canu A, Simoncini T. Regulatory effects of estetrol on the endothelial plasminogen pathway and endothelial cell migration. Maturitas. 2017;99:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Schoergenhofer C, Matzneller P, Mußbacher M, Schmid JA, Jilma-Stohlawetz P, Zeitlinger M, Jilma B. Colistin dampens fibrinolysis and endothelial activation during endotoxaemia. A randomised, double blind trial. Thromb Haemost. 2017;117:1714-1721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Chu CK, Farnell MB, Nguyen JH, Stauffer JA, Kooby DA, Sclabas GM, Sarmiento JM. Prosthetic graft reconstruction after portal vein resection in pancreaticoduodenectomy: a multicenter analysis. J Am Coll Surg. 2010;211:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Gong Y, Zhang L, He T, Ding J, Zhang H, Chen G, Zhang D, Wu Z, Chen Q, Fan H, Wang Q, Bie P, Wang H. Pancreaticoduodenectomy combined with vascular resection and reconstruction for patients with locally advanced pancreatic cancer: a multicenter, retrospective analysis. PLoS One. 2013;8:e70340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |