Published online Apr 27, 2023. doi: 10.4240/wjgs.v15.i4.621
Peer-review started: November 11, 2022
First decision: January 23, 2023
Revised: February 8, 2023
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 27, 2023
Processing time: 163 Days and 3.1 Hours
Previous reports have focused on muscle mass as a prognostic factor in esophageal cancer.
To investigate how preoperative body type influences the prognosis of patients with esophageal squamous cell carcinoma who underwent neoadjuvant chemotherapy (NAC) and surgery.
The subjects were 131 patients with clinical stage II/III esophageal squamous cell carcinoma who underwent subtotal esophagectomy after NAC. Skeletal muscle mass and quality were calculated based on computed tomography images prior to NAC, and their statistical association with long-term outcomes was examined retrospectively in this case-control study.
The disease-free survival rates in the low psoas muscle mass index (PMI) group vs the high PMI group were 41.3% vs 58.8% (P = 0.036), respectively. In the high intramuscular adipose tissue content (IMAC) group vs the low IMAC group, the disease-free survival rates were 28.5% vs 57.6% (P = 0.021), respectively. The overall survival (OS) rates for the low PMI group vs the high PMI group were 41.3% vs 64.5% (P = 0.008), respectively, and for the high IMAC group vs the low IMAC group, they were 29.9% vs 61.9% (P = 0.024), respectively. Analysis of the OS rate revealed significant differences in patients aged 60 years or older (P = 0.018), those with pT3 or above disease (P = 0.021), or those with lymph node metastasis (P = 0.006), aside from PMI and IMAC. Multivariate analysis demonstrated that pT3 or above [hazard ratio (HR): 1.966, 95% confidence interval (CI): 1.089-3.550, P = 0.025), lymph node metastasis (HR: 2.154, 95%CI: 1.118-4.148, P = 0.022), low PMI (HR: 2.266, 95%CI: 1.282-4.006, P = 0.005), and high IMAC (HR: 2.089, 95%CI: 1.036-4.214, P = 0.022) were significant prognostic factors for esophageal squamous cell carcinoma.
Skeletal muscle mass and quality before NAC in patients with esophageal squamous cell carcinoma are significant prognostic factors for postoperative OS.
Core Tip: Esophageal cancer patients are often nutritionally malnourished, and their muscle mass is often decreased. In addition to loss of muscle mass, it is often associated with loss of muscle quality. In this study, the prognosis of esophageal squamous cell carcinoma patients was found to be influenced by muscle composition before preoperative chemotherapy. The prognosis is not only affected by muscle mass but also by muscle quality.
- Citation: Ichinohe D, Muroya T, Akasaka H, Hakamada K. Skeletal muscle mass and quality before preoperative chemotherapy influence postoperative long-term outcomes in esophageal squamous cell carcinoma patients. World J Gastrointest Surg 2023; 15(4): 621-633
- URL: https://www.wjgnet.com/1948-9366/full/v15/i4/621.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i4.621
Esophageal cancer continues to have a poor prognosis, with a low 5-year survival rate of 20%[1]. Poor prognostic factors are due to the tendency of the cancer to metastasize at an early stage[2,3] and to easily invade nearby vital organs such as the lungs, large blood vessels, heart, and trachea, indicating that the cancer is already advanced at the time of diagnosis[4]. Therefore, the standard treatment is a combination of chemotherapy and radiotherapy in addition to surgery[5]. However, the prognosis remains poor.
In recent years, preoperative sarcopenia has been identified as a factor that reduces short-term postoperative prognosis and outcomes after gastrointestinal cancer surgery[6]. Sarcopenia is defined as the loss of function associated with muscle mass loss and quality[7]. Factors such as cancer status, underlying disease, advanced age, and sex are involved. Preoperative muscle mass loss has been reported as a postoperative complication or prognostic factor in gastric[8], hepatocellular[9], biliary[10], pancreatic[11], and colorectal cancers[12]. Recently, it has been suggested that, in addition to muscle mass, fatty degeneration of muscle and muscle quality changes also affect prognosis[13]. Low skeletal muscle mass has been reported to influence the occurrence of postoperative respiratory complications in esophageal cancer[14-16] and is a factor for poor short-term outcomes[17,18].
Esophageal cancer is often complicated by preoperative nutritional deficits due to reduced oral intake caused by stenosis. Therefore, sarcopenia is often complicated preoperatively[19]. In addition, eso
Multidisciplinary treatment for esophageal cancer is available in a variety of forms, including pre- and postoperative chemotherapy[22] and preoperative chemoradiotherapy[23,24]. The multidisciplinary approach is used in Europe and the United States for adenocarcinoma; in Japan and East Asian countries, however, this is more common for squamous cell carcinoma[4]. In Japan, preoperative chemotherapy and subtotal esophagectomy with three-field lymph node dissection is the standard treatment[5]. Therefore, assessing the impact of sarcopenia on short-term and long-term outcomes after esophageal cancer surgery requires a consistent examination of the disease and treatment context.
The present study included Japanese patients with squamous cell carcinoma of the esophagus who underwent preoperative chemotherapy and subtotal esophagectomy with three-field lymph node dissection as the standard therapy. We examined the effect of muscle mass and quality before preoperative chemotherapy on long-term prognosis in these patients.
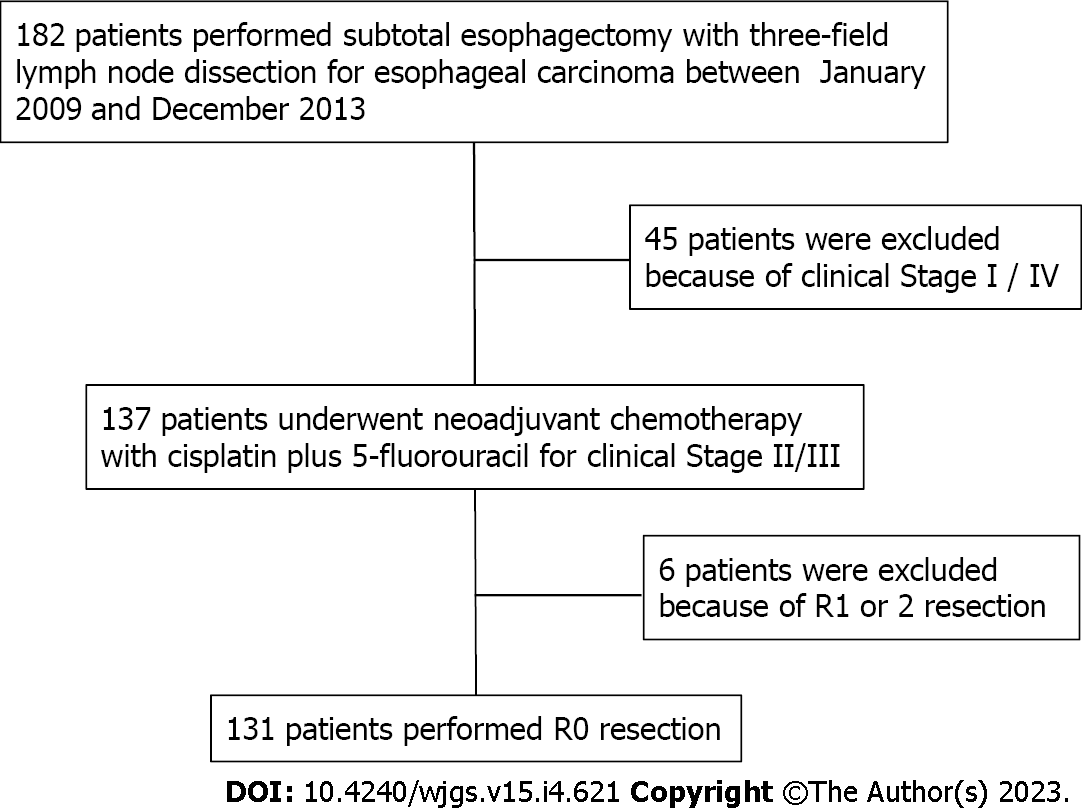
Of the 182 consecutive esophageal cancer patients who underwent esophagectomy between January 2009 and December 2013 at our hospital in Aomori, Japan, 131 were recruited for the study. In our hospital, one surgeon specializes in upper gastrointestinal surgery and performs 30 or more esophageal cancer surgeries per year. The selected subjects underwent subtotal esophagectomy with three-field lymph node dissection after completion of two courses of 5-fluorouracil plus cisplatin as neoadjuvant chemotherapy (NAC) for clinical stage II/III esophageal squamous cell carcinoma. They had no residual tumors. Six patients with positive resection margins were excluded (Figure 1).
All patients were examined by esophagogastroduodenoscopy and diagnosed histologically with esophageal squamous cell carcinoma (adenocarcinoma was excluded because the inclusion criterion for NAC is squamous cell carcinoma) confirmed by biopsy. They were then examined by routine 1-mm slice contrast-enhanced computed tomography (CT) and positron emission tomography-CT, and staged according to the TNM classifications (7th edition)[25] of the Union for International Cancer Control.
Two courses of 5-fluorouracil plus cisplatin therapy were administered. NAC for clinical stage II or III esophageal squamous cell carcinoma was administered according to the Japan Clinical Oncology Group 9907 trial[5]. The regimen was: (1) Day 1: Cisplatin 80 mg/m2 intravenous infusion; (2) days 1-5: 5-fluorouracil 800 mg/m2 intravenous infusion; and (3) cycle frequency every 21 d for 2 cycles.
The effect of chemotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumors guidelines (version 1.1[26]). Right thoraco-laparotomic subtotal esophagectomy and three-field lymph node dissection were performed after two courses of NAC. For reconstruction, retrosternal route gastric tube reconstruction was performed unless the patient was post-gastrectomy. Postoperatively, the patient was treated in the intensive care unit for systemic management. After discharge, blood examinations were performed every 3 mo and radiological examinations every 6 mo.
Age, body mass index (BMI), performance status, and American Society of Anesthesiologists physical status were determined from the medical records of the patients. The white blood cell count, neutrophil count, lymphocyte count, high sensitivity C-reactive protein level, and serum albumin level were investigated according to preoperative blood chemistry data. Neutrophil-to-lymphocyte ratio (used as a nutrition index), Prognostic Nutritional Index, Geriatric Nutritional Risk Index, and modified Glasgow prognostic score were calculated as evaluation criteria.
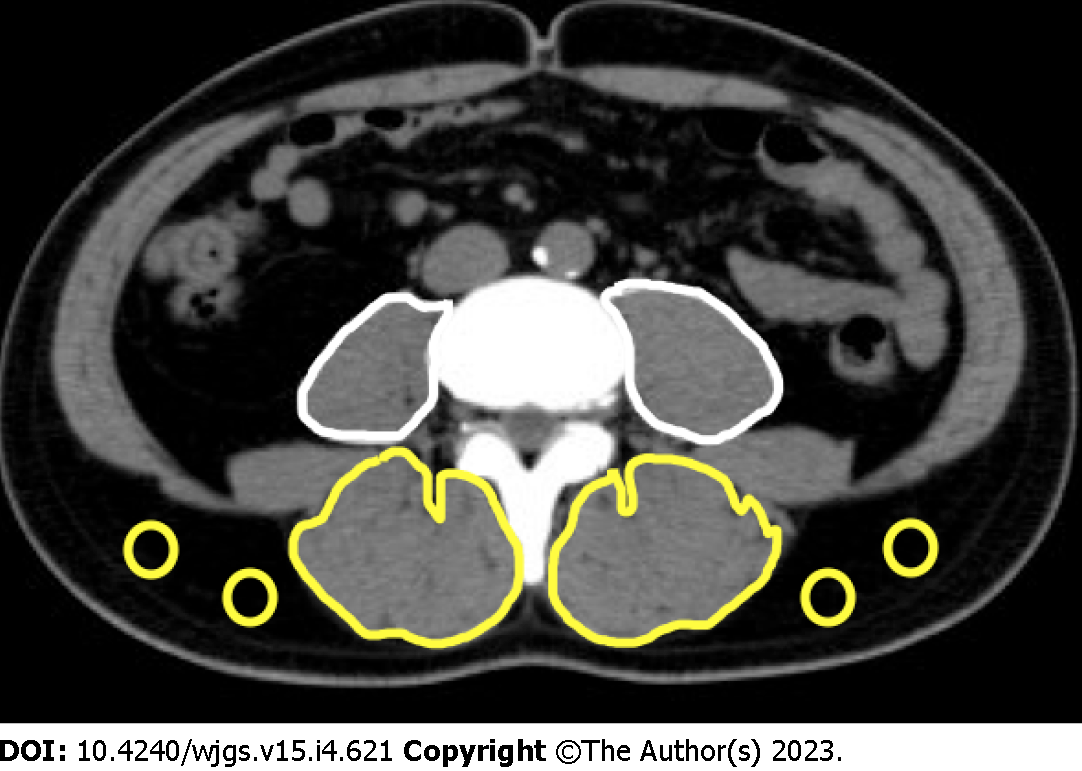
For skeletal muscle mass measurement, computed tomography (CT) images before NAC were used. The bilateral psoas muscle areas were measured at the third lumbar vertebral level through tracing using Digital Imaging and Communications in Medicine viewer software, EV Invite® (PSP Corporation, Tokyo, Japan) (Figure 2). The value calculated by dividing the psoas muscle area by the square of the height was determined as the psoas muscle mass index (PMI) [= (cross-sectional area of bilateral psoas muscle)/(height)2 (cm2/m2)].
For skeletal muscle quality measurement using CT values, the bilateral multifidus muscles were traced at the third lumbar vertebral level (the same as the level of the psoas muscle cross-sectional area measurement), and the mean CT value of this region was calculated. In addition, subcutaneous fat was traced at four sites at the same level, and the mean CT value was determined. The mean CT value of the multifidus muscle was divided by the mean CT value of the subcutaneous fat at the four sites, and the calculated value was regarded as the intramuscular adipose tissue content (IMAC) [= mean CT value of bilateral multifidus muscle (HU)/mean CT value of four points of subcutaneous fat (HU)][27,28].
In this study, a receiver operating characteristic curve against overall survival (OS) was prepared using precalculated PMI with the optimum cutoff value of PMI set at 4 (area under the curve: 0.538, sensitivity: 61.0%, specificity: 78.3%). Patients with PMI < 4 and PMI ≥ 4 were designated as belonging to the low and high PMI groups, respectively, and compared.
Similarly, a receiver operating characteristic curve for IMAC was prepared, and the optimum cutoff value was set at 0.36 (area under the curve: 0.538, sensitivity: 61.0%, specificity: 78.3%). Patients with IMAC ≥ -0.36 and IMAC < -0.36 were designated as belonging to the high and low IMAC groups, respectively.
Postoperative complications were defined according to the Clavien-Dindo classification[29], with Clavien-Dindo grade ≥ 3 defined as the presence of complications. For analysis of outcomes, OS and disease-free survival (DFS) rates were used.
For statistical analysis, SPSS® Statistics (Version 22.0; IBM Corp., Armonk, NY, United States) was used. All variables are presented as median values. In univariate analysis, continuous and non-continuous variables were analyzed using the Mann-Whitney U test and χ2test, respectively. Survival curves were prepared using the Kaplan-Meier method. For multivariate analysis, the log rank test was used, and analysis was performed using the Cox proportional hazards model. P values < 0.05 were regarded as significant.
Although this study was a retrospective study, ethical considerations required approval because of the use of biometric data. This study is registered with the Research Registry (Unique Identifying Number 7880) and was approved by the Ethics Committee (Approval number: 2020-38). This article is reported in line with the STROBE criteria[30].
The median age of the 131 patients was 64 years (range: 44-78 years), and the median BMI was 21.4 kg/m2 (range: 14.7-27.7 kg/m2). The clinical stages were stage II in 68 patients and stage III in 63 patients. The determination of the effect of NAC following the Response Evaluation Criteria in Solid Tumors guidelines was complete response in 2 patients, partial response in 79, stable disease in 42, and progressive disease in 8. The rate of response to NAC was 61.8% compared with the disease control rate of 93.9%. The median number of postoperative hospitalization days was 18 (range: 11-225); reoperation was performed on 4 patients (3.1%). There was no postoperative mortality at the hospital nor was there any mortality within 90 d following surgery. The median duration of postoperative follow-up was 60.9 mo (range: 3.9-100.3 mo).
The median PMI value was 4.94 (2.12-8.98). When the cases were classified setting the cutoff value of PMI at 4, the low and high PMI groups included 36 (27.5%) and 95 (72.5%) patients, respectively. In the between-group comparison, BMI and Geriatric Nutritional Risk Index were significantly lower in the low PMI group compared to the high PMI group, but no significant difference was noted for age, nutrition index, or chemotherapy response rates.
Similarly, in the comparison of IMAC, representing muscle quality, age was significantly higher in the high IMAC group. Details are presented in Table 1.
| All | Preoperative | Preoperative | P value | Preoperative | Preoperative | P value | |
| n = 131 | Low PMI, n = 36 | High PMI, n = 95 | High IMAC, n = 17 | Low IMAC, n = 114 | |||
| Age, yr | 64 (44-78) | 63 (50-75) | 65 (44-78) | 0.749 | 68 (44-74) | 64 (45-78) | 0.034 |
| Gender (male/female) | 120/11 | 28/8 | 92/3 | 0.001 | 15/2 | 105/9 | 0.635 |
| Preoperative body mass index | 21.4 (14.7-27.7) | 19.9 (14.7-24.2) | 21.6 (15.9-27.7) | 0.001 | 21.6 (18.4-25.6) | 21.2 (14.7-27.7) | 0.194 |
| PS ≥ 1 | 12 (9.2%) | 4 (11.1%) | 8 (8.4%) | 0.736 | 1 (5.9%) | 16 (9.6%) | 0.708 |
| ASA-PS (2/3) | 112/19 | 30/6 | 82/13 | 0.782 | 14/3 | 98/16 | 0.713 |
| Albumin (g/dL) | 4.1 (2.9-4.9) | 3.9 (3.1-4.8) | 4.1 (2.9-4.9) | 0.127 | 4.0 (3.5-4.5) | 4.1 (2.9-4.9) | 0.471 |
| CRP (mg/dL) | 0.11 (0.01-7.37) | 0.11 (0.02-7.37) | 0.11 (0.01-6.48) | 0.905 | 0.16 (0.20-4.53) | 0.10 (0.01-7.37) | 0.160 |
| Neutrophil-lymphocyte ratio | 1.66 (0.24-22.33) | 2.10 (0.29-14.06) | 1.54 (0.24-22.33) | 0.293 | 1.95 (0.59-7.73) | 1.54 (0.24-22.33) | 0.171 |
| Prognostic nutritional index | 49.05 (35.70-106.15) | 46.65 (35.70-68.00) | 49.30 (37.30-106.15) | 0.098 | 48.40 (40.75-53.35) | 49.33 (35.70-106.15) | 0.135 |
| mGPS (0/1/2) | 105/21/5 | 27/8/1 | 78/13/4 | 0.515 | 12/5/0 | 93/16/5 | 0.232 |
| GNRI | 105.9 (81.7-121.4) | 99.0 (86.8-108.8) | 107.2 (81.7-121.4) | 0.001 | 106.1 (86.1-121.4) | 105.4 (97.3-111.6) | 0.356 |
| Clinical T-stage (1/2/3/4) | 3/68/57/3 | 1/14/20/1 | 2/54/37/2 | 0.390 | 1/6/10/0 | 2/62/47/3 | 0.090 |
| Clinical N-stage (0/1/2/3) | 51/35/36/9 | 13/11/9/3 | 38/24/27/6 | 0.889 | 7/5/3/2 | 44/30/33/7 | 0.735 |
| Clinical stage (II/III) | 68/63 | 18/18 | 50/45 | 0.846 | 10/7 | 58/56 | 0.609 |
| Tumor response to chemotherapy | |||||||
| CR/PR/SD/PD | 2/79/42/8 | 0/21/13/2 | 2/58/29/6 | 0.852 | 0/6/9/2 | 2/73/33/6 | 0.090 |
| Pre NAC PMI | 4.94 (2.40-8.86) | 3.66 (2.40-4.43) | 5.39 (3.34-8.86) | 0.001 | 4.76 (3.28-6.64) | 5.01 (2.40-8.86) | 0.558 |
| Pre NAC IMAC | -0.46 (-1.07--0.19) | -0.47 (-1.07--0.28) | -0.46 (-1.06--0.19) | 0.248 | -0.32 (-0.75--0.19) | -0.48 (-1.07--0.33) | 0.001 |
| Operative time (min) | 443 (328-882) | 444 (328-882) | 443 (339-786) | 0.495 | 452 (328-882) | 440 (350-786) | 0.472 |
| Intraoperative bleeding (mL) | 730 (150-3015) | 652 (330-2550) | 732 (150-3015) | 0.258 | 750 (450-2550) | 718 (150-3015) | 0.247 |
| Postoperative complications (C-D ≥ 3) | |||||||
| Any complication | 53 (40.5%) | 16 (44.4%) | 37 (38.9%) | 0.690 | 5 (29.4%) | 48 (42.1%) | 0.430 |
| Respiratory complication | 28 (21.4%) | 8 (22.2%) | 20 (21.1%) | 1.000 | 4 (23.5%) | 24 (21.1%) | 1.000 |
| Anastomotic leakage | 3 (2.3%) | 2 (5.6%) | 1 (1.1%) | 0.183 | 0 (0%) | 3 (2.6%) | 1.000 |
| Pathological tumor grading | |||||||
| G1/G2/G3/Gx | 14/77/38/2 | 3/24/8/1 | 11/53/30/1 | 0.534 | 1/9/7/0 | 13/68/31/2 | 0.600 |
| pT-Stage (0/1/2/3/4) | 7/27/21/73/3 | 1/7/5/21/1 | 6/19/16/52/2 | 0.985 | 0/2/4/11/0 | 7/25/17/62/3 | 0.796 |
| pN-Stage (0/1/2/3) | 46/17/30/38 | 11/8/11/6 | 35/9/19/32 | 0.059 | 3/2/5/7 | 43/15/25/31 | 0.399 |
| pStage (0/1/2/3) | 4/18/36/73 | 0/6/10/20 | 4/12/26/53 | 0.640 | 0/2/2/13 | 4/16/34/60 | 0.279 |
| Reoperation | 4 (3.1%) | 1 (2.8%) | 3 (3.2%) | 1.000 | 0 (0%) | 4 (3.5%) | 0.651 |
| Length of hospital stay (d) | 18 (11-225) | 18 (11-225) | 18 (11-112) | 0.630 | 20 (11-225) | 18 (11-112) | 0.432 |
| 30 d mortality | 0 | 0 | 0 | 0 | 0 | ||
| 90 d mortality | 0 | 0 | 0 | 0 | 0 | ||
| Harvested number of LNs | 91 (42-194) | 102 (44-194) | 90 (42-184) | 0.360 | 82 (49-130) | 91 (42-194) | 0.092 |
A Clavien-Dindo classification of three or more severe complications was noted in 53 patients (40.5%). Failure of sutures was seen in 3 patients (2.3%), and respiratory complications were noted in 28 (21.4%). When comparing PMI, no significant difference was noted in operative time, blood loss, or postoperative complication. There were no significant differences in tumor-associated factors. The results of IMAC were similar (Table 1).
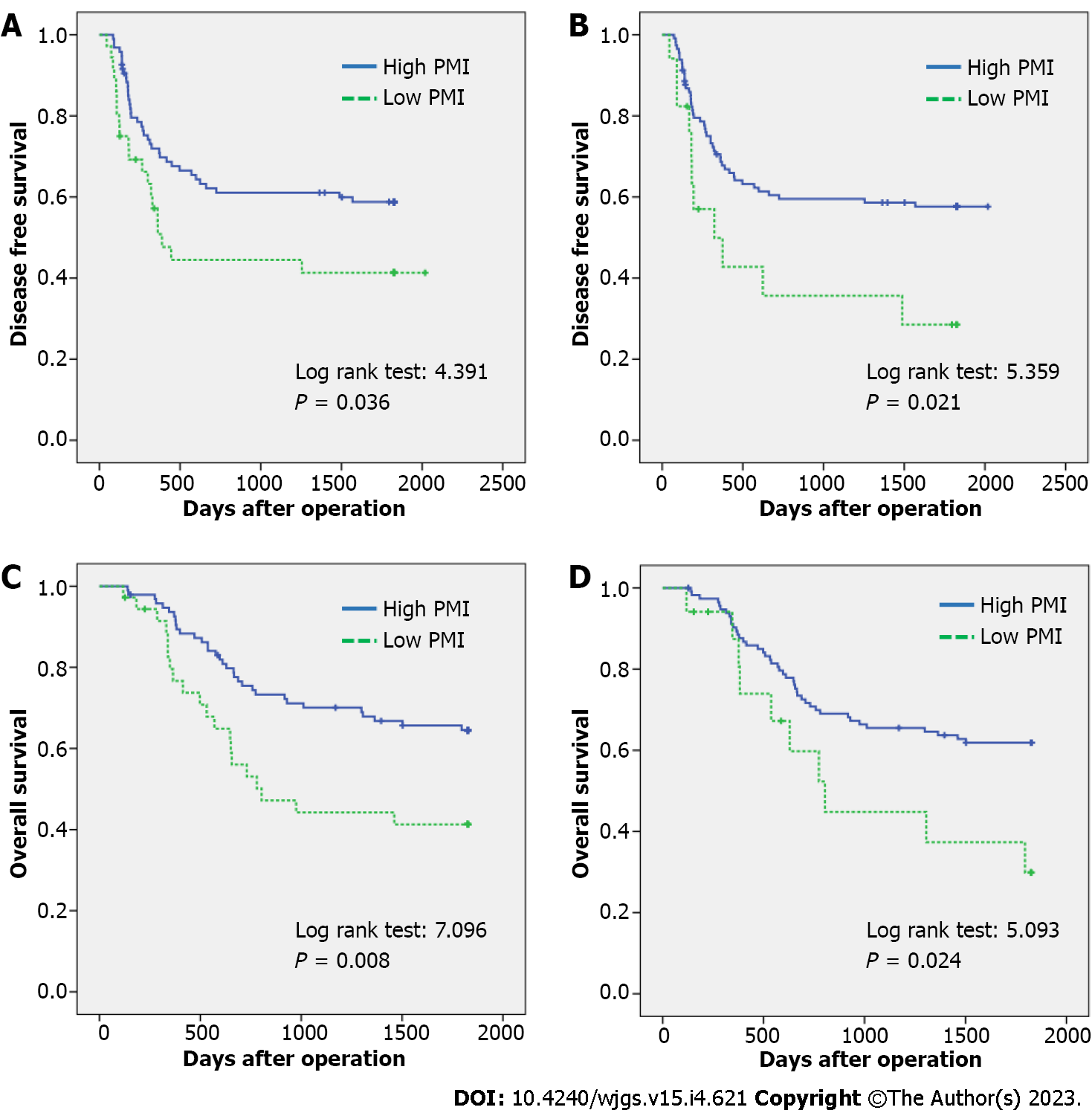
The 5-year DFS rates in the low and high PMI groups were 41.3% and 58.8%, respectively (P = 0.036). For IMAC, the 5-year DFS rates were 28.5% and 57.6% in the high and low IMAC groups, respectively (P = 0.021) (Figure 3A and B).
The 5-year OS rates in the low and high PMI groups were 41.3% and 64.5%, respectively (P = 0.008), showing a significant difference between the two groups. Regarding IMAC, the 5-year OS rates of the high group vs the low group were 29.9% and 61.9%, respectively (P = 0.024), which were significantly different (Figure 3C and D).
Univariate analysis of the OS rate revealed significant differences in patients aged 60 years or older (P = 0.018), those with pT3 or above disease (P = 0.021), and those with lymph node metastasis (P = 0.006). When these factors were subjected to multivariate analysis using the Cox proportional hazards model, pT3 or above [hazard ratio (HR): 1.966, 95% confidence interval (CI): 1.089-3.550, P = 0.025], low PMI (HR: 2.266, 95%CI: 1.282-4.006, P = 0.005), and high IMAC (HR: 2.089, 95%CI: 1.036-4.214, P = 0.022) were significantly different and regarded as independent poor prognostic factors (Table 2).
| 1-year survival | 3-year survival | 5-year survival | Univariate analysis | P | Multivariate analysis | P | ||
| Log rank | HR, 95%CI | |||||||
| Age, yr | ≥ 60 | 88.0 | 58.0 | 51.1 | 5.604 | 0.018 | 2.031 | 0.054 |
| < 60 | 91.9 | 75.7 | 75.7 | 0.986-4.183 | ||||
| BMI | < 22 | 92.0 | 64.0 | 59.8 | 0.108 | 0.743 | ||
| ≥ 22 | 87.3 | 62.6 | 57.4 | |||||
| PS | 0 | 89.8 | 65.7 | 59.6 | 1.640 | 0.200 | ||
| 1 | 81.8 | 45.5 | 45.5 | |||||
| ASA | 2 | 87.2 | 60.4 | 55.7 | 1.812 | 0.178 | ||
| 3 | 100.0 | 78.9 | 73.3 | |||||
| Stage | II | 86.4 | 65.2 | 59.0 | 0.051 | 0.821 | ||
| III | 91.9 | 61.0 | 57.6 | |||||
| PNI | ≥ 50 | 87.5 | 58.9 | 58.9 | 0.006 | 0.937 | ||
| < 50 | 90.3 | 66.5 | 57.9 | |||||
| NLR | ≥ 2.5 | 93.5 | 54.2 | 54.2 | 0.600 | 0.439 | ||
| < 2.5 | 87.7 | 66.0 | 59.8 | |||||
| mGPS | 0 | 88.4 | 64.9 | 59.9 | 0.493 | 0.483 | ||
| 1-2 | 92.0 | 56.0 | 52.0 | |||||
| GNRI | ≥ 98 | 87.8 | 62.1 | 56.8 | 0.498 | 0.480 | ||
| < 98 | 93.3 | 66.7 | 63.3 | |||||
| Operative time (min) | ≥ 450 | 89.1 | 63.2 | 55.4 | 0.039 | 0.843 | ||
| < 450 | 89.1 | 63.1 | 60.3 | |||||
| Blood loss (mL) | ≥ 730 | 93.7 | 64.8 | 61.4 | 0.570 | 0.450 | ||
| < 730 | 84.6 | 61.5 | 55.4 | |||||
| Postoperative complication | Present | 88.1 | 63.7 | 57.5 | 0.026 | 0.873 | ||
| Absent | 89.7 | 62.8 | 58.9 | |||||
| pT | 0-2 | 90.9 | 80.0 | 69.1 | 5.350 | 0.021 | 1.966 | 0.025 |
| 3- | 87.7 | 50.3 | 50.3 | 1.089-3.550 | ||||
| pN | 0 | 93.5 | 82.6 | 73.7 | 7.465 | 0.006 | 2.154 | 0.022 |
| 1- | 86.7 | 52.1 | 49.6 | 1.118-4.148 | ||||
| IMAC | < -0.40 | 89.4 | 65.5 | 61.9 | 5.093 | 0.024 | 2.089 | 0.022 |
| ≥ -0.40 | 87.4 | 44.8 | 29.9 | 1.036-4.214 | ||||
| PMI | ≥ 4.0 | 93.6 | 70.1 | 64.5 | 7.096 | 0.008 | 2.266 | 0.005 |
| < 4.0 | 76.7 | 44.2 | 41.3 | 1.282-4.006 |
The first finding of the present study was that lower skeletal muscle mass (low PMI) and changes in skeletal muscle quality (high IMAC) before preoperative chemotherapy had an impact on OS. To assess skeletal muscle mass, a cross-sectional area of the psoas muscle at the level of the lumbar spine L3 in the abdominal CT images before preoperative chemotherapy was used. Dual-energy X-ray absorptiometry and bioelectrical impedance analysis are methods to measure skeletal muscle mass. However, unlike CT imaging before preoperative chemotherapy, these methods require additional examination and raise the issue of invasive radiation exposure[31]. These measurement methods are not standardized for measuring muscle mass[32]. In addition, it has been reported that it is difficult to standardize and assess muscle quality[33-35].
In this study, the cross-sectional area of the psoas muscle at the lumbar L3 level was used to assess muscle mass. Essentially, it was necessary to assess muscle mass by volume rather than area. The cross-sectional area of the psoas muscle is maximal at the level of the lumbar spine L3 and thus can be assessed as representative of the volume[36,37]. There are systematic reviews/meta-analyses of sarcopenia using a technique that measures skeletal muscle mass at L3 in patients undergoing abdominal surgery. The method used to measure muscle mass in this study was reasonable because it is cited as a factor affecting perioperative complications and prognosis in previous reports[38,39].
The same lumbar spine L3 level in CT images used to assess skeletal muscle mass was also used to assess skeletal muscle. We calculated the degree of fat content within the multifidus muscle in those CT images based on the CT values. For this method, muscle quantity and quality were assessed at the same L3 level as abdominal CT imaging studies. The advantage was that no additional metrics were needed to assess changes in quality as a new parameter.
One modality of assessing muscle quality from CT images is the IMAC method[27,28], which evaluates the degree of fat content in muscle and quantifies the degree of fat degeneration. Fat degeneration of muscle has been reported to correlate with muscle weakness and loss of function[40,41]; for this reason, it can be used to assess muscle quality. In fact, muscle quality changes, determined by the IMAC method, have been reported as poor prognostic factors in nonalcoholic fatty liver disease[27], liver transplantation[42,43], hepatocellular carcinoma[44,45], pancreatic cancer[46], and cholangiocarcinoma diseases[13,47]. As mentioned above, it is reasonable to employ this same technique to assess the status of fatty degenerative changes in muscle and the relationship to the prognosis of patients undergoing preoperative chemotherapy for esophageal cancer.
In the present study, the results showed that a decrease in skeletal muscle mass and muscle quality changes affected OS. However, from previous reports on sarcopenia, the mechanism by which it affects the prognosis remains unclear. A cancer-bearing state is considered a systemic, chronic inflammatory condition. This may lead to the secretion of inflammatory cytokines interleukin (IL)-6, IL-8, tumor necrosis factor-alpha (TNF-α), and myostatin, and this may affect the entire body[48]. Increased secretion of the proinflammatory cytokine IL-6 itself and IL-6 mediated by TNF-α has been reported to reduce skeletal muscle mass[49]. It has also been reported that myostatin is a cytokine that potently reduces skeletal muscle, and its secretion increases in chronic inflammatory conditions, resulting in a decrease in skeletal muscle mass[50]. We consider that the combination of the effects of these cytokines leads to a malignant cycle of decreased skeletal muscle mass in a cancer-bearing state.
From an immunological point of view, IL-6, IL-8, and TNF-α cytokines are involved. IL-6 decreases the function of dendritic cells and T lymphocytes[51]. IL-8 and TNF-α also induce immunosuppressive myeloid-derived suppressor cells[52,53]. The actions of these cytokines are thought to suppress host immunity. Conversely, the secretion of IL-15, which is important for the maintenance of natural killer cell function, is reduced as a result of a decrease in the skeletal muscle, which is a secretory organ[54]. This inhibits the function of natural killer cells[55]. A decrease in IL-15 has been reported to increase adipose tissue[56], which may be linked to fat degeneration in muscle. We hypothesize that the chronic inflammatory state, which is a cancer-bearing state as described above, reduces skeletal muscle from inflammatory cytokines and, at the same time, suppresses immunity, which may worsen the prognosis.
Fat degeneration of muscle (a change in muscle quality) causes an increase in adipose tissue and the secretion of transforming growth factor-beta (TGF-β)[57]. It has been shown that TGF-β has an inhibitory effect on immune system cells such as T cells, B cells, natural killer cells, and dendritic cells, resulting in a suppression of immunity against cancer[58,59]. We presume that the long-term immunosuppressed state caused by the muscle mass loss and muscle quality changes may have resulted in a poor prognosis. These results suggest that muscle-strengthening interventions for patients with poor muscle composition may improve their prognosis in the future.
This study was limited to surgical cases of esophageal squamous cell carcinoma in Japanese patients who received NAC. Therefore, other races and adenocarcinomas were not included. The number of subjects analyzed was 131, which is not a large survey, and there is a male/female ratio imbalance.
Changes in skeletal muscle mass and muscle quality before NAC in esophageal squamous cell carcinoma in Japanese is a prognostic factor of OS.
Recently, muscle has been reported as an important prognostic factor. Not only muscle mass but also muscle quality has been reported to affect prognosis. Therefore, it is important to reveal how muscle composition is affected in patients undergoing preoperative chemotherapy for esophageal squamous cell carcinoma.
Esophageal cancer has a poor prognosis, and perioperative complications can be serious. It is important to consider prognostic factors in patients with esophageal cancer.
If body composition is a factor affecting prognosis, then preoperative chemotherapy and preoperative interventions can improve prognosis. In other words, a program to improve body composition before chemotherapy or before surgery can improve the prognosis of esophageal cancer patients. The objective was to determine the effect of muscle mass and quality on overall survival (OS) in esophageal squamous cell carcinoma.
In this study, we measured a cross-sectional area of the psoas muscle from computed tomography images. We evaluated muscle quality based on computed tomography values of the psoas muscle and subcutaneous fat. This was novel because both muscle mass and muscle quality were measured from the same image.
In this study, prognostic factors were found in patients who received preoperative chemotherapy for esophageal squamous cell carcinoma. Muscle mass as well as muscle quality and body composition before chemotherapy impacted disease-free survival and OS.
In this study, body composition was a prognostic factor for esophageal squamous cell carinoma patients. This suggests that muscle itself may be an immune system. Furthermore, the prognosis may be improved noninvasively if body composition is improved before chemotherapy or surgery.
Further studies are required to support our data. Randomized controlled trials to examine the prognostic change with and without the intervention of body composition improvement programs before chemotherapy and before surgery should be conducted.
Many thanks to Shari Joy Berman for her help in proofreading the English.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lin Q, China; Liu D, China; Liu Y, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15317] [Article Influence: 3063.4] [Reference Citation Analysis (4)] |
| 2. | Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360-7; discussion 368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 376] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Collard JM, Otte JB, Fiasse R, Laterre PF, De Kock M, Longueville J, Glineur D, Romagnoli R, Reynaert M, Kestens PJ. Skeletonizing en bloc esophagectomy for cancer. Ann Surg. 2001;234:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598-5606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 736] [Cited by in RCA: 747] [Article Influence: 62.3] [Reference Citation Analysis (8)] |
| 5. | Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil vs preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 1052] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 6. | Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, Svendsen LB, Christensen JF. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann Surg. 2018;268:58-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 252] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 7. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8471] [Article Influence: 564.7] [Reference Citation Analysis (0)] |
| 8. | Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2019;22:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 9. | Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 565] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 10. | Yoon SB, Choi MH, Song M, Lee JH, Lee IS, Lee MA, Hong TH, Jung ES, Choi MG. Impact of preoperative body compositions on survival following resection of biliary tract cancer. J Cachexia Sarcopenia Muscle. 2019;10:794-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Ninomiya G, Fujii T, Yamada S, Yabusaki N, Suzuki K, Iwata N, Kanda M, Hayashi M, Tanaka C, Nakayama G, Sugimoto H, Koike M, Fujiwara M, Kodera Y. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: A retrospective cohort study. Int J Surg. 2017;39:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle. 2019;10:111-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 13. | Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Fujimoto Y, Iida T, Yagi S, Taura K, Hatano E, Okajima H, Uemoto S. Impact of Skeletal Muscle Mass, Muscle Quality, and Visceral Adiposity on Outcomes Following Resection of Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2017;24:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 14. | Ida S, Watanabe M, Yoshida N, Baba Y, Umezaki N, Harada K, Karashima R, Imamura Y, Iwagami S, Baba H. Sarcopenia is a Predictor of Postoperative Respiratory Complications in Patients with Esophageal Cancer. Ann Surg Oncol. 2015;22:4432-4437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Nishigori T, Okabe H, Tanaka E, Tsunoda S, Hisamori S, Sakai Y. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol. 2016;113:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Ishida T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, Kurokawa Y, Motoori M, Kimura Y, Nakajima K, Mori M, Doki Y. Impact of measurement of skeletal muscle mass on clinical outcomes in patients with esophageal cancer undergoing esophagectomy after neoadjuvant chemotherapy. Surgery. 2019;166:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Makiura D, Ono R, Inoue J, Fukuta A, Kashiwa M, Miura Y, Oshikiri T, Nakamura T, Kakeji Y, Sakai Y. Impact of Sarcopenia on Unplanned Readmission and Survival After Esophagectomy in Patients with Esophageal Cancer. Ann Surg Oncol. 2018;25:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Nakashima Y, Saeki H, Hu Q, Tsuda Y, Zaitsu Y, Hisamatsu Y, Ando K, Kimura Y, Oki E, Mori M. Skeletal Muscle Loss After Esophagectomy Is an Independent Risk Factor for Patients with Esophageal Cancer. Ann Surg Oncol. 2020;27:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Mariette C, De Botton ML, Piessen G. Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol. 2012;19:2128-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 3839] [Article Influence: 274.2] [Reference Citation Analysis (0)] |
| 21. | Qiu Y, You J, Wang K, Cao Y, Hu Y, Zhang H, Fu R, Sun Y, Chen H, Yuan L, Lyu Q. Effect of whole-course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: A randomized control trial. Nutrition. 2020;69:110558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy vs surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 23. | Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, Ackland S, Gotley DC, Joseph D, Millar J, North J, Walpole ET, Denham JW; Trans-Tasman Radiation Oncology Group; Australasian Gastro-Intestinal Trials Group. Surgery alone vs chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 704] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 24. | Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 1053] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 25. | Sobin GM, Wittekind CH. Oesopgagus including Oesophagastric Junction. In: International Union Against Cancer TNM Classification of Malignant Tumors. 7nd ed. 2009: 66-72. |
| 26. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21618] [Article Influence: 1351.1] [Reference Citation Analysis (1)] |
| 27. | Kitajima Y, Eguchi Y, Ishibashi E, Nakashita S, Aoki S, Toda S, Mizuta T, Ozaki I, Ono N, Eguchi T, Arai K, Iwakiri R, Fujimoto K. Age-related fat deposition in multifidus muscle could be a marker for nonalcoholic fatty liver disease. J Gastroenterol. 2010;45:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Kitajima Y, Hyogo H, Sumida Y, Eguchi Y, Ono N, Kuwashiro T, Tanaka K, Takahashi H, Mizuta T, Ozaki I, Eguchi T, Kimura Y, Fujimoto K, Anzai K; Japan Nonalcoholic Fatty Liver Disease Study Group (JSG-NAFLD). Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol. 2013;28:1507-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24843] [Article Influence: 1183.0] [Reference Citation Analysis (0)] |
| 30. | Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M; STROBE initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163-W194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 1322] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 31. | Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D(3) -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 32. | Boshier PR, Heneghan R, Markar SR, Baracos VE, Low DE. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta-analysis. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015;74:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 34. | Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, Bautmans I, Bertière MC, Brandi ML, Al-Daghri NM, Burlet N, Cavalier E, Cerreta F, Cherubini A, Fielding R, Gielen E, Landi F, Petermans J, Reginster JY, Visser M, Kanis J, Cooper C. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 519] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 35. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7801] [Article Influence: 1300.2] [Reference Citation Analysis (1)] |
| 36. | Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1652] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 37. | Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018;8:11369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 334] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 38. | Jones K, Gordon-Weeks A, Coleman C, Silva M. Radiologically Determined Sarcopenia Predicts Morbidity and Mortality Following Abdominal Surgery: A Systematic Review and Meta-Analysis. World J Surg. 2017;41:2266-2279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 39. | Yang Z, Zhou X, Ma B, Xing Y, Jiang X, Wang Z. Predictive Value of Preoperative Sarcopenia in Patients with Gastric Cancer: a Meta-analysis and Systematic Review. J Gastrointest Surg. 2018;22:1890-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 40. | Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging. 2014;18:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | West MA, van Dijk DPJ, Gleadowe F, Reeves T, Primrose JN, Abu Hilal M, Edwards MR, Jack S, Rensen SSS, Grocott MPW, Levett DZH, Olde Damink SWM. Myosteatosis is associated with poor physical fitness in patients undergoing hepatopancreatobiliary surgery. J Cachexia Sarcopenia Muscle. 2019;10:860-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Hamaguchi Y, Kaido T, Okumura S, Fujimoto Y, Ogawa K, Mori A, Hammad A, Tamai Y, Inagaki N, Uemoto S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014;20:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 43. | Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, Kamo N, Okajima H, Uemoto S. Impact of Skeletal Muscle Mass Index, Intramuscular Adipose Tissue Content, and Visceral to Subcutaneous Adipose Tissue Area Ratio on Early Mortality of Living Donor Liver Transplantation. Transplantation. 2017;101:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 44. | Hamaguchi Y, Kaido T, Okumura S, Ito T, Fujimoto Y, Ogawa K, Mori A, Hammad A, Hatano E, Uemoto S. Preoperative intramuscular adipose tissue content is a novel prognostic predictor after hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2015;22:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Fujimoto Y, Ogawa K, Mori A, Hammad A, Hatano E, Uemoto S. Muscle Steatosis is an Independent Predictor of Postoperative Complications in Patients with Hepatocellular Carcinoma. World J Surg. 2016;40:1959-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, Hammad A, Mori A, Takaori K, Uemoto S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 47. | Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Kobayashi A, Iida T, Yagi S, Taura K, Hatano E, Uemoto S. Impact of the preoperative quantity and quality of skeletal muscle on outcomes after resection of extrahepatic biliary malignancies. Surgery. 2016;159:821-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 49. | Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 287] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 50. | Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 602] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 51. | Tsukamoto H, Fujieda K, Senju S, Ikeda T, Oshiumi H, Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018;109:523-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 52. | Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735-6741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1625] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 53. | Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, Wu H, Xu X, Erben U, Wu P, Syrbe U, Sieper J, Qin Z. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122:4094-4104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 54. | Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1093] [Cited by in RCA: 1118] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 55. | Patidar M, Yadav N, Dalai SK. Interleukin 15: A key cytokine for immunotherapy. Cytokine Growth Factor Rev. 2016;31:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 56. | Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 405] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 57. | Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3:37-48. [PubMed] |
| 58. | Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 1726] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 59. | Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 2012;4:535-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |