Published online Mar 27, 2023. doi: 10.4240/wjgs.v15.i3.488
Peer-review started: November 26, 2022
First decision: January 3, 2023
Revised: January 15, 2023
Accepted: February 16, 2023
Article in press: February 16, 2023
Published online: March 27, 2023
Processing time: 120 Days and 16.3 Hours
Xanthogranulomatous inflammation (XGI) is an uncommon process involving an accumulation of inflammatory cells, commonly lipid-laden macrophages. XGI has been described to occur throughout the body but only rarely in the lower gastrointestinal tract. We describe a case of XGI contributing to chronic ob
We report the case of a 42-year-old female who presented with intermittent epigastric pain and subjective fevers. She had undergone a laparoscopic small bowel resection for Meckel’s diverticulum five years prior. Her workup was notable for computed tomography scan demonstrating mild inflammation and surrounding stranding at the level of the prior anastomosis. She underwent a laparotomy, resection of the prior anastomosis and re-anastomosis, with final histopathological examination findings consistent with mural XGI.
XGI can occur at the site of a prior bowel anastomosis and cause chronic obstructive symptoms.
Core Tip: Xanthogranulomatous inflammation (XGI) is an uncommon inflammatory condition characterized by foamy histiocytes and other inflammatory cells. We report a rare case of XGI that occurred in the terminal ileum. Moreover, this is the first reported case of XGI associated with a prior bowel anastomosis. This case enhances our understanding of XGI and provides more insight into the pathophysiology of the condition.
- Citation: Wang W, Korah M, Bessoff KE, Shen J, Forrester JD. Xanthogranulomatous inflammation requiring small bowel anastomosis revision: A case report. World J Gastrointest Surg 2023; 15(3): 488-494
- URL: https://www.wjgnet.com/1948-9366/full/v15/i3/488.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i3.488
Xanthogranulomatous inflammation (XGI) is a rare benign condition involving an inflammatory response that can occur in multiple areas throughout the body[1,2]. Clinical presentations and imaging are generally nonspecific since XGI symptoms can vary depending on the organ system; as such, histopathological examination is necessary for a definitive diagnosis[3,4].
Though XGI is a benign process, it is important to consider this diagnosis in the differential for atypical masses because XGI can be challenging to distinguish from cancer, which may lead to avoidable radical treatments[1,3,5]. Therefore, a better understanding of the characteristics of XGI is urgently needed.
Herein, we present a case of a 42-year-old female with XGI at a prior small bowel anastomosis. While XGI has been reported in many organs[1,6], only a few cases of XGI in the lower gastrointestinal (GI) tract have been reported[1,7]. In addition, there have been no cases reported for XGI associated with a bowel anastomosis.
Intermittent mid-epigastric abdominal pain.
A 42-year-old female started having intermittent recurrent mid-epigastric abdominal pain episodes that resulted in multiple emergency department (ED) visits. Six months prior, she had presented to the ED with two days of intermittent epigastric pain and subjective fevers. At that time, she had no nausea or vomiting, her last bowel movement was the day prior to presentation, and she was passing flatus. On exam, she was hemodynamically stable with a benign physical exam. Her abdomen was soft, slightly distended, with moderate tenderness to palpation in the epigastric region, with no evidence of peritonitis. Her white blood cell count was 8.0 K/μL. She had a computed tomography (CT) scan of her abdomen and pelvis (AP) without contrast demonstrating her prior bowel anastomosis was focally dilated, with abnormal surrounding inflammatory stranding, along with mesenteric swirling around the anastomosis which raised suspicion for possible obstruction due to internal hernia. She underwent a diagnostic laparoscopy where an adhesive band of omentum to the prior small bowel anastomosis was lysed. All bowel was viable.
Following that surgery, her epigastric pain recurred, along with some nausea and vomiting. She had Helicobacter pylori (H. pylori) serum and stool antigen and antibody tests that were negative, and an abdominal radiograph demonstrated non-obstructive bowel gas pattern. Esophagogastroduodenoscopy (EGD) demonstrated a few localized non-bleeding erosions in the gastric antrum, with no stigmata of recent bleeding. A CT AP with intravenous contrast given after premedication demonstrated no acute pathologies that could explain her ongoing abdominal pain. She was discharged after she started tolerating oral intake and her pain improved. At a postoperative clinic visit a week later, her pain had completely resolved, and she was tolerating a regular diet without issues.
Unfortunately, her symptoms returned months later and led to multiple ED visits. During these episodes, she had no nausea or vomiting and no particular precipitating factors. She had been having dark stools, which had resolved after stopping ibuprofen, and otherwise had normal stools and flatus. Her recurrent symptoms prompted a thorough outpatient work-up.
Her past history was notable for depression, anxiety, iron deficiency anemia, cystitis, infectious mononucleosis, H. pylori infection, an unprovoked pulmonary embolism for which she had previously been on warfarin for a year, and prior Epstein-Barr virus (EBV) infection. Five years prior, she had a laparoscopic small bowel resection with a stapled side-to-side anastomosis for Meckel’s diverticulum. She also had a history of left ovarian cyst removal and bilateral inguinal hernia repairs. Three years prior, she had a colonoscopy and EGD performed for bright red blood per rectum, heartburn, epigastric and lower abdominal pain, nausea, and bilious emesis. These studies demonstrated very small internal hemorrhoids and erosive gastropathy but were otherwise normal.
The patient had never smoked, had used methamphetamine previously but quit 13 years prior, and drank one glass of wine a week. Her family history was notable for diabetes and stroke in her parents. She was allergic to penicillins, iodinated contrast media, and fish-containing products.
Her vital signs during her ED visits were within normal limits. Her abdominal exam was unremarkable at office visits when she was not experiencing an abdominal pain episode, but during her symptomatic episodes, she had mid-epigastric tenderness to palpation with no rebound or guarding.
During each ED visit, she was noted to have mild leukocytosis (11.8-15.9 K/μL), with otherwise overall unremarkable laboratory findings.
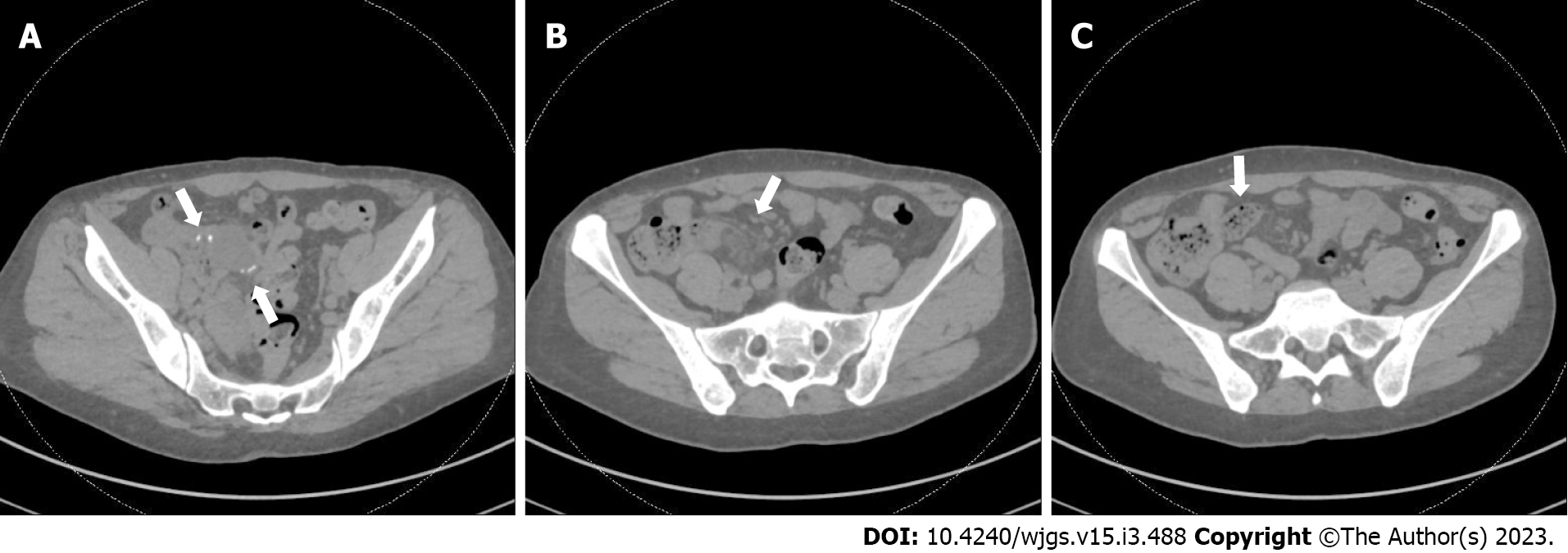
During one ED visit, a CT AP demonstrated mild inflammation and surrounding stranding at the level of the prior anastomosis (Figure 1A and B), along with small bowel fecalization (Figure 1C) concerning for possible infectious or inflammatory enteritis with nonspecific prominent mesenteric nodes.
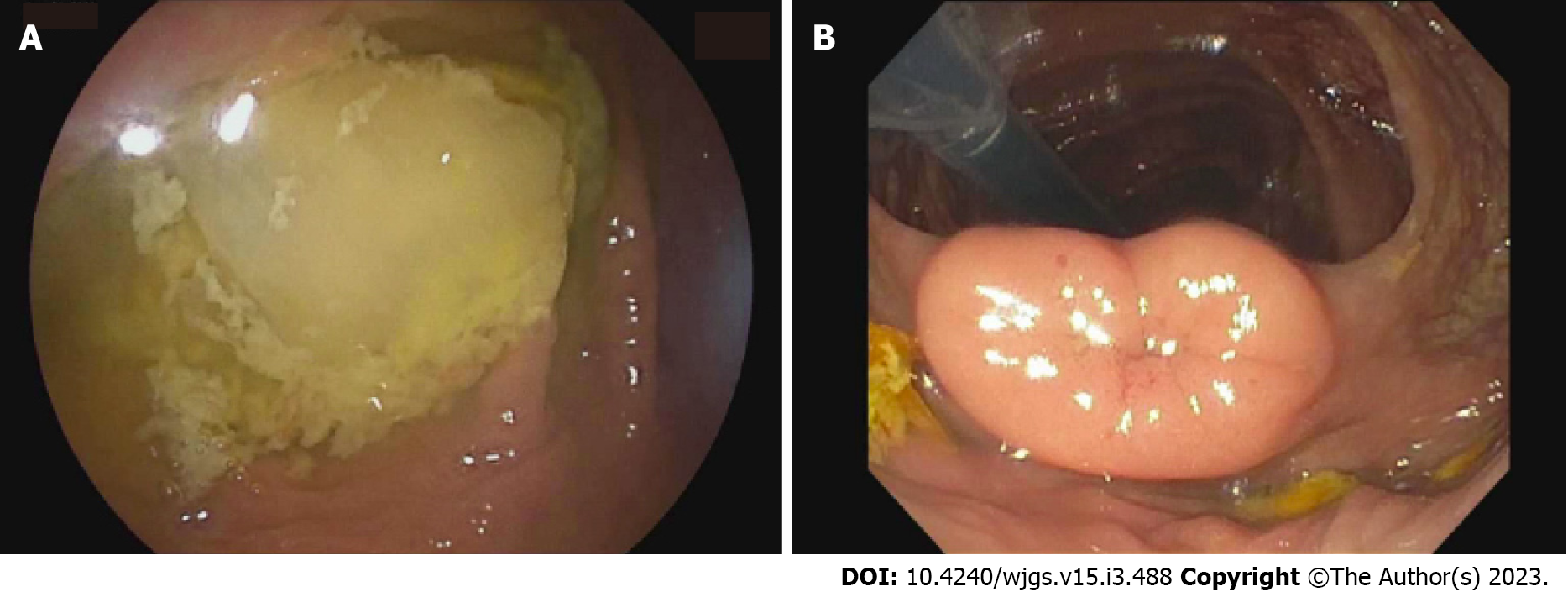
The patient underwent a lower double-balloon enteroscopy, where neither a double nor single enteroscope could be advanced beyond the terminal ileum (TI), though the examined colon and visualized approximately 3 cm of TI appeared normal (Figure 2).
Given that she failed nonoperative attempts to access her possible stricture, she underwent a laparotomy, resection of her prior small bowel anastomosis, and had a handsewn re-anastomosis.
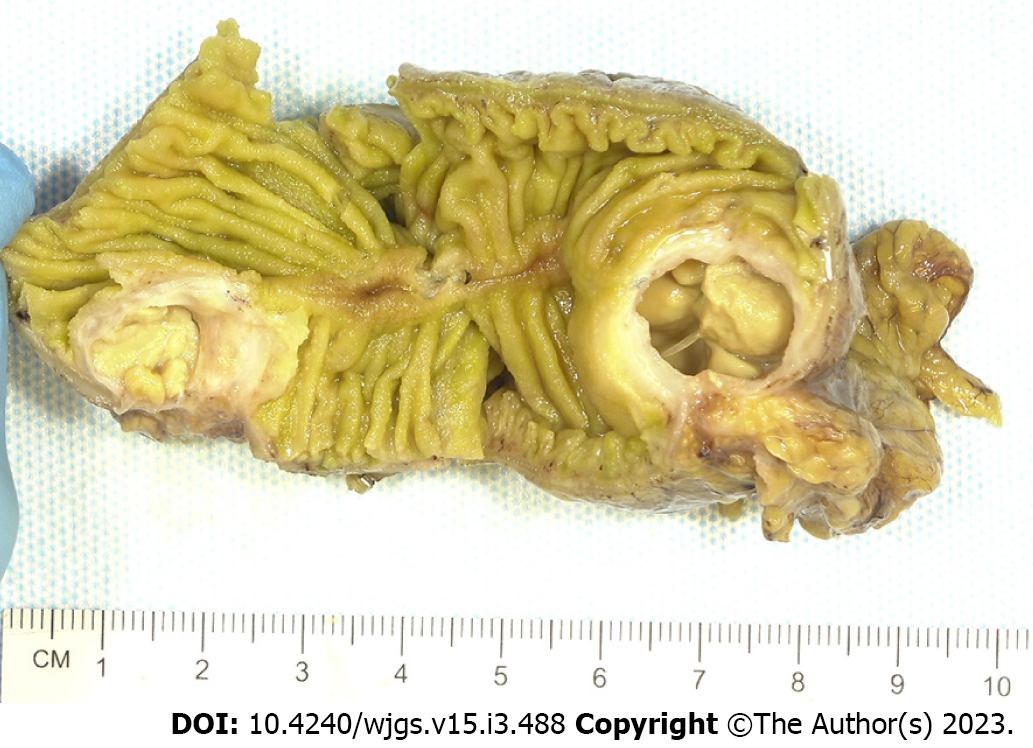
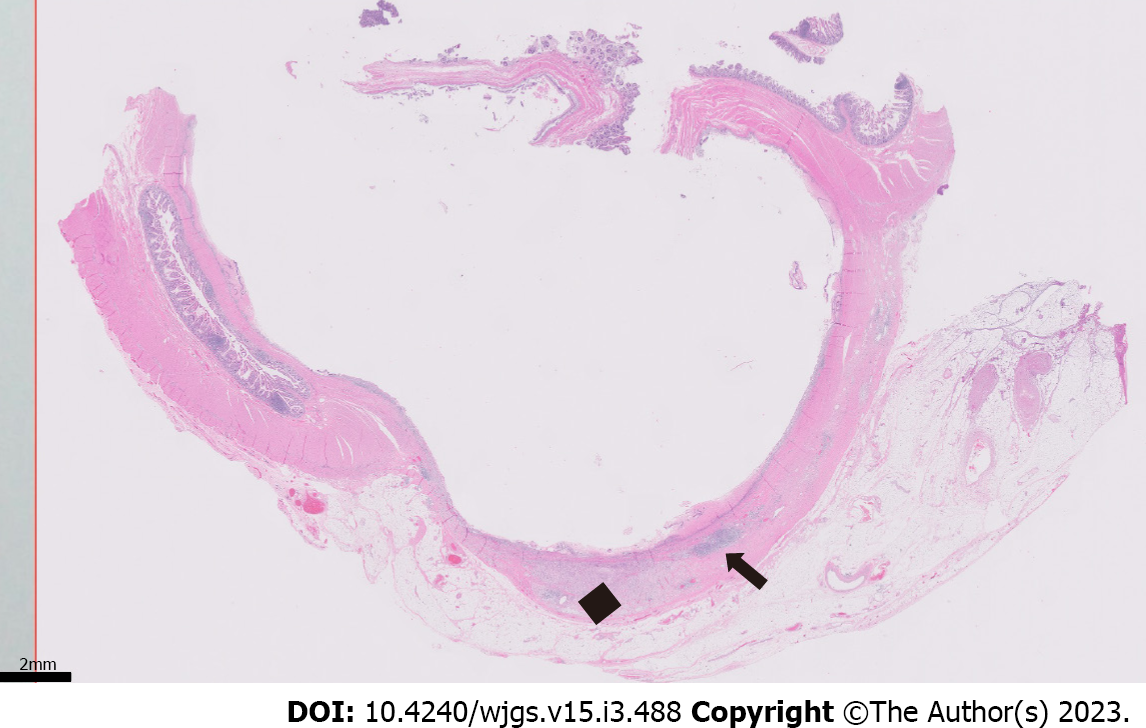
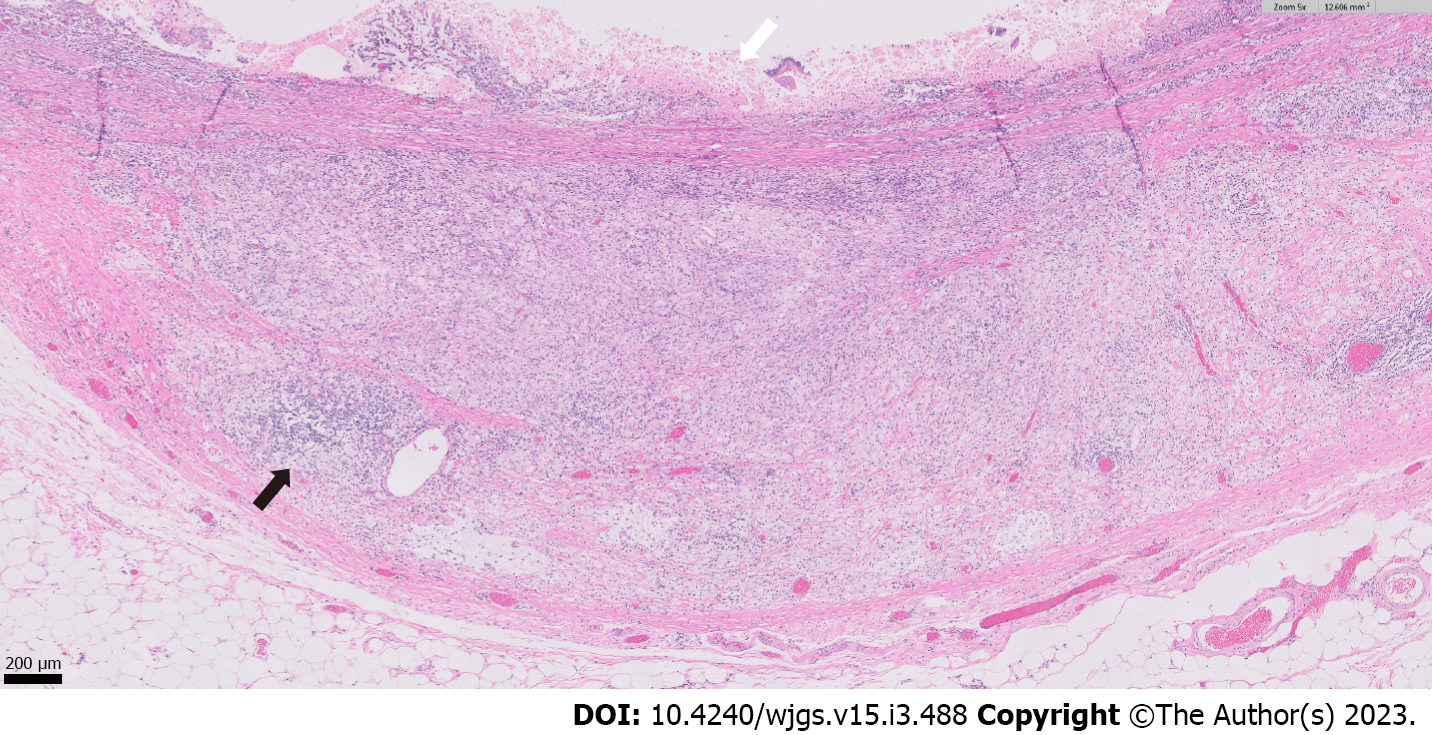
Histopathologic examination of the resected small bowel segment demonstrated a 7.5 cm × 5.3 cm × 4 cm portion of small bowel, with smooth, tan-brown serosa and tan-green mucosa with normal appearing folds (Figure 3). The prior anastomosis site was tan-pink with puckered area of mucosa. There was a cystic cavity abutting the prior anastomosis, which was tan-yellow with copious yellow-tan, viscous purulent cystic material, suggesting significant chronic inflammation underlying the anastomosis site. Microscopic analysis revealed significant infiltration by foamy macrophages, lymphocytes, and plasma cells with no neoplastic cells concerning for carcinoma (Figures 4 and 5). Overall, the histopathological examination findings were consistent with mural XGI.
Postoperatively, she developed ileus that self-resolved and was discharged eight days after surgery. At her postoperative clinic visit three weeks later, she was tolerating oral intake, having normal bowel movements, and only having some incisional pain with no obstructive symptoms. Eight months after that follow-up, she developed an incisional hernia that was repaired with mesh. At an outpatient follow-up clinic visit two weeks after the hernia repair surgery, she had some incisional pain but continued to have no obstructive symptoms.
XGI is a rare inflammatory process that requires histopathological examination for definitive diagnosis. Specifically, XGI usually presents, as it did in our patient, with yellow lesions on gross pathologic examination and is histologically defined by foamy histiocytes and various inflammatory cells, including activated plasma cells, lymphocytes, and sometimes multinucleated giant cells[6,8].
Although XGI has been reported in many organs, the majority of reported cases of XGI have been in the gallbladder, followed by occasional cases in the kidney and less commonly in the GI tract or other organs, such as the female genital tract or the head and neck[1,6]. Only a few cases of XGI in the lower GI tract have been reported[1,7]. In one, a patient presented with chronic abdominal pain mimicking appendiceal cancer on abdominal CT[1]. In that case, laparoscopic hemicolectomy revealed several golden-yellow and brown lesions, and histology confirmed XGI in the TI[1]. Interestingly, pathogenesis was attributed to chronic inflammation caused by microscopic perforations from a possible fish bone[1]. In another report, a patient presented with abdominal pain with ultrasound findings concerning for acute appendicitis. Diagnostic laparoscopy and midline laparotomy revealed an ileocecal mass concerning for invasive cancer. The mass was resected via hemicolectomy followed by a primary side-to-side anastomosis and was revealed to be XGI upon histologic examination. In that case presentation, no clear pathogenesis was established[7].
The exact pathophysiology of XGI is currently unknown, and various theories have been proposed, including abscess, hemorrhage, and necrosis, which may instigate the XGI response[2]. Chronic infection, abnormal lipid transport, and immunological disorders may also play a role[9]. Furthermore, recurrent inflammation due to a foreign body and obstruction has also been hypothesized and may have played a role in our patient[1,9].
Our patient’s XGI lesion was in the TI, and it is especially unique because the lesion was associated with a prior anastomosis. Though the exact mechanism by which this patient’s XGI developed is unclear, she had multiple risk factors that may have acted as triggers that elicited this response. For example, as described earlier, foreign body has been hypothesized to play a role in the development of XGI by causing microperforations leading to chronic inflammation and tissue damage[1]. In this patient’s case, the anastomosis itself may have triggered a foreign body response that led to XGI.
To our knowledge, there are no other cases where a stapled anastomosis was associated with XGI. However, it is possible the patient’s immune system reacted to the staples in such a way as to trigger XGI. This unusual reaction to the foreign body may be related to the patient’s prior EBV infection, which can impact the immune system, lead to inflammation, and trigger several autoimmune diseases[10,11]. It is unknown if performing a hand-sewn anastomosis initially could have prevented this. In fact, meta-analyses comparing both anastomosis techniques in emergency laparotomy and colorectal surgery have generally demonstrated little significant difference in outcomes[12,13]. However, further studies are needed to understand specific variations in foreign body responses that may be triggered by the two different techniques[12,13].
Another potential explanation for our patient’s XGI is that an obstructive process triggered it, perhaps from stricture at the anastomosis independent of the XGI lesion. Obstruction is recognized as a cause of xanthogranulomatous appendicitis, pyelonephritis, and cholecystitis[9]. On the other hand, the large inflammatory XGI mass may have formed as a response to another process, and the lesion then may have then led to this patient’s obstructive symptoms rather than the XGI lesion being a consequence of obstruction.
In conclusion, XGI is an uncommon inflammatory condition characterized by foamy histiocytes and other inflammatory cells. XGI occurs very rarely in the lower GI tract, and we describe such a case in the TI. To our knowledge, this is the first case report describing XGI associated with a prior anastomosis. This case is also an example where repeated pain and obstructive symptoms may necessitate anastomosis revision for definitive diagnosis and clinical resolution.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dayan D, Israel; Zhao K, China S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Yoon JS, Jeon YC, Kim TY, Han DS, Sohn JH, Nam KW, Nam YS, Pyo JY. Xanthogranulomatous inflammation in terminal ileum presenting as an appendiceal mass: case report and review of the literature. Clin Endosc. 2013;46:193-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Rammohan A, Cherukuri SD, Sathyanesan J, Palaniappan R, Govindan M. Xanthogranulomatous cholecystitis masquerading as gallbladder cancer: can it be diagnosed preoperatively? Gastroenterol Res Pract. 2014;2014:253645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Chen TP, Yi WL, Liu CS, Lin YH. Neck xanthogranuloma mimicking malignancy in a patient with diabetes mellitus: A case report and literature review. Medicine (Baltimore). 2018;97:e12615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Bourm KS, Menias CO, Ali K, Alhalabi K, Elsayes KM. Spectrum of Xanthogranulomatous Processes in the Abdomen and Pelvis: A Pictorial Review of Infectious, Inflammatory, and Proliferative Responses. AJR Am J Roentgenol. 2017;208:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Krishna M, Dayal S. Xanthogranulomatus inflammatory lesion mimicker of malignancy: A clinicopathological study from rural India. North Clin Istanb. 2021;8:485-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Wong KC, Tsui WM, Chang SJ. Xanthogranulomatous inflammation of terminal ileum: report of a case with small bowel involvement. Hong Kong Med J. 2015;21:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Zhang XS, Dong HY, Zhang LL, Desouki MM, Zhao C. Xanthogranulomatous inflammation of the female genital tract: report of three cases. J Cancer. 2012;3:100-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Dhawan S, Jain D, Kalhan SK. Xanthogranulomatous inflammation of ascending colon with mucosal involvement: report of a first case. J Crohns Colitis. 2011;5:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Chen MR. Epstein-barr virus, the immune system, and associated diseases. Front Microbiol. 2011;2:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Houen G, Trier NH. Epstein-Barr Virus and Systemic Autoimmune Diseases. Front Immunol. 2020;11:587380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 12. | Naumann DN, Bhangu A, Kelly M, Bowley DM. Stapled versus handsewn intestinal anastomosis in emergency laparotomy: a systemic review and meta-analysis. Surgery. 2015;157:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Neutzling CB, Lustosa SA, Proenca IM, da Silva EM, Matos D. Stapled versus handsewn methods for colorectal anastomosis surgery. Cochrane Database Syst Rev. 2012;CD003144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |