Published online Mar 27, 2023. doi: 10.4240/wjgs.v15.i3.420
Peer-review started: January 20, 2023
First decision: February 10, 2023
Revised: February 16, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: March 27, 2023
Processing time: 66 Days and 8 Hours
Emerging studies indicate the critical involvement of microorganisms, such as Epstein-Barr virus (EBV), in the pathogenesis of inflammatory bowel disease (IBD). Immunosuppressive therapies for IBD can reactivate latent EBV, com
To explore the clinical significance of latent EBV infection in IBD patients.
Latent EBV infection was determined by double staining for EBV encoded RNA and CD20 in colon specimens of 43 IBD patients who underwent bowel resection. Based on the staining results, the patients were divided into two groups, according to their latent EBV infection states - negative (n = 33) and positive (n = 10). Illness severity of IBD were assigned according to Crohn’s disease activity index (ulcerative colitis) and Mayo staging system (Crohn’s disease). The clinic-pathological data were analyzed between the two different latent EBV groups and also between the mild-to-moderate and severe disease groups.
Systolic pressure (P = 0.005), variety of disease (P = 0.005), the severity of illness (P = 0.002), and pre-op corticosteroids (P = 0.025) were significantly different between the EBV-negative and EBV-positive groups. Systolic pressure (P = 0.001), variety of disease (P = 0.000), pre-op corticosteroids (P = 0.011) and EBV infection (P = 0.003) were significantly different between the mild-to-moderate and severe disease groups.
IBD patients with latent EBV infection may manifest more severe illnesses. It is suggested that the role of EBV in IBD development should be further investigated, latent EBV infection in patients with serious IBD should be closely monitored, and therapeutic course should be optimized.
Core Tip: Inflammatory bowel disease (IBD) patients with latent Epstein-Barr virus (EBV) infection may manifest more severe illnesses. It is suggested that the role of EBV in IBD development should be further investigated, latent EBV infection in patients with serious IBD should be closely monitored, and therapeutic course should be optimized.
- Citation: Wei HT, Xue XW, Ling Q, Wang PY, Zhou WX. Positive correlation between latent Epstein-Barr virus infection and severity of illness in inflammatory bowel disease patients. World J Gastrointest Surg 2023; 15(3): 420-429
- URL: https://www.wjgnet.com/1948-9366/full/v15/i3/420.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i3.420
Inflammatory bowel disease (IBD) collectively refers to the chronic inflammation of the intestinal lining due to the altered immune response of the gut microbiota. The cases of IBD are increasing globally at an alarming rate in the 21st century[1]. Two major types of IBD, namely Crohn’s disease (CD) and ulcerative colitis (UC) are the most commonly occurring ones, seriously threatening global health burden. Therefore, understanding the pathogenesis of IBD plays an important role in the prevention and disease management. Emerging studies have indicated the critical involvement of microorganisms in the IBD pathogenesis and progression[2].
Epstein-Barr virus (EBV), also called human herpesvirus 4, infection accounts for one of the leading viral infections (approximately 90%) in humans. While most infections have been found to be involved in the oral route, other infection pathways like sexual transmission, transmission during blood transfusion, and organ transplantation have also been noted. EBV primarily targets resting B lymphocytes inducing their proliferation and polyclonal activities. Under this condition, adaptive immunity-associated cytotoxic T cells play important roles in regulating the EBV infection in the host. Notably, acutely infected individuals may present with infectious mononucleosis in a small cohort. Mostly, EBV integrates its DNA element into the memory B lymphocytes and establishes a lifelong latent infection status. While the virus can enter the lytic stage in individuals with compromised immune systems. In such cases, EBV may be reactivated, promoting the onset of virus-related malignancies, including Burkitt's lymphoma, Hodgkin's lymphoma, gastric cancer, and nasopharyngeal cancer subtypes[3]. In addition, EBV infection is associated with autoimmune diseases, such as multiple bone marrow fibrosis[4].
Moreover, the pathological connection between EBV and IBD has been receiving increasing attention in recent times[5,6]. Notably, immunosuppressive therapies, including anti-tumor necrosis factor alpha therapy, have been found to induce IBD pathogenesis and reactivate latent EBV[7], thereby increasing the susceptibility toward lymphocyte proliferation diseases. There is currently a lot of controversy regarding the direct involvement of EBV in inducing IBD and whether to include the EBV screening prior to IBD treatment initiation[8,9]. On the other hand, EBV co-infection may complicate the clinical course of IBD by aggravating the severity, chronicity, refraction to therapy, and increasing the recurrence rate of IBD[10]. However, most studies on EBV infection and IBD severity included a relatively small number of cases[6,11], and the clinical significance of EBV expression in B lymphocytes in the diseased intestinal tissue of IBD patients has not been discussed in detail.
Based on the findings from previous studies and the mode of EBV pathogenesis in relation to IBD, in this study, we examined 43 patients who underwent surgical resection, using EBV encoded RNA (EBER) and B lymphocyte (CD20) double staining technique to correlate the latent EBV infection and clinic-pathological data of IBD patients. Furthermore, we explored the clinical value of latent EBV infection in predicting the severity of IBD.
This study involved the retrospective review of 43 IBD patients’ demographics and basic clinical information, including characteristics, medical history, clinical data, biochemical data, and pathological information. The enrolled patients underwent bowel resection surgery in the Department of Surgery at Peking Union Medical College Hospital between July 2010 and September 2013. These patients were diagnosed with either CD or UC by both pathological and clinical examinations. Exclusion criteria of the study included: (1) Extraintestinal chronic diseases, such as chronic renal insufficiency, heart failure, cirrhosis and severe chronic obstructive pulmonary disease, etc.; (2) Medical history of immunodeficiency diseases, such as chronic infections and/or history of inflammatory diseases, including vasculitis, systemic lupus erythematosus, and rheumatologic condition; and (3) History of synchronous malignancies.
Collection data included the following parameters, patient age at the time of surgery, gender, past medical history, variety and severity of disease, cause of surgery, surgery procedure, symptoms, signs, complications, accompanying diseases, treatment course, and biochemical data. Illness severity of CD and UC were assigned according to CD activity index and Mayo staging system.
EBV latent infection was diagnosed based on the results of double staining of EBER and CD20 (specific biomarker of B lymphocytes) markers. The double staining was performed following the pre-optimized staining protocol. Briefly, the IBD samples were fixed in 10% buffered formalin, dehydrated in alcohol, and embedded in paraffin. Paraffin blocks were sectioned at 4 μm thickness. Routine hematoxylin and eosin staining was performed for histopathological examinations. For double staining with EBER and CD20, in situ hybridization (ISH) using 3,3'-diaminobenzidine (DAB) chromogen was first performed, followed by immunohistochemistry (IHC) for CD20 using Fast Red DAB chromogen. IHC was performed on an automated immunostainer (BOND-MAX, Leica Microsystems), according to the manufacturer’s protocols (Bond Polymer Refine RNA ISH protocol and Bond Polymer Refine Red IHC protocol, Leica Microsystems).
For each test sample, a second section (consecutive section wherever possible) was hybridized with a mixture of sense (non-complimentary) EBER probe as the negative control. The number of EBER positive cells, which were stained in the cell nucleus, were manually counted in the high-power field (HPF) for each optical field. B-lymphocytes cytomembrane showed positive staining for CD20 expression in IHC analysis.
Based on the double staining results, patients from the EBV latent infection (EBER positive) and EBV non-latent infection (EBER negative) were compared in terms of demographics, clinical characteristics, and biochemical findings. The clinic-pathological results were also compared between the patients with mild-to-moderate and severe disease symptoms. To analyze categorical variables, the χ2 test was used. Measurement data that met the normal distribution were compared using the t-test between the two groups, and measurement data that did not conform to the normal distribution were compared using the Mann-Whitney U test between the two groups. To perform statistical analyses, SPSS 25.0 (SPSS for Window, SPSS Inc, Chicago, IL) was adopted. A P < 0.05 indicated significant differences.
All clinic-pathological data are detailed in Table 1 and Table 2. Among the 43 IBD patients recruited to this study, there were 34 male- and 9 female patients. The age of the patients ranged from 13 to 70 years, and the mean age was 43.6 years. Among these patients, 20 patients had a history of smoking, and 17 patients had an alcohol drinking habit. Twenty-seven patients were diagnosed with CD and 16 patients with UC. The number of mild, moderate and severely affected patients were 10, 21, and 12, respectively. Three patients underwent surgery as they were seriously required, 9 patients were non-responsive to internal medicine therapy, and the other patients were medically required. Regarding the surgical procedures, 14 patients received laparoscopic partial enterectomy, and the other 29 patients had partial enterectomy through open surgery. The mean heart rate of these patients was 87.2 times/min, the median systolic pressure was 101 mmHg, and the mean diastolic pressure was 66.2 mmHg. At admission, 17 patients complained of fever, 42 patients reported abdominal pain, 29 patients had diarrhea, 20 patients had fecal occult blood, 15 patients had mucus or bloody purulent stool, 39 patients had a history of losing weight, 14 patients had abdominal mass, 2 patients had toxic megacolon, 14 patients had a gastrointestinal hemorrhage, 24 patients had intestinal obstruction, 9 patients had intestinal perforation, 8 patients had a perianal disease, and 16 patients had extraintestinal manifestations. Before surgery, 35 patients received aminosalicylic acid, 19 patients had corticosteroids, and 9 patients undertook immunosuppressive therapy. The mean treatment course was 73.6 d.
| Variables | Data |
| Characteristics | |
| Sex (Male/female) | 34/9 |
| Age (yr), mean ± SD | 43.6 ± 2.7 |
| Smoking (No/Yes) | 23/20 |
| Drinking (No/Yes) | 26/17 |
| Clinical data | |
| Crohn’s disease/ulcerative colitis | 27/16 |
| Location | |
| Crohn disease (L1/L2/L3/L4) | 9/0/18/0 |
| Ulcerative colitis (E1/E2/E3) | 0/2/14 |
| Severity of illness (Mild/moderate/severe) | 10/21/12 |
| Cause of surgery | |
| Patient required | 3 |
| Non-response to intern medicine therapy | 9 |
| Medical required | 31 |
| Intestinal obstruction | 18 |
| Fistula | 5 |
| Definite diagnosis requires | 1 |
| Gastrointestinal bleeding | 3 |
| Intestinal stenosis | 1 |
| Gastrointestinal perforation | 2 |
| Carcinogenesis | 1 |
| Pre-op aminosalicylic acid (No/Yes) | 8/35 |
| Pre-op corticosteroids (No/Yes) | 24/19 |
| Pre-op immunosuppressive therapy (No/Yes) | 34/9 |
| Surgical procedures (Laparoscopic/open surgery) | 14/29 |
| Course (d), mean ± SD | 73.6 ± 11.7 |
| Indicators to be explored | |
| Latent Epstein-Barr virus infection (Negative/positive) | 33/10 |
| Variables | Data |
| Vital signs | |
| Heart rate (time/min), mean ± SD | 87.2 ± 3.1 |
| Systolic pressure (mmHg), median (Q1, Q3) | 101.0 (94.5, 113.0) |
| Diastolic pressure (mmHg), mean ± SD | 66.2 ± 1.7 |
| Clinical manifestations | |
| Fever (No/Yes) | 26/17 |
| Abdominal pain (No/Yes) | 1/42 |
| Diarrhea (No/Yes) | 14/29 |
| Fecal occult blood (No/Yes) | 23/20 |
| Mucus or bloody purulent stool (No/Yes) | 28/15 |
| Lose weight (≥ 5 kg) (No/Yes) | 4/39 |
| Abdominal mass (No/Yes) | 29/14 |
| Toxic megacolon (No/Yes) | 41/2 |
| Gastrointestinal hemorrhage (No/Yes) | 29/14 |
| Intestinal obstruction (No/Yes) | 19/24 |
| Intestinal perforation (No/Yes) | 34/9 |
| Perianal disease (No/Yes) | 35/8 |
| Extraintestinal manifestations (No/Yes) | 27/16 |
| Oral ulcer (Yes) | 13 |
| Pyoderma gangrenosum (Yes) | 1 |
| Peripheral spondyloarthritis (Yes) | 6 |
| Others (Yes) | 2 |
| Biochemical data | |
| White blood cell (× 109/L), mean ± SD | 6.7 ± 0.6 |
| Neutrophil count (× 109/L), mean ± SD | 4.6 ± 0.5 |
| Hemoglobin (g/L), mean ± SD | 103.6 ± 4.5 |
| Platelets (× 109/L), mean ± SD | 343.2 ± 25.7 |
| Alanine aminotransferase (U/L), median (Q1, Q3) | 11.0 (8.0, 14.0) |
| Aspartate aminotransferase (U/L), median (Q1, Q3) | 14.0 (10.3, 18.8) |
| Albumin (g/L), mean ± SD | 30.6 ± 1.5 |
| Lactate dehydrogenase (U/L), median (Q1, Q3) | 127.0 (102.0, 140.0) |
| Creatinine (μmol/L), mean ± SD | 60.7 ± 3.0 |
| Potassium (mmol/L), mean ± SD | 3.8 ± 0.1 |
| Prothrombin time (s), mean ± SD | 12.2 ± 0.2 |
| Activated partial thromboplastin time (s), median (Q1, Q3) | 29.3 (26.5, 32.9) |
| C-reactive protein (g/L), median (Q1, Q3) | 26.3 (16.3, 71.4) |
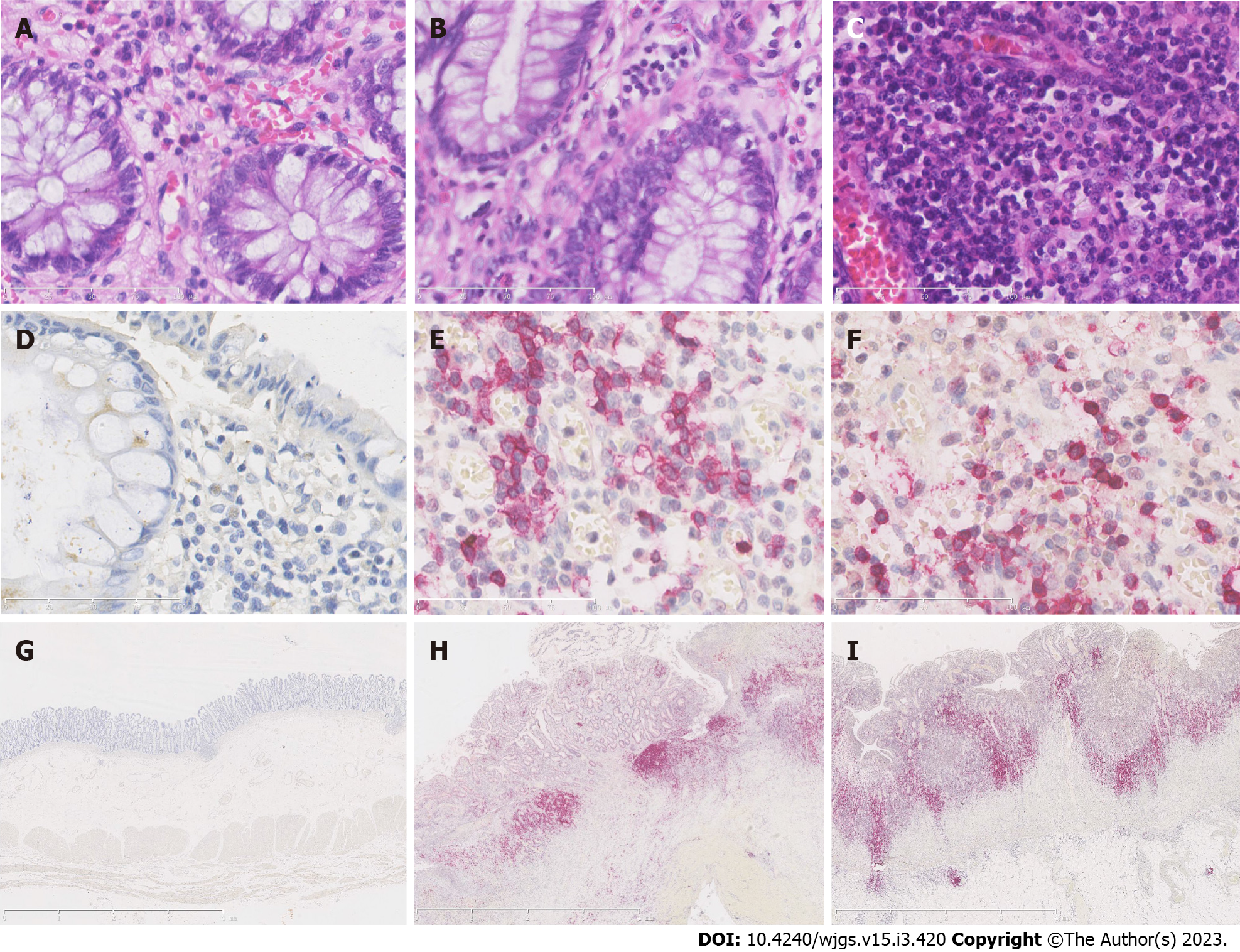
EBER positive staining, mainly distributed in the nucleus of B lymphocytes, suggested that the EBV latent infection status in the diagnosed patients. We found ten cases (23.3%) of IBD with EBV positive diagnosis, including 2 (7.4%) CD and 8 (50%) UC patients. Figure 1A, D and G show the normal control colon tissue.
In two cases of CD patients with EBV latent infection, numbers of EBER-positive B lymphocytes ranged from 8 to15 cells per HPF, and the positively stained cells were scattered throughout the field, accounting for 80%-90% EBER-positive B lymphocytes. The CD pathology was manifested as the full-wall inflammation, but EBER-positive B lymphocytes were mainly concentrated in the mucosa and submucosa (Figure 1B, E, and H).
In cases of 8 UC patients with EBV latent infection, numbers of EBER-positive B lymphocytes ranged from13 to 60 cells/HPF, and with a random distribution of the EBER-positive cells, accounting for 30%-70% cells per HPF. Like CD patients, EBER-positive B lymphocytes in UC patients were also concentrated in the mucosa and submucosa (Figure 1C, F, and I). Importantly, all cases of UC with EBV latent infection showed a full colon type pathology.
Clinico-pathological data of all patients were compared between the EBER-positive and EBER-negative expressions in B lymphocyte groups, according to the IHC results, as demonstrated in Table 3. Systolic pressure (P = 0.005), variety of disease (P = 0.005), the severity of illness (P = 0.002), and pre-op corticosteroids (P = 0.025) were significantly different between the two groups. While no significant difference was found between the two groups in terms of other characteristics, clinical and biochemical data.
| Variables | Epstein-Barr virus-negative | Epstein-Barr virus-positive | Statistical value (t/χ2) | P value |
| Systolic pressure (mmHg), mean ± SD | 106.56 ± 11.51 | 94.33 ± 7.71 | 2.991 (t) | 0.0051 |
| Variety of disease | 7.965 (Adjusted) | 0.0051 | ||
| Crohn disease | 25 | 2 | ||
| Ulcerative colitis | 8 | 8 | ||
| Severity of illness | 12.293 (Likelihood ratio) | 0.0021 | ||
| Mild | 8 | 2 | ||
| Moderate | 20 | 1 | ||
| Severe | 5 | 7 | ||
| Pre-op corticosteroids | 5.017 (Adjusted) | 0.0251 | ||
| No | 22 | 2 | ||
| Yes | 11 | 8 |
Clinico-pathological data of all patients were also compared between the mild-to-moderate and severe patient groups, as demonstrated in Table 4. Systolic pressure (P = 0.001), variety of disease (P = 0.000), pre-op corticosteroids (P = 0.011) and latent EBV infection (P = 0.003) were significantly different between the two groups, and no significant difference was found between the two groups in terms of other categories of characteristics, clinical and biochemical data.
| Variables | Mild/moderate (n = 31) | Severe (n = 12) | Statistical value (t/χ2) | P value |
| Systolic pressure (mmHg), mean ± SD | 107.33 ± 11.698 | 94.45 ± 7.71 | 3.482 (t) | 0.0011 |
| Variety of disease | 12.542 (Adjusted) | 0.0001 | ||
| Crohn disease | 25 | 2 | ||
| Ulcerative colitis | 6 | 10 | ||
| Pre-op corticosteroids | 6.408 | 0.0111 | ||
| No | 21 | 3 | ||
| Yes | 10 | 9 | ||
| Latent Epstein-Barr virus infection | 8.911 (Adjusted) | 0.0031 | ||
| Negative | 28 | 5 | ||
| Positive | 3 | 7 |
After excluding some confounding factors, our results showed that the latent EBV infection detected in colon tissue of IBD patients was positively related to the severity of the disease, and patients with latent EBV infection in their colon tissues were severely ill. Studies have confirmed that EBV can cause immune disorders, and IBD is one of the autoimmune diseases. Therefore, we hypothesized that EBV infection could aggravate the severity of IBD through complex immune mechanisms. In addition, advanced stage IBD patients with altered immune response and immunosuppressive therapy could also show increased susceptibility to EBV. Eventually, the immune imbalance following EBV infection and the resulting deterioration of the IBD stage are most likely to be mutually causal, leading to a vicious cycle of disease aggressiveness and poor prognosis. However, unlike the effect of EBV on multiple myeloma, we observed that not all the patients with severe IBD had EBV-positive diagnosis, which led us to postulate that EBV infection could be one of the critical factors in inducing severe IBD symptoms, but was not an indispensable etiological factor. In addition to the involvement of EBV, the severity of IBD must have involved other complex pathophysiological mechanisms. Based on our findings, we suggest that patients with latent EBV infection should be closely monitored, and the effect of EBV infection on the IBD patients should be given attention, and the pathogenesis between them should be clearly defined.
The clinic-pathological relationship between EBV and IBD has always attracted the attention of researchers and clinicians. For example, Dimitroulia et al[12], and Li et al[13], have shown that the prevalence of EBV in the intestinal tissue of patients with IBD is significantly higher than that in the control group. Moreover, patients with a high prevalence of EBV infection exhibit worsening disease symptoms, as compared to non-EBV infected patients who present remission incidences, suggesting that the severity of IBD may be related to the EBV infection[12,13]. In addition, it has also been found that the positive expression of EBV in IBD patients is higher in refractory patients than in the control group. Further, the higher EBV positive expression has been linked to the mucous damage and high clinical indexes of activity[6,11,14]. Our results were consistent with these findings, suggesting that the positive expression of EBV in latent infection might be related to the severity of IBD. However, most of the above studies used specimens from patients' serum or colonoscopy biopsies, while the specimens used in our study were specimens removed from bowel surgery. Relative to the determination of EBV in serum, EBV in bowel resection specimens can better reflect the direct role of EBV in the IBD pathology. In addition, compared to the EBV determination in colonoscopy biopsy specimens, the EBV of the bowel resection specimens can better reflect the EBV infiltration of the entire layer of the bowel wall. Therefore, our results based on the bowel resection specimens were more reliable and precise. We also obtained morphological data of EBV by IHC analysis of the colon wall of IBD patients to determine the exact location of EBV in the B lymphocytes of intestinal tissue, which was also the verification and supplementary to the previous studies.
In our study, we found that latent EBV infection rates were related to the pre-op corticosteroid administration. Crosstalk between EBV-positivity and corticosteroid administration has also been found in another study[14]. We thought two aspects should be considered to explain these reasons. Firstly, latent EBV infection might be related to the severity of the disease, and the disease severity was directly proportional to the increasing dose of corticosteroids, indicating EBV infection was related to corticosteroids. Secondly, following the corticosteroid therapy, the immunosuppressive condition of patients might have increased their susceptibility toward EBV infection. However, the exact reason is needed to be confirmed by cohort research in the future.
Interestingly, we also found that the proportion of latent EBV infection in UC patients was higher than that in CD patients. In addition, all UC patients with latent EBV infection exhibited full colon type pathology. The reason for the difference in the proportion of EBV latent infection in the UC and CD patients and the relationship between latent EBV infection and subtypes in UC patients are worthy of further research and discussion.
So far, there is no consensus on whether all IBD patients mandatorily require EBV infection testing at the early stage. At present, the detection of EBV infection in IBD patients is mainly focused on patients who require azathioprine treatment, which may increase the activation of EBV and the incidence of related lymphatic system proliferative diseases. Studies suggest that before starting to use azathioprine, detection of EBV serology should be performed first, following the counting of natural killer cells during treatment to determine whether the patient has the risk of developing an abnormally serious primary EBV infection and EBV-related malignancies[5,8,15-17]. But other studies have also shown that the activation of EBV has no direct connection with the impact of immunosuppressive therapy[13,18]. Notably, the exposure to EBV in adulthood is almost universal, and the incidences of hematological malignancies in IBD are rare. In the cost-benefit analysis, it seems that the value of EBV detection before the IBD patient starts treatment is limited[9,19-21]. Our results suggest that it is important not only to consider the activation of EBV by immunosuppressive drugs that can lead to related malignant tumors but also to understand the relationship between EBV infection and the severity of IBD. With regard to the question of whether to carry out EBV testing first in IBD patients, we suggest that determination should be made after the role of EBV infection on IBD progression rate and deterioration are clarified, or it can be used in high-risk patient populations (such as patients with full colon UC or those who require corticosteroid therapy for the disease).
In addition to the aforementioned obstacles, there are still several unsolved questions about the relation of EBV infection with IBD severity. For example, whether IBD patients with latent EBV infection should be treated for EBV. And recent studies have shown that different EBV strains have inconsistent pathogenic effects on nasopharyngeal carcinoma[22]. However, the exact causal relationship between different EBV strains and the progression of IBD is not clear yet.
Small sample size and one-center study were also considerable drawbacks of this retrospective study. A large sample size and multi-center studies to explain the serious relationship between latent EBV infection and the condition of IBD patients are needed for detailed investigation in the future.
In conclusion, our findings indicated that IBD patients with latent EBV infection might manifest severe symptoms. We suggest that the role of EBV in IBD development should be further investigated, and latent EBV infection in patients with serious IBD should be closely monitored and optimized treatment.
Emerging studies indicate the critical involvement of microorganisms, such as Epstein-Barr virus (EBV), in the pathogenesis of inflammatory bowel disease (IBD). Immunosuppressive therapies for IBD can reactivate latent EBV, complicating the clinical course of IBD.
This study explored the clinical significance of EBV expression in B lymphocytes derived from IBD patients’ intestinal tissues in detail.
This study aimed to explore the clinical significance of latent EBV infection in IBD patients.
Double staining for EBV encoded RNA and CD20 to determine latent EBV infection. The clinic-pathological data were analyzed between the two different latent EBV groups and also between the mild-to-moderate and severe disease groups.
Systolic pressure, variety of disease, the severity of illness, and pre-op corticosteroids were significantly different between the EBV-negative and EBV-positive groups. Systolic pressure, variety of disease, pre-op corticosteroids and EBV infection were significantly different between the mild-to-moderate and severe disease groups.
Latent EBV infection is positively related to severity of IBD illness.
The role of EBV in IBD development should be further investigated; latent EBV infection in patients with serious IBD should be closely monitored.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dash S, India; Yumiba T, Japan S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4105] [Article Influence: 513.1] [Reference Citation Analysis (110)] |
| 2. | Azimi T, Nasiri MJ, Chirani AS, Pouriran R, Dabiri H. The role of bacteria in the inflammatory bowel disease development: a narrative review. APMIS. 2018;126:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Dunmire SK, Verghese PS, Balfour HH Jr. Primary Epstein-Barr virus infection. J Clin Virol. 2018;102:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 281] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 4. | Dugan JP, Coleman CB, Haverkos B. Opportunities to Target the Life Cycle of Epstein-Barr Virus (EBV) in EBV-Associated Lymphoproliferative Disorders. Front Oncol. 2019;9:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | de Francisco R, Castaño-García A, Martínez-González S, Pérez-Martínez I, González-Huerta AJ, Morais LR, Fernández-García MS, Jiménez S, Díaz-Coto S, Flórez-Díez P, Suárez A, Riestra S. Impact of Epstein-Barr virus serological status on clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Pezhouh MK, Miller JA, Sharma R, Borzik D, Eze O, Waters K, Westerhoff MA, Parian AM, Lazarev MG, Voltaggio L. Refractory inflammatory bowel disease: is there a role for Epstein-Barr virus? Hum Pathol. 2018;82:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Lapsia S, Koganti S, Spadaro S, Rajapakse R, Chawla A, Bhaduri-McIntosh S. Anti-TNFα therapy for inflammatory bowel diseases is associated with Epstein-Barr virus lytic activation. J Med Virol. 2016;88:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Honkila M, Niinimäki R, Taskinen M, Kuismin O, Kettunen K, Saarela J, Turunen S, Renko M, Tapiainen T. A nearly fatal primary Epstein-Barr virus infection associated with low NK-cell counts in a patient receiving azathioprine: a case report and review of literature. BMC Infect Dis. 2019;19:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Barnes EL, Herfarth HH. The Usefulness of Serologic Testing for Epstein-Barr Virus Before Initiation of Therapy for Inflammatory Bowel Disease. Gastroenterology. 2017;153:1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Wu S, He C, Tang TY, Li YQ. A review on co-existent Epstein-Barr virus-induced complications in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2019;31:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Ciccocioppo R, Racca F, Scudeller L, Piralla A, Formagnana P, Pozzi L, Betti E, Vanoli A, Riboni R, Kruzliak P, Baldanti F, Corazza GR. Differential cellular localization of Epstein-Barr virus and human cytomegalovirus in the colonic mucosa of patients with active or quiescent inflammatory bowel disease. Immunol Res. 2016;64:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Dimitroulia E, Pitiriga VC, Piperaki ET, Spanakis NE, Tsakris A. Inflammatory bowel disease exacerbation associated with Epstein-Barr virus infection. Dis Colon Rectum. 2013;56:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Li X, Chen N, You P, Peng T, Chen G, Wang J, Li J, Liu Y. The Status of Epstein-Barr Virus Infection in Intestinal Mucosa of Chinese Patients with Inflammatory Bowel Disease. Digestion. 2019;99:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Ciccocioppo R, Racca F, Paolucci S, Campanini G, Pozzi L, Betti E, Riboni R, Vanoli A, Baldanti F, Corazza GR. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: need for mucosal viral load measurement. World J Gastroenterol. 2015;21:1915-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Hyams JS, Dubinsky MC, Baldassano RN, Colletti RB, Cucchiara S, Escher J, Faubion W, Fell J, Gold BD, Griffiths A, Koletzko S, Kugathasan S, Markowitz J, Ruemmele FM, Veereman G, Winter H, Masel N, Shin CR, Tang KL, Thayu M. Infliximab Is Not Associated With Increased Risk of Malignancy or Hemophagocytic Lymphohistiocytosis in Pediatric Patients With Inflammatory Bowel Disease. Gastroenterology. 2017;152:1901-1914.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 16. | de Francisco R, Castaño-García A, Riestra S. Editorial: which inflammatory bowel disease patients should be screened for Epstein-Barr virus infection? Aliment Pharmacol Ther. 2018;48:1159-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Goetgebuer RL, van der Woude CJ, de Ridder L, Doukas M, de Vries AC. Clinical and endoscopic complications of Epstein-Barr virus in inflammatory bowel disease: an illustrative case series. Int J Colorectal Dis. 2019;34:923-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Rodríguez-Lago I, Merino O, López de Goicoechea MJ, Aranzamendi M, Zubiaurre L, Muro N, Ortiz de Zárate J, Cilla G, Cabriada JL. Immunosuppression for inflammatory bowel disease does not influence Epstein-Barr viral load in the short-term. Gastroenterol Hepatol. 2019;42:542-547. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Chapman S, El-Matary W. Screening for Epstein-Barr Virus Status and Risk of Hemophagocytic Lymphohistiocytosis in Children With Inflammatory Bowel Disease on Azathioprine. Gastroenterology. 2017;153:1167-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Hans AK, Scott FI. Editorial: which inflammatory bowel disease patients should be screened for Epstein-Barr virus infection? Aliment Pharmacol Ther. 2018;48:1158-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Ahmed T, Brown F, Ahmed R, Shah A, Whitehead S, Steed H, Brookes MJ. Letter: impact of Epstein-Barr virus serological status on clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:476-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 22. | Xu M, Yao Y, Chen H, Zhang S, Cao SM, Zhang Z, Luo B, Liu Z, Li Z, Xiang T, He G, Feng QS, Chen LZ, Guo X, Jia WH, Chen MY, Zhang X, Xie SH, Peng R, Chang ET, Pedergnana V, Feng L, Bei JX, Xu RH, Zeng MS, Ye W, Adami HO, Lin X, Zhai W, Zeng YX, Liu J. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat Genet. 2019;51:1131-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |