Published online Mar 27, 2023. doi: 10.4240/wjgs.v15.i3.398
Peer-review started: November 24, 2022
First decision: December 10, 2022
Revised: December 18, 2022
Accepted: February 14, 2023
Article in press: February 14, 2023
Published online: March 27, 2023
Processing time: 123 Days and 5.6 Hours
Hepatic alveolar echinococcosis (HAE) is a serious zoonotic infection that affects humans. It may have a tumor-like appearance at times. Percutaneous treatment of HAE patients is extremely relaxing for them. HAE is a significant human zoonotic infection caused by the fox tapeworm Echinococcus Multilocularis larvae. It possesses the characteristics of an invasive tumor-like lesion due to its infiltrative growth pattern and protracted incubation period. The disease is endemic over central Europe, Asia, and North America.
To characterize HAE patients who were treated percutaneously, their outcomes, and the major technical features of percutaneous treatment in HAE.
Patients who were treated with percutaneous cyst drainage and/or percutaneous biliary drainage were included in the study. Uncorrected abnormal coagulation values and solid or non-infected HAE with minor necrotic change were excluded.
Thirty-two patients underwent percutaneous cyst drainage, two patients underwent percutaneous biliary drainage, and four patients underwent percutaneous biliary drainage alone. Interventional radiology is utilized to drain echinococcal necrosis and abscesses within/without the liver, as well as diseased and clogged bile ducts.
Percutaneous drainage of cyst contents and/or biliary channels using a minimally invasive technique is a very beneficial. Percutaneous cyst drainage with albendazole therapy improves quality of life in patients who are unable to undergo surgery, even when the mass resolves with long-term treatment.
Core Tip: Interventional radiology is utilized to drain echinococcal necrosis and abscesses within/without the liver either as palliative operations or as a bridge to radical resection. Percutaneous cyst drainage with albendazole therapy improves quality of life in patients who are unable to undergo surgery, even when the mass resolves with long-term treatment.
- Citation: Eren S, Aydın S, Kantarci M, Kızılgöz V, Levent A, Şenbil DC, Akhan O. Percutaneous management in hepatic alveolar echinococcosis: A sum of single center experiences and a brief overview of the literature. World J Gastrointest Surg 2023; 15(3): 398-407
- URL: https://www.wjgnet.com/1948-9366/full/v15/i3/398.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i3.398
Hepatic alveolar echinococcosis (HAE) is a significant human zoonotic infection caused by the fox tapeworm Echinococcus Multilocularis larvae (EM). It possesses the characteristics of an invasive tumor-like lesion due to its infiltrative growth pattern and protracted incubation period. The disease is endemic over central Europe, Asia, and North America[1,2].
Percutaneous sterilization procedures, surgery, medication treatment, a “wait-and-see” approach, or a combination of these are available for management. In comparison, the clinical signs of alveolar echinococcosis (AE) are similar to those of a malignant, silently progressive liver disease, with local tissue infiltration and metastasis. Structured treatment is critical for AE management, which involves WHO staging, pharmacological therapy, and at least a decade of follow-up[3].
While excision of lesions or liver transplantation (LT) is the most successful treatment option when operable, the majority of patients require palliative care prior to open surgery due to the presence of comorbidities. Typically, the disease is identified at an irreversible stage. In certain instances, invasion of the bile ducts and arteries, as well as necrosis in the lesion's center, result in serious consequences such as cholangitis and liver abscesses. Palliative surgery has been shown to have little advantage in terms of care, while percutaneous and endoscopic techniques have increased in popularity. In these patients, percutaneous draining of the complex cyst and biliary tree may be employed as a minimally invasive technique[2,4-6].
The purpose of this study is to characterize the methods to treat HAE patients, the outcomes of the treatment options, and the major technical features of percutaneous treatment in HAE.
Electronic archives were retrospectively evaluated to define the treatment options of the HAE patients between January 2012-December 2021. Patients were classified under two main subgroups: (1) surgical treatment: complete surgical excision and antihelmintic therapy, partial resection and antihelmintic therapy, LT; and (2) Interventional radiologic treatment: percutaneous cyst drainage, percutaneous cyst drainage with percutaneous biliary drainage, percutaneous biliary drainage only.
The first diagnosis was made mostly on the basis of conventional imaging findings such as computed tomography (CT) scans in three phases (hepatic artery, portal vein, and hepatic vein); ultrasound (US); immunoserologic testing with enzyme-linked immunosorbent assay; and, in some cases, magnetic resonance imaging (MRI).
Age, gender data and the presence/frequency of the complications were noted.
Interventional radiologic treatment: Uncorrected abnormal coagulation values and solid or non-infected HAE with minor necrotic change were the main contraindications for interventional radiologic treatment. The big necrotic cyst or infected cyst with or without mass effect on the biliary tree and surrounding arteries were the selection criteria for percutaneous cyst drainage.
All cases were reviewed for percutaneous access to the cyst, application route, and selection of the appropriate imaging modality for guidance prior to draining. Generally, we preferred US entry advice. We chose CT guidance for cysts that were difficult to visualize with US (due to thick calcification of the cyst wall or conspicuous gas within the cyst). Patients with abnormal coagulation parameters were handled as soon as possible after hematologic correction. In situations of cholangitis or biliary obstruction due to mass invasion, percutaneous biliary drainage was performed. Seldinger's method was used to install drainage catheters (8-10 Fr). We employed both intercostal and subcostal techniques.
The Statistical Package for Social Sciences for Windows 20 software was used to analyze the data (IBM SPSS Inc., Chicago, IL, United States). The Kolmogorov-Smirnov test was used to determine whether the data conformed to a normal distribution. Numerical variables with a normal distribution were represented as mean ± SD values and categorical variables as number (n) and percentage values (%). Age was compared across groups using the student's t test, and the frequency of complications was analyzed using the Chi-square test according to subgroups.
The current study included 125 patients, 67 (53.6%) of whom were female and 58 (46.4%) of whom were male. Mean age of the population was 53.6 ± 8.4 years, median age was 63 years (min-max; 41-82 years).
Table 1 shows the detailed distribution of patients based on treatment options.
| Surgical treatment | Interventional radiologic treatment | ||
| Complete surgical excision and antihelmintic therapy | 60 (48) | Percutaneous cyst drainage | 32 (25.6) |
| Partial resection and antihelmintic therapy | 23 (18.4) | Percutaneous cyst drainage with percutaneous biliary drainage | 2 (1.6) |
| Liver transplantation | 4 (3.2%) | Percutaneous biliary drainage only | 4 (3.2) |
| Total | 87 (69.6) | Total | 38 (30.4) |
Mean age of the patients was 48.8 ± 3.4 years. 45 patients (51.7%) were female and 42 (48.2%) patients were male.
Complications were discovered in 15 (17.2%) patients: Fever (8 patients), hemorrage (4 patients), subphrenic infection (2 patient), bile leakage (1 patient).
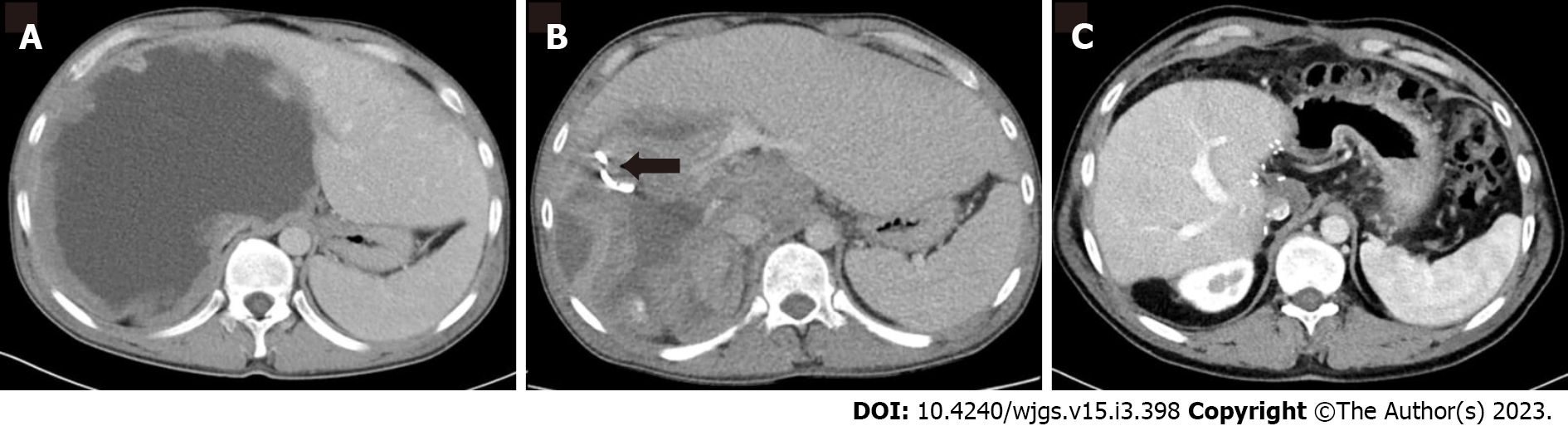
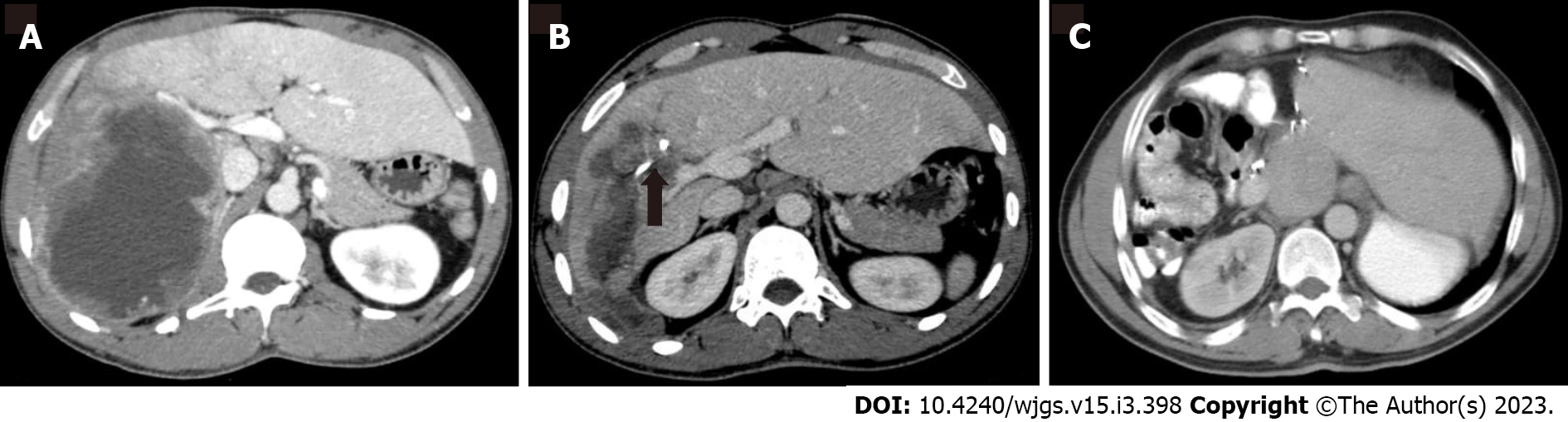
Figures 1 and 2 demonstrate the significant radiologic findings of surgically treated patients.
Mean age of the patients was 64.5 ± 6.1 years, 20 patients (52.6%) were female and 18 (47.3%) patients were male.
Twenty-eight lesions mostly located in the right lobe (73.6 %) and 9 lesions primarily located in the left lobe (32.1%). There was bilateral involvement in one case (2.6%). Cyst infection was detected at 11 (28.9%) cases.
Intercostal route was used in six patients (15.7 %), whereas subcostal approach was preferred in the rest. All cysts were effectively drained, and no significant complications associated with catheter drainage were observed during follow-up. Complication rate was significantly higher in surgical treatment group than interventional radiologic treatment (P = 0.001). Catheters were replaced due to blockage or stenosis in six patients. All patients admitted prophylactic antibiotics and albendazole.
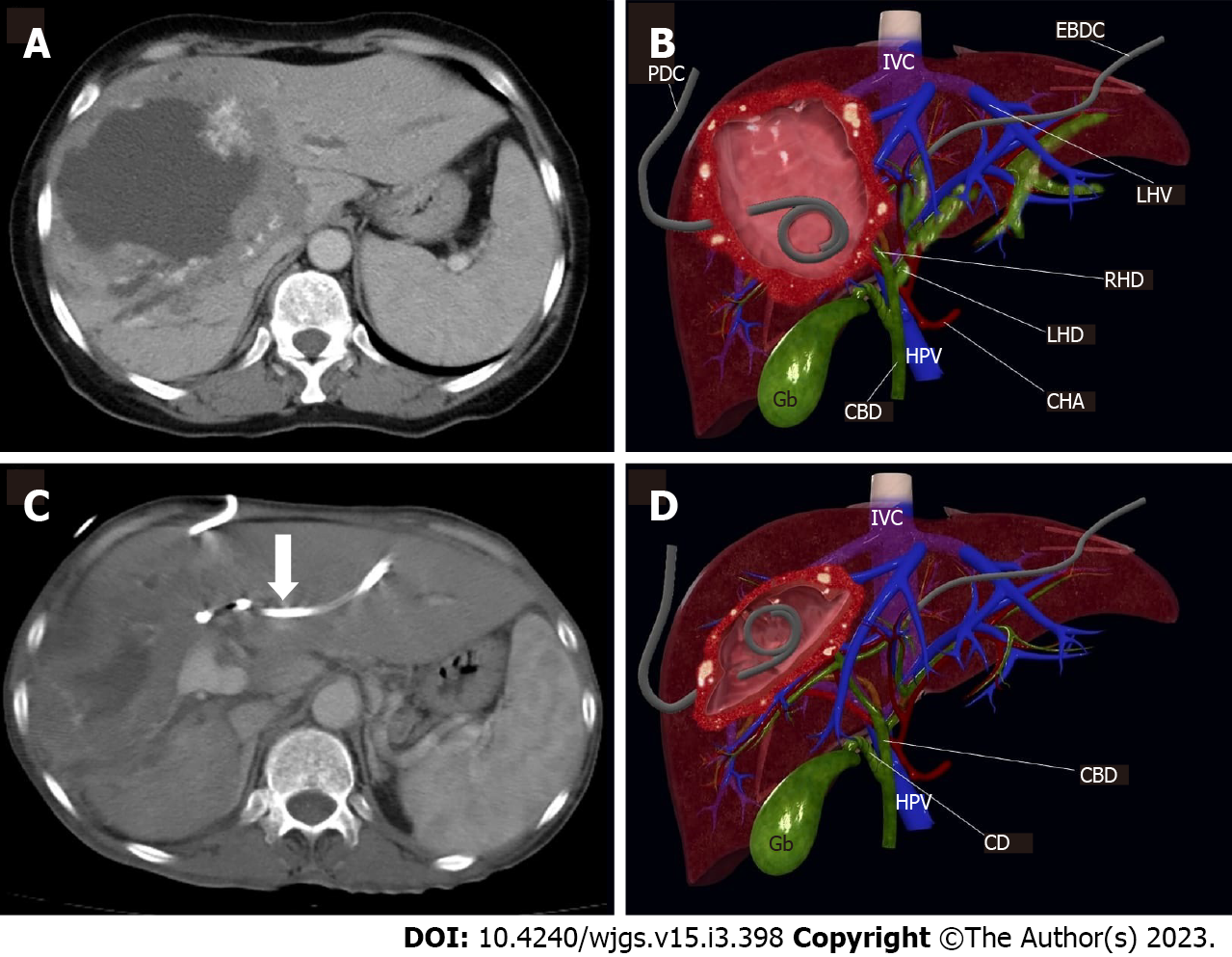
Figure 3 demonstrates the significant findings of interventional radiologic treatment. Table 2 contains further information about the patients covered.
| No. | Age/gender | Location of cyst | Cystic content | Percutaneous treatment | Surgery |
| 1 | 35/M | RL | Infected | PD | LT |
| 2 | 47/F | LL | Non-infected | PD | No |
| 3 | 58/F | RL | Non-infected | PD-PBD | No |
| 4 | 60/F | LL | Non-infected | PD | No |
| 5 | 37/M | RL | Non-infected | PD | Right lobectomy |
| 6 | 66/F | RL | Non-infected | PD | No |
| 7 | 33/F | RL | Non-infected | PD | LT |
| 8 | 36/F | RL | Non-infected | PD | LT |
| 9 | 36/M | RL | Non-infected | PD | LT |
| 10 | 52/F | RL | Infected | PD-PBD | LT |
| 11 | 57/M | RL | Non-infected | PD | LT |
| 12 | 33/F | RL | Infected | PD | Right lobectomy |
| 13 | 26/F | RL | Infected | PD | Right lobectomy |
| 14 | 62/M | RL | Non-infected | PD | No |
| 15 | 39/M | LL | Infected | PD | No |
| 16 | 59/M | LL | Non-infected | PD | No |
| 17 | 66/F | LL | Non-infected | PD | No |
| 18 | 21/F | LL | Infected | PD | Left lobectomy |
| 19 | 24/M | RL | Non-infected | PD | LT |
| 20 | 35/M | RL | Non-infected | PD | LT |
| 21 | 51/M | RL | Non-infected | PD | Right lobectomy |
| 22 | 44/F | RL | Non-infected | PD | No |
| 23 | 28/M | RL | Infected | PD | LT |
| 24 | 57/F | RL | Non-infected | PD | LT |
| 25 | 71/F | RL | Non-infected | PD | No |
| 26 | 17/M | RL | Non-infected | PD | No |
| 27 | 39/F | RL | Non-infected | PD | No |
| 28 | 71/F | RL | Infected | PD | No |
| 29 | 50/F | RL | Infected | PD | Right lobectomy |
| 30 | 34/M | Bilateral | Non-infected | PD | No |
| 31 | 28/M | RL | Non-infected | PD | No |
| 32 | 50/M | LL | Non-infected | PD | Left lobectomy |
| 33 | 15/M | LL | Infected | PD | Inoperable |
| 34 | 61/M | RL | Infected | PD | Inoperable |
| 35 | 45/F | LL | Non-infected | PBD | No |
| 36 | 53/F | RL | Non-infected | PBD | LT |
| 37 | 25/F | RL | Non-infected | PBD | LT |
| 38 | 43/F | RL | Non-infected | PBD | LT |
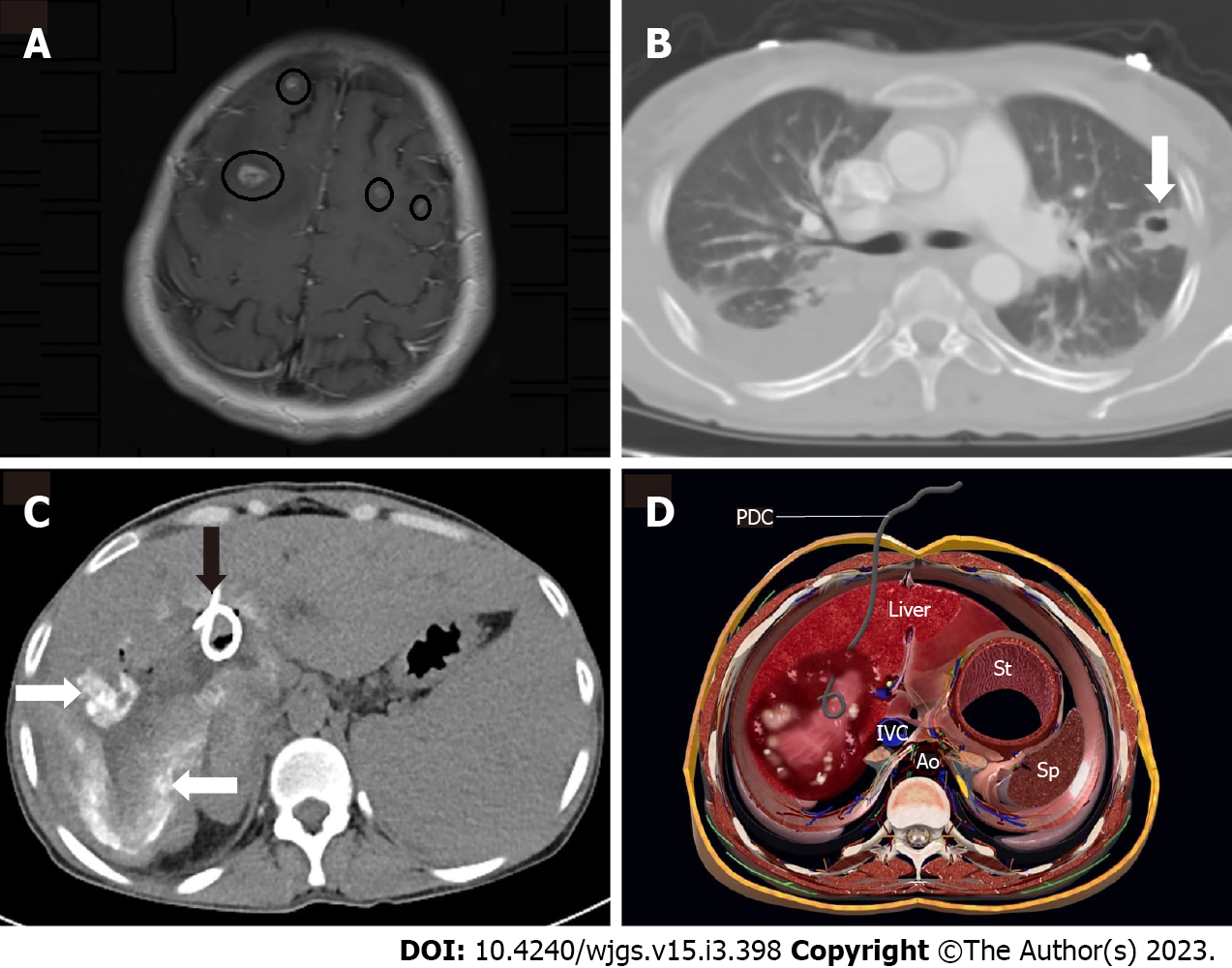
There were no cases of extrahepatic alveolar echinococcosis in the surgical therapy group. Whereas, in the interventional radiologic treatment subgroup, six patients were diagnosed with extrahepatic alveolar echinococcosis: three in the lung, one in the adrenal gland, one in the brain (Figure 4), and one in the peritoneal cavity.
Alveolar echinococcosis is one of the most dangerous and potentially fatal zoonoses on the planet, and it appears to be spreading across Europe[1,7]. In 97 percent of patients, lesions begin in the liver. Due to the larva's slow growth rate, it behaves similarly to a slow-growing invasive tumor that eventually invades the liver parenchyma, arteries, and bile ducts. In severe HAE, the mass may invade all neighboring organs and spread hematogenously to distant organs such as the lungs and brain[8,9].
There are studies in the literature that show that familial factors may influence susceptibility to alveolar echinococcosis[10]. However, family clustering is extremely uncommon in alveolar echinococcus. The rate of blood ties between patients was found to be 13% in a study of 153 people. As a result, in the event of alveolar echinococcosis, the family should be screened with US[11].
Immunoserologic studies based on the use of EM are useful in diagnosing and determining the EM agent. In the United States, HAE lesions frequently present as ill-defined heterogeneous infiltrations. While necrosis and infection cause hypoechoic foci inside the mass, hyperechoic zones are associated with fibro-parasitic tissue and dispersed calcifications. CT demonstrates the invading mass's characteristic calcifications more clearly. Triphasic contrast enhanced CT imaging is very beneficial for determining the vascular and biliary extension and invasion of neighboring tissue. With its high sensitivity to soft tissue, MRI is extremely useful for detecting satellite liver lesions, invasions, and central nervous system lesions, as well as examining the biliary tract[12].
Due to the sluggish growing rate of cysts, there is typically an asymptomatic phase before diagnosis of several years. The clinical appearance is similar to that of slow-growing liver cancer, and severe illness almost always involves invasion of the biliary and vascular walls. Although it is always fatal if not properly treated, early detection and treatment offer a better prognosis[13,14].
Although radical liver resection is the preferred method of treatment in order to prevent palliative surgical procedures, total excision of the mass is frequently not possible. LT should be regarded as a viable option for life-saving treatment. LT, on the other hand, is not always feasible and is contraindicated in patients with residual or metastatic HAE[15,16]. Depending on the degree of liver surgery, documented complication rates ranged from 15% to 36%, and fatality rates after excision ranged from 3% to 4.2%[17]. Similarly to the literature, the complication rate of surgical treatment in our sample was 17.2%, but we identified no issues in the interventional radiologic treatment subgroup, despite having a higher mean age. This suggests that interventional radiological therapy may be a viable treatment option for alveolar echinococcosis.
Despite all major surgical procedures, only half of the patients recovered completely. Interventional radiological treatments have been developed over time and have replaced palliative surgeries[18,19]. Drugs that inhibit parasitic growth are also crucial in the treatment of alveolar echinococci. In a series of 37 patients, Bresson-Hadni et al[14] used a multidisciplinary approach. In comparison to the past, only one patient received a liver transplant, and palliative surgery rates dropped by 80% in the literature.
HAE lesions are divided into three types: solid, pseudocystic, and mixed. While percutaneous cyst drainage can be performed in pseudocystic and mixed forms, percutaneous biliary drainage can be performed in any form as a palliative treatment for biliary stasis[20].
Cyst enlargement may result in compression or obstruction of the circulatory and biliary systems. Cyst necrosis and infection are two primary factors that contribute to fast cyst growth. The mass's inadequate vascularization frequently results in necrosis in the center portion of the lesion. Necrosis increases the intra-cystic pressure and mass effect, which may result in biliary stasis, cysto-biliary fistula, or necrotic cyst content rupture into the peritoneal and/or pleural space. With lower intracystic pressure, catheter drainage of necrotic material minimizes these problems[21].
Cyst infection is a significant consequence of HAE and may present acutely as cholangitis and septicemia, mimicking a liver abscess. Catheter drainage of infected cysts, such as liver abscess, should be performed until favorable conditions for major surgery are achieved. Because surgical therapy is contraindicated in acutely infected cysts, transcatheter drainage of a life-threatening bacterial or fungal infection within the cyst may be performed as a bridge operation in symptomatic patients prior to a curative surgical procedure. Catheter drainage can alleviate both the symptoms associated with abscess and the compressive symptoms on the arteries and biliary tree[8,21]. Eleven of our patients with infected cystic content were successfully treated with drainage.
In cases of infectious manifestations of centro-parasitic abscess or cholangitis, radiological interventional procedures are extremely useful. Percutaneous drainage of massive centro-parasitic abscesses, combined with systemic antibiotics, significantly improves the patient's clinical status. It is especially useful in elderly patients for whom a partial hepatectomy is not an option. Radiological interventional procedures are also very helpful in cases of cholangitis caused by parasitic tissue infiltration of the biliary tree and the resulting fibro-inflammatory reaction[22].
Biliary blockage symptoms typically emerge as a result of direct invasion of the major bile ducts or as a result of HAE's mass effect. Cholangitis symptoms may also be present as a result of parasite mass connection with bile ducts or pigment stones accumulating above a parasitic biliary stenosis[8]. Biliary blockage and cholangitis result in a more rapid decline in liver function, as well as mass destruction of the liver tissue. In these instances, if the patient has a large necrotic mass, we choose cyst drainage to alleviate tension on the biliary tree and major arteries. In certain cases, biliary stasis symptoms may improve with cyst draining alone due to the cyst's reduction in size. If biliary invasion and extensive cysto-biliary fistulas are present, the bile content of the cyst can be drained concurrently with the cyst content without extra biliary drainage. If these individuals benefit just from cyst drainage, this approach may be beneficial in avoiding the use of several catheters and may be sufficient in the interim till surgery. If patients have not demonstrated sufficient benefit from cyst draining, catheterization of one or two sides of the biliary tree should alleviate symptoms of biliary stasis.
In patients who are unable to undergo surgery, percutaneous cyst drainage and/or percutaneous biliary drainage with albendazole medication are the only treatment options available to protect these patients from re-infection, rupture, and to alleviate compressive symptoms. Biliary stenting may be combined with percutaneous biliary drainage or cyst draining if necessary[2]. We also have examples extrahepatic alveolar echinococcosis. Percutaneous cyst draining was used to treat one incidence of metastases to peritoneal cavity due to infected cystic material. Another case with cerebral metastases was treated with percutaneous draining of the infected cyst and albendazole (10 mg/kg) medical treatment. Extrahepatic involvement was not uncommon in patients treated with interventional radiologic procedures, according to our patient sample. The relevance of the radiologic workup before deciding on the sort of treatment is highlighted at this point. Extensive exams for extrahepatic involvement, particularly with CT or MRI, can aid in determining the best course of treatment.
Although percutaneous drainage is extremely beneficial in HAE patients, our cases had certain restrictions. Transcatheter draining of alveolar cysts is more challenging than conventional cyst drainage. To begin, we generally opted to apply US instructions. Nonetheless, the cyst's appearance was obscured in some cases due to significant calcification or strong fibrosis. In some patients, CT guidance for needle entry and catheter placement may be required. Second, the cyst capsule proved difficult to puncture due to its rigid nature. As a result, we are required to employ a thick needle for entrance and to dilate the tract prior to catheter drainage installation. Prior to catheter implantation, it is beneficial to lower positive pressure within the cyst via cystic content aspiration to minimize peritoneal seeding in these circumstances. Nonetheless, catheter exchange is required in the majority of patients undergoing follow-up.
Interventional radiology is utilized to drain echinococcal necrosis and abscesses within/without the liver, as well as diseased and clogged bile ducts, in HAE cases, either as palliative operations or as a bridge to radical resection. These techniques not only relieve pressure on the hepatic arteries and biliary path, but also make surgical resection easier by minimizing peripheral granulation tissue. In acutely infected patients, percutaneous drainage of cyst contents and/or biliary channels using a minimally invasive technique is a very beneficial, if not required, surgery that can save lives in some circumstances. Percutaneous cyst drainage with albendazole therapy improves quality of life in patients who are unable to undergo surgery, even when the mass resolves with long-term treatment.
Alveolar echinococcus is a zoonotic infection that can be fatal in humans. In our study, we conducted a brief assessment of the role of interventional radiology in the treatment of alveolar echinococcus.
Despite radical surgical procedures, the rate of complete recovery from alveolar echinococcus is quite low. Patients can benefit greatly from an increase in the success rates of treatments performed with interventional radiology.
The goal of our research is to compare the success rates of interventional radiological methods to surgical methods.
Our clinic's experience and those mentioned in the literature, as well as surgical methods, were compared.
Interventional radiology can be used to treat infected alveolar echinococci in particular.
Interventional radiology can be used to treat infected alveolar echinococci in particular. Palliative surgery rates may thus fall.
In our study, it was aimed to provide convenience for the patient and save time and money by advancing interventional radiological treatment methods in the treatment of alveolar echinococcus.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corrales FJ, Spain; Tavan H, Iran S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, Wassermann M, Takahashi K, de la Rue M. Ecology and Life Cycle Patterns of Echinococcus Species. Adv Parasitol. 2017;95:213-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 301] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 2. | Kantarci M, Bayraktutan U, Karabulut N, Aydinli B, Ogul H, Yuce I, Calik M, Eren S, Atamanalp SS, Oto A. Alveolar echinococcosis: spectrum of findings at cross-sectional imaging. Radiographics. 2012;32:2053-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C, Vuitton DA. The Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv Parasitol. 2017;96:259-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 4. | Bulakçı M, Kartal MG, Yılmaz S, Yılmaz E, Yılmaz R, Şahin D, Aşık M, Erol OB. Multimodality imaging in diagnosis and management of alveolar echinococcosis: an update. Diagn Interv Radiol. 2016;22:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Dagoglu-Kartal MG, Ciftci T, Ozer C, Akinci D, Akhan O. Case Report: Role of Interventional Radiology in the Management of Patients with Alveolar Echinococcus: Successful Management of Three Cases. Am J Trop Med Hyg. 2018;98:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Koroglu M, Akhan O, Gelen MT, Koroglu BK, Yildiz H, Kerman G, Oyar O. Complete resolution of an alveolar echinococcosis liver lesion following percutaneous treatment. Cardiovasc Intervent Radiol. 2006;29:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Brehm K. The role of evolutionarily conserved signalling systems in Echinococcus multilocularis development and host-parasite interaction. Med Microbiol Immunol. 2010;199:247-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Bresson-Hadni S, Miguet JP, Mantion G, Giraudoux P, Vuitton DA. L'échinococcose alvéolaire: une maladie comparable à un cancer du foie à marche lente [Alveolar echinococcosis: a disease comparable to a slow growing cancer]. Bull Acad Natl Med. 2008;192:1131-1138. [PubMed] |
| 9. | Moray G, Shahbazov R, Sevmis S, Karakayali H, Torgay A, Arslan G, Savas N, Yilmaz U, Haberal M. Liver transplantation in management of alveolar echinococcosis: two case reports. Transplant Proc. 2009;41:2936-2938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Vuitton DA, Zhang SL, Yang Y, Godot V, Beurton I, Mantion G, Bresson-Hadni S. Survival strategy of Echinococcus multilocularis in the human host. Parasitol Int. 2006;55 Suppl:S51-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Yang YR, Ellis M, Sun T, Li Z, Liu X, Vuitton DA, Bartholomot B, Giraudoux P, Craig PS, Boufana B, Wang Y, Feng X, Wen H, Ito A, McManus DP. Unique family clustering of human echinococcosis cases in a chinese community. Am J Trop Med Hyg. 2006;74:487-94. [PubMed] |
| 12. | Bresson-Hadni S, Delabrousse E, Grenouillet F, Mantion G, Vuitton DA. [Alveolar echinococcosis: how to confirm the diagnosis? Bull Acad Natl Med. 2008;192:1141-1149. [PubMed] |
| 13. | Piarroux M, Piarroux R, Giorgi R, Knapp J, Bardonnet K, Sudre B, Watelet J, Dumortier J, Gérard A, Beytout J, Abergel A, Mantion G, Vuitton DA, Bresson-Hadni S. Clinical features and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a survey in 387 patients. J Hepatol. 2011;55:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Bresson-Hadni S, Delabrousse E, Blagosklonov O, Bartholomot B, Koch S, Miguet JP, Mantion GA, Vuitton DA. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol Int. 2006;55 Suppl:S267-S272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Kawamura N, Kamiyama T, Sato N, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamaga S, Matsushita M, Todo S. Long-term results of hepatectomy for patients with alveolar echinococcosis: a single-center experience. J Am Coll Surg. 2011;212:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Vuitton DA, Bresson-Hadni S, Giraudoux P, Bartholomot B, Laplante JJ, Delabrousse E, Blagosklonov O, Mantion G. [Alveolar echinococcosis: from an incurable rural disease to a controlled urban infection]. Presse Med. 2010;39:216-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Salm LA, Lachenmayer A, Perrodin SF, Candinas D, Beldi G. Surgical treatment strategies for hepatic alveolar echinococcosis. Food Waterborne Parasitol. 2019;15:e00050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Bresson-Hadni S, Vuitton DA, Bartholomot B, Heyd B, Godart D, Meyer JP, Hrusovsky S, Becker MC, Mantion G, Lenys D, Miguet JP. A twenty-year history of alveolar echinococcosis: analysis of a series of 117 patients from eastern France. Eur J Gastroenterol Hepatol. 2000;12:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Roche G, Canton P, Gerard A, Colin D, Boissel P, Chaulieu C, Dureux JB. Essai de traitement de l'échinococcose alvéolaire par le flubendazole. A propos de 7 observations. Med Mal Infect. 1982;12:218-230. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Liu YH, Wang XG, Gao JS, Qingyao Y, Horton J. Continuous albendazole therapy in alveolar echinococcosis: long-term follow-up observation of 20 cases. Trans R Soc Trop Med Hyg. 2009;103:768-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Matsumoto J, Yagi K. Experimental studies on Echinococcus multilocularis in Japan, focusing on biohazardous stages of the parasite. Exp Parasitol. 2008;119:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |